Extracellular adenosine triphosphate affects the maturation of human monocyte–derived dendritic cells (DCs), mainly by inhibiting T-helper 1 (Th1) cytokines, promoting Th2 cytokines, and modulating the expression of costimulatory molecules. In this study, we report that adenosine triphosphate (ATP) can induce immunosuppression through its action on DCs, defining a new role for extracellular nucleotides. Microarray analysis of ATP-stimulated human DCs revealed inter alia a drastic up-regulation of 2 genes encoding mediators involved in immunosuppression: thrombospondin-1 (TSP-1) and indoleamine 2,3-dioxygenase (IDO). The release of TSP-1 by DCs in response to ATP was confirmed at the protein level by enzyme-linked immunosorbent assay (ELISA), immunodetection, and mass spectrometry analysis, and has an antiproliferative effect on T CD4+ lymphocytes through TSP-1/CD47 interaction. Our pharmacologic data support the involvement of purinergic receptor P2Y11 in this ATP-mediated TSP-1 secretion. We demonstrate also that ATP significantly potentiates the up-regulation of IDO—a negative regulator of T lymphocyte proliferation—and kynurenine production initiated by interferon-γ (IFN-γ) in human DCs.
Thus, extracellular ATP released from damaged cells and previously considered as a danger signal is also a potent regulator of mediators playing key roles in immune tolerance. Consequently, nucleotides' derivatives may be considered as useful tools for DC-based immunotherapies.
Introduction
Dendritic cells (DCs) are antigen-presenting cells (APCs) playing a crucial role in the induction and regulation of immune responses. In response to danger signals like proinflammatory cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β); pathogen-related molecules (lipopolysaccharide [LPS], double-stranded RNA, bacterial DNA); and T cell–derived signals such as CD40 ligand, immature dendritic cells undergo maturation. Maturation of DCs induces the loss of endocytosis, the surface expression of stable major histocompatibility complex (MHC)–peptide complexes and costimulatory molecules (CD80, CD86) and the production of cytokines such as IL-12.1 Maturation is also accompanied by a shift in the expression of chemokines and their receptors, allowing DC migration to lymphoid organs. High secretion of IL-12 by mature DCs induces differentiation of CD4+ T cells into T-helper 1 (Th1) cells secreting interferon-γ (IFN-γ), whereas low IL-12 release combined with IL-10 production induces a Th2 response or a T regulatory response associated with induced tolerance.1,2
In the past several years, our concepts about the role of DCs as the most potent initiators of the immune response against foreign antigens have evolved and the existence of an intermediate state between immaturity and maturation, called semimaturation, has been proposed.3 An increasing number of reports provide strong evidence that APCs, and particularly DCs, are also involved in central and peripheral tolerance.4,5 This tolerogenicity seems to be mediated by both specialized regulatory DCs and DCs undergoing semimaturation.
Extracellular nucleotides are released inter alia from necrotic cells and can therefore alert the immune system of abnormal cell death.6 In human monocyte-derived DCs, adenosine triphosphate (ATP) induces the up-regulation of costimulatory molecules (such as CD80, CD83, and CD86)6-8 and adhesion molecules such as CD54,9 and regulates various chemokines (CXC chemokine ligand 10 [CXCL10], CC chemokine ligand 5 [CCL5], CCL22) and chemokine receptors (CXC chemokine receptor 4 [CXCR4], CC chemokine receptor 7 [CCR7], CCR5).9 DCs exposed to ATP show an increased capacity to migrate to lymph nodes and a reduced ability to attract Th1 lymphocytes.9,10 ATP regulates also the action of LPS on human DCs: inhibition of the production of proinflammatory cytokines such as IL-12, IL-1β, TNF-α, and IL-6, and potentiation of anti-inflammatory IL-10.6,8,11 This profile of action is similar to that of cyclic adenosine monophosphate (cAMP)–elevating agents (ie, prostaglandin E2 [PGE2]) and is indeed associated with an increase in cAMP, presumably mediated by the purinergic receptor P2Y11.8,10 ATP, via inhibition of IL-12 and potentiation of IL-10, will thus impair the initiation of a Th1 response and favors a Th2 response or tolerance.6,11 Recently, Schnurr et al12 found that ATP also inhibited IL-27 expression but enhanced IL-23 expression—2 IL-12 family members—induced by intact Escherichia coli.
In this article, we report for the first time the critical role of ATP-mediated signal transduction in up-regulating 2 targets (thrombospondin-1 [TSP-1] and indoleamine 2,3-dioxygenase [IDO]) involved in T-cell immunosuppression, to investigate the potential role of extracellular nucleotides in immune tolerance.
Materials and methods
Reagents
ATP, adenosine 5′-O-(3-thiotriphosphate) (ATPγS), adenosine 5′-O-(2-thiodiphosphate) (ADPβS), 2′-3′-O-(4-benzoylbenzoyl)-ATP (BzATP), prostaglandin E2 (PGE2), LPS, forskolin, A23187, apyrase, adenosine deaminase, and pertussis toxin were obtained from Sigma (Saint Louis, MO). 2-Propylthio-β,γ-dichloromethylene-d-ATP (AR-C67085MX) was a generous gift from Drs J. D. Turner and D. Shah (AstraZeneca, Wilmington, DE). The anti-CD3 mAb OKT3 (Orthoclone OKT3) was provided by Janssen-Cilag (Berchem, Belgium), and the anti-CD28 monoclonal antibody (mAb) (clone CD28.2) was supplied by BD PharMingen (San Diego, CA). [3H]-thymidine (92.5 × 1010 Bq/mM [25 Ci/mM]) was from Moravek Biochemicals (Brea, CA). The C6.7 mAb that interferes with the ligation of human TSP-1 to CD47 was from LabVision (Fremont, CA).
Preparation of monocyte-derived dendritic cells
Immature human DCs were derived from adherent peripheral blood monocytes obtained from buffy coats of healthy volunteer donors as described previously.13 After 5 or 6 days of culture in the presence of 800 U/mL of granulocyte-macrophage colony-stimulating factor (GM-CSF) and 500 U/mL of IL-4, cells were replated at 1 × 106 cells/mL in 24 multiwells in complete medium with GM-CSF and IL-4. Nucleotides or other tested agents were then added for different periods of time.
Flow cytometric analysis
The purity of each cell preparation was evaluated using fluorescein isothiocyanate (FITC)–conjugated anti–human CD3, CD4, CD8, CD45RA (for lymphocytes), and CD1a, CD3, CD14, CD80, CD83, CD86 and human leukocyte antigen (HLA)–DR (for DCs) supplied by PharMingen (San Diego, CA) on 2 × 105 cells in 100 μL of phosphate-buffered saline (PBS) with 0.1% sodium azide for 30 minutes in the dark at 4°C. Cells were washed with 1 mL of PBS and analyzed on a FACSort (Becton Dickinson, Franklin Lakes, NJ). Data were analyzed using Cellquest software (Becton Dickinson); the number of events was at least 10 000.
Microarray analysis
Immature DCs were stimulated by ATPγS (100 μM) for 6 hours in the complete RPMI medium. The stimulation was stopped by addition of TRIZOL reagent (Life Technologies, Groningen, The Netherlands). RNA was extracted using TRIZOL reagent and purified on a RNeasy kit column (Qiagen, Hilden, Germany). Amplified antisense RNA (5 μg) was labeled by reverse transcription using SuperScript II (Invitrogen, Carlsbad, CA) and hybridized to an array containing on average 21 000 human cDNA fragments (Vlaams Institut voor Biotechnologie [VIB] Microarray Facility, Leuven, Belgium).14 The experiments were made including colorflip with 2 preparations of DCs from 2 independent donors.
ELISA
Human thrombospondin (TSP-1) was measured in DC supernatants by enzyme-linked immunosorbent assay (ELISA) using a commercially available kit from Chemicon International (Temecula, CA). IL-10 and IFN-γ were measured in supernatants by ELISA using commercially available kits from BioSource (Nivelles, Belgium).
Western blotting experiments
Immature DCs were seeded (106 cells/mL) and treated for different periods of time with or without different agents (PGE2,ATP,ATPγS, and/or IFN-γ). For TSP-1 detection, supernatants were collected at the end of incubation, and centrifuged to remove nonadherent cells. Each supernatant (40 μL) was loaded on a 7.5% sodium dodecyl sulfate (SDS)–polyacrylamide gel. For IDO detection, cells were washed once with PBS and lysed on ice in 150 μL Laemmli buffer. The protein concentration was determined using the method of Minamide and Bamburg.15 The same amount of protein (25 μg) for each condition was loaded and separated on a 12% SDS–polyacrylamide gel. Proteins were then transferred overnight at 60 V and 4°C onto a nitrocellulose membrane using 20 mM Tris (tris(hydroxymethyl)aminomethane), 154 mM glycine, 20% (vol/vol) methanol as a transfer buffer. Immunodetection was achieved with anti–TSP-1 (1:200; Abcam, Cambridge, United Kingdom) or anti-IDO antibody (1:500; a generous gift from B. van den Eynde, Université Catholique de Louvain, Belgium), antibodies using the Western Lightning Chemiluminescence Reagent Plus detection system (PerkinElmer, Brussels, Belgium) with a biotinylated secondary rabbit antibody (Amersham Biosciences, Freiburg, Germany).
Mass spectrometry analysis
Immature DCs were washed 4 times with serum-free medium, seeded (106 cells/mL) in RPMI without serum, and treated for different periods of time with or without ATPγS (100 μM). Supernatants (4 mL/condition) were collected and centrifuged to remove floating cells. Proteins were extracted from supernatants with StrataClean resin (Stratagene, La Jolla, CA) and loaded on an 8% SDS–polyacrylamide gel. After colloidal Coomassie blue staining, proteins of interest were excised and in-gel digested using trypsin (Promega, Madison, WI).16 Mass spectrometry analysis was performed on a quadrupole time-of-flight (Q-TOF) Ultima Global mass spectrometer equipped with a MALDI (matrix-assisted laser desorption/ionization) source (Micromass, Manchester, United Kingdom). Microsequencing was performed by Argon-induced fragmentation after selection of the parent ion.
T-cell proliferation assays
DCs were prepared as described and were stimulated by 300 μM ATP in complete medium. After 24 hours, supernatants of untreated and ATP-treated DCs were collected. In parallel, naive T CD4+ lymphocytes were prepared using T CD4+ isolation kit II and CD45RO microbeads (Miltenyi Biotec, Bergish Gladbach, Germany) as previously described.6 The CD4+ CD45RA+ T cells (2 × 105/well) were resuspended in 100 μL fresh medium and mixed with 100 μL DC supernatant. Then, they were activated in flat-bottom 96-well plates precoated with the anti-CD3 mAb (10 μg/mL) alone or in combination with soluble anti-CD28 mAb (1 μg/mL). After 48 hours of culture, proliferation was assessed by [3H]-thymidine (0.037 MBq/well [1 μCi/well]; ICN Biochemicals, Asse, Belgium) uptake during the next 16 hours. Each experimental condition was tested in triplicate.
Kynurenine measurements
DCs were stimulated with IFN-γ (10 and 100 U/mL) alone or in combination with ATP or ATPγS for 24 hours. Cells were then washed and resuspended in red-phenol–free complete medium supplemented with 300 μM L-tryptophan. After 5 hours, supernatants were collected and kynurenine concentration was quantified by high-performance liquid chromatography (HPLC).17 Culture supernatants (400 μL) were extracted with 80 μL 10% trichloroacetic acid; the precipitate was removed by centrifugation; and the supernatant was diluted in the initial mobile phase, composed of deionized water, 5% (vol/vol) methanol, 1% (vol/vol) acetic acid, and 5 mM of hexane sulfonic acid. Samples were injected onto a μBondapak C18 reverse phase (3.9 × 300 mm; Waters, Milford, MA) and eluted with a linear gradient of methanol (5%-40% over 35 minutes) at 1 mL/min. Absorbance was measured at 370 nm and compared against a standard curve of L-kynurenine.
Statistical analysis
Student t test was performed using Prism software (GraphPad, San Diego, CA).
Results
Identification of major target genes of adenosine triphosphates in human DCs
Microarray experiments were performed to select relevant target genes in the gene expression profile of adenosine triphosphates in human monocyte–derived DCs. ATPγS, which is more resistant to degradation by ectonucleotidases, was used instead of ATP to avoid additional gene regulations due to its degradation products, more particularly ADP and adenosine. Immature monocyte–derived DCs (106/mL) were incubated for 6 hours with or without 100 μM ATPγS. RNAs were then extracted, amplified, and labeled to hybridize an array containing 21.005 human sequences. The experiment was reproduced for 2 independent donors. From these experiments, it appeared that about 200 sequences were up-regulated or down-regulated at least 2 times in response to ATPγS. Table 1 shows regulated sequences reflecting DC maturation: up-regulation of CXCR4, CCR7, CD83, CD86 mRNAs, and down-regulation of CCR1, CCR5, and DC-specific transmembrane protein (DC-STAMP; DCST1/2) mRNAs (Table 1). Table 2 shows regulated genes involved in interactions between DCs and T lymphocytes such as interleukins (IL-10 [IL10] and IL-23 [IL23A]) or chemokines (CXCL10). We decided to focus the following experiments on 2 major target genes up-regulated by ATPγS that are involved in immune tolerance:18-23 TSP-1 (THBS1), which was clearly the most up-regulated gene, and IDO (INDO) (Table 2).
ATPγS-regulated markers of DC maturation
Origin . | GeneBank no. . | Unigene no. . | Name . | Description . | Ratio . |
|---|---|---|---|---|---|
| RZPD | AA479357 | Hs.421986 | CXCR4 | Chemokine (C-X-C motif) receptor 4 | 39.2 |
| RZPD | AI682706 | Hs.1652 | CCR7 | Chemokine (C-C motif) receptor 7 | 3.9 |
| RZPD | AA443748 | Hs.79197 | CD83 | CD83 antigen | 3.5 |
| RZPD | H16746 | Hs.27954 | CD86 | CD86 antigen | 1.8 |
| RZPD | AI268407 | Hs.211458 | DCST1/2 | DC-specific transmembrane protein | 0.18 |
| RZPD | AI417775 | Hs.511796 | CCR5 | Chemokine (C-C motif) receptor 5 | 0.13 |
| GS | D10925 | Hs.301921 | CCR1 | Chemokine (C-C motif) receptor 1 | 0.13 |
Origin . | GeneBank no. . | Unigene no. . | Name . | Description . | Ratio . |
|---|---|---|---|---|---|
| RZPD | AA479357 | Hs.421986 | CXCR4 | Chemokine (C-X-C motif) receptor 4 | 39.2 |
| RZPD | AI682706 | Hs.1652 | CCR7 | Chemokine (C-C motif) receptor 7 | 3.9 |
| RZPD | AA443748 | Hs.79197 | CD83 | CD83 antigen | 3.5 |
| RZPD | H16746 | Hs.27954 | CD86 | CD86 antigen | 1.8 |
| RZPD | AI268407 | Hs.211458 | DCST1/2 | DC-specific transmembrane protein | 0.18 |
| RZPD | AI417775 | Hs.511796 | CCR5 | Chemokine (C-C motif) receptor 5 | 0.13 |
| GS | D10925 | Hs.301921 | CCR1 | Chemokine (C-C motif) receptor 1 | 0.13 |
RZPD indicates Deutsches Resourcenzentrum für Genomforschung (Berlin, Germany); GS, Genome Systems (Wilmington, DE).
ATPγS-regulated genes involved in DC-T lymphocyte interactions
Origin . | GeneBank no. . | Unigene no. . | Name . | Description . | Ratio . |
|---|---|---|---|---|---|
| RZPD | AA043285 | Hs.164226 | THBS1 | Thrombospondin-1 | 199.6 |
| GS | AU138239 | Hs.840 | INDO | Indoleamine-pyrrole-2,3 dioxygenase | 7.8 |
| RZPD | AA418747 | Hs.98309 | IL23A | Interleukin 23, p19 | 3.1 |
| GS | AA382010 | Hs.193717 | IL10 | Interleukin 10 | 2.3 |
| RZPD | AA150307 | Hs.413924 | CXCL10 | Chemokine (C-X-C motif) ligand 10 | 0.5 |
Origin . | GeneBank no. . | Unigene no. . | Name . | Description . | Ratio . |
|---|---|---|---|---|---|
| RZPD | AA043285 | Hs.164226 | THBS1 | Thrombospondin-1 | 199.6 |
| GS | AU138239 | Hs.840 | INDO | Indoleamine-pyrrole-2,3 dioxygenase | 7.8 |
| RZPD | AA418747 | Hs.98309 | IL23A | Interleukin 23, p19 | 3.1 |
| GS | AA382010 | Hs.193717 | IL10 | Interleukin 10 | 2.3 |
| RZPD | AA150307 | Hs.413924 | CXCL10 | Chemokine (C-X-C motif) ligand 10 | 0.5 |
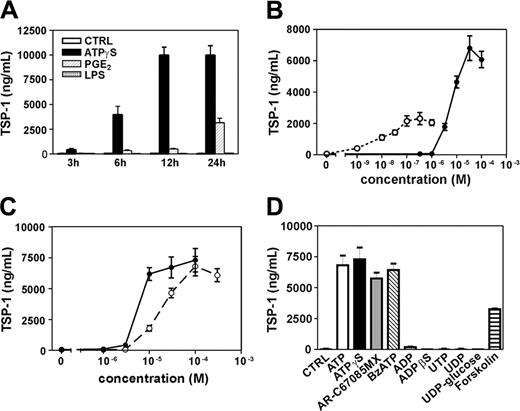
ATP is a potent activator of TSP-1 release in human DCs
We have quantified TSP-1 production from stimulated DCs by ELISA. DCs were stimulated for various periods of time with ATPγS, PGE2, and LPS. These last 2 agents were chosen because LPS is a major DC maturation signal and PGE2 displays effects similar to ATP in DCs through cAMP activation. As shown in Figure 1A and B, ATPγS is more efficient than PGE2 in stimulating TSP-1 release, whereas LPS had no effect. Moreover, TSP-1 production was observed already after 3 hours for ATPγS and rose to its maximum after 12 hours of stimulation. At 24 hours of stimulation, PGE2 effect represents 34.8% ± 6.0% of the ATPγS effect, which represents a peak of 10 μg/mL of TSP-1 (mean ± SD of 3 independent experiments). In addition, concentration-action curves of ATPγS and the physiologic ligand ATP have been compared (Figure 1C). ATPγS was more potent than ATP, with effective concentration at 50% (EC50) values of 6.1 ± 1.3 μM for ATPγS and 17.4 ± 3.2 μM for ATP (Figure 1C) (mean ± range of 2 independent experiments). We have also observed comparable TSP-1 production in response to other ATP derivatives such as AR-C67085MX (2-propyl-β,γ-dichloromethylene-D-ATP) and BzATP (2′-and 3′-O-(4-benzoyl-benzoyl)ATP), known to be P2Y11 agonists, while ADP, ADPβS (adenosine 5′-O-(2-thiodiphosphate), uridine triphosphate (UTP), uridine diphosphate (UDP), and UDP-glucose were inactive (Figure 1D). The cAMP-elevating agent forskolin (10 μM) was also able to initiate TSP-1 release (Figure 1D), whereas a preincubation of DCs with pertussis toxin (100 ng/mL; 16 hours before stimulation with ATP) did not affect ATP-mediated TSP-1 production (data not shown). These pharmacologic data support the involvement of P2Y11 receptor in ATP-mediated TSP-1 production. We then investigated whether the strong effect of ATPγS and ATP compared with PGE2 or forskolin on TSP-1 production was due to activation of both cAMP and phosphoinositide pathways. Calcium-elevating agents such as UTP or calcium ionophore A23187 were not able to induce any TSP-1 production (Figure 1D and data not shown) or to potentiate PGE2-mediated TSP-1 production (data not shown).
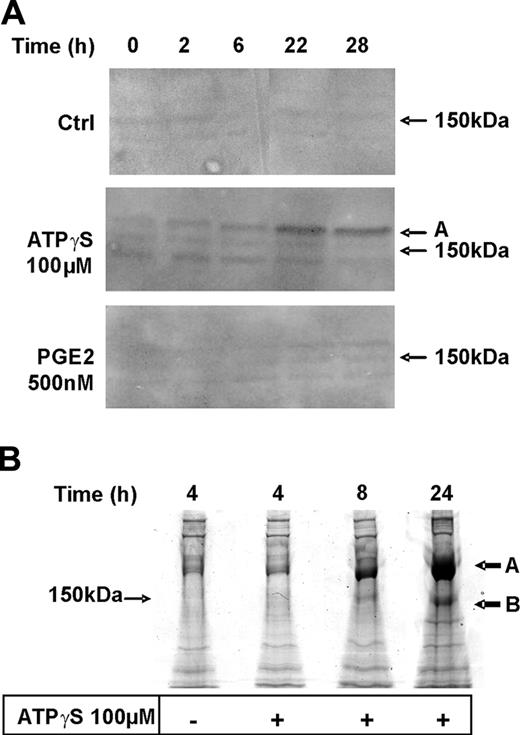
Proteomics analysis of nucleotide-mediated TSP-1 production by DCs
We then compared time courses of TSP-1 secretion in response to ATPγS and PGE2 by Western blotting experiments (Figure 2A). Three bands were detected using a polyclonal anti–TSP-1 antibody. A detectable signal (band A; 180 kDa) was observed after 6 hours and a significant signal after 22 hours of stimulation with ATPγS, whereas a weak signal was obtained only after 22 hours of stimulation with PGE2.
To confirm that the bands revealed in our immunodetection experiments (Figure 2A) correspond to human TSP-1, we performed an 8% SDS–polyacrylamide gel using supernatants from unstimulated DCs and DCs stimulated for various periods of time with ATPγS. As shown in Figure 2B, colloidal Coomassie blue staining revealed 1 highly modulated band (A) presenting an apparent molecular weight (MW) around 180 000 (band A) and another modulated band with an apparent MW of 150 000 (band B). A third lower band was slightly regulated and is likely to correspond to the lower band detected with anti–TSP-1 antibody. Intensities of bands A and B were sufficient to expect protein identification by mass spectrometry. They were excised and in-gel digested with trypsin. Peptide mass fingerprinting and microsequencing after fragmentation (sequenced peptide: N1114ALWHTGNTPGQVR1127) permitted to unambiguously identify bands A and B as intact human TSP-1, most probably with distinct posttranslational modifications.
Production of TSP-1 by human monocyte–derived DCs in response to nucleotides. (A) DCs were stimulated for various periods of time with either ATPγS (100 μM), PGE2 (500 nM), or LPS (100 ng/mL). (B) DCs were challenged with various concentrations of ATPγS (•) or PGE2 (○) for 24 hours. (C) DCs were challenged with various concentrations of ATPγS (•) or ATP (○) for 24 hours. (D) DCs were stimulated for 24 hours with ATPγS, AR-C67085MX, BzATP, and ADPβS at 100 μM and with ATP, ADP, UTP, UDP, and UDP-glucose at 300 μM. Supernatants of treated DCs were harvested for ELISA measurements of human TSP-1. Results are expressed as nanograms per 106 cells/mL and represent the mean ± range of duplicate points in 1 representative out of 3 (A) or 2 (B-D) independent experiments.
Production of TSP-1 by human monocyte–derived DCs in response to nucleotides. (A) DCs were stimulated for various periods of time with either ATPγS (100 μM), PGE2 (500 nM), or LPS (100 ng/mL). (B) DCs were challenged with various concentrations of ATPγS (•) or PGE2 (○) for 24 hours. (C) DCs were challenged with various concentrations of ATPγS (•) or ATP (○) for 24 hours. (D) DCs were stimulated for 24 hours with ATPγS, AR-C67085MX, BzATP, and ADPβS at 100 μM and with ATP, ADP, UTP, UDP, and UDP-glucose at 300 μM. Supernatants of treated DCs were harvested for ELISA measurements of human TSP-1. Results are expressed as nanograms per 106 cells/mL and represent the mean ± range of duplicate points in 1 representative out of 3 (A) or 2 (B-D) independent experiments.
Proteomics analysis of TSP-1 production by DCs in response to ATPγS. (A) DCs were either untreated (Ctrl) or treated with ATPγS (100 μM) or PGE2 (500 nM) for different periods of time in complete medium. Supernatants were collected at the end of incubation to perform Western blotting using an anti–TSP-1 rabbit polyclonal antibody (1:200). (B) DCs were treated with ATPγS (100 μM) for 4, 8, or 24 hours in serum-free medium. At the end of incubation, supernatants were collected and proteins were extracted and loaded on an 8% SDS–polyacrylamide gel. After colloidal Coomassie blue staining, proteins of interest (black arrows A and B) were excised and in-gel digested by trypsin.
Proteomics analysis of TSP-1 production by DCs in response to ATPγS. (A) DCs were either untreated (Ctrl) or treated with ATPγS (100 μM) or PGE2 (500 nM) for different periods of time in complete medium. Supernatants were collected at the end of incubation to perform Western blotting using an anti–TSP-1 rabbit polyclonal antibody (1:200). (B) DCs were treated with ATPγS (100 μM) for 4, 8, or 24 hours in serum-free medium. At the end of incubation, supernatants were collected and proteins were extracted and loaded on an 8% SDS–polyacrylamide gel. After colloidal Coomassie blue staining, proteins of interest (black arrows A and B) were excised and in-gel digested by trypsin.
ATP inhibits T CD4+ lymphocyte proliferation through TSP-1 release by DCs
To investigate the effect of soluble factors like TSP-1 secreted by DCs in response to ATP, the capacity of supernatants of these DCs to modulate proliferation of activated naive T CD4+ cells has been evaluated (Figure 3). We have used ATP instead of ATPγS in these assays to avoid the remaining presence of nucleotide in supernatants of treated DCs that are mixed with lymphocyte suspensions so that the inhibition of proliferation observed was thus not due to a direct negative effect of ATPγS on lymphocytes proliferation as already described,24 but to the release of soluble factors by DCs. T-cell activation was obtained using coated anti-CD3 mAb (10 μg/mL) alone (Figure 3A-B)—to mimic T-cell receptor (TCR) activation—or in combination with soluble anti-CD28 mAb (1 μg/mL; Figure 3C-G)—to mimic full activation. In both cases, supernatants from ATP-treated DCs reduced the proliferation of activated T CD4+ (Figure 3A,C): 86.7% ± 29.7% and 72.3% ± 25.9% of inhibition in the presence of anti-CD3 mAb alone or the combination of anti-CD3 and anti-CD28 mAbs, respectively (mean ± SD of 5 independent experiments; P < .01 according to the Student t test). The effect of human recombinant TSP-1 (7 μg/mL)—diluted in fresh medium—on T-cell proliferation was tested using the same preparation of DCs (Figure 3B,D).
CD47 receptor mediates inhibitory effects of TSP-1 on T lymphocyte early activation and proliferation.25 We have evaluated whether TSP-1 was one of the soluble factors responsible for the observed immunosuppressive effect mediated by ATP: we have preincubated DCs supernatants with an anti–human TSP-1–neutralizing antibody named C6.7 (Figure 3E), which blocks binding of TSP-1 to CD47 receptors.21 There was a consistent reduction of the ATP-mediated inhibition of T lymphocyte proliferation in the presence of anti–TSP-1 antibody (C6.7): from 71.5% ± 3.5% of inhibition to 23.3% ± 16.5% of inhibition (mean ± range from 2 independent experiments; P < .05 according to the Student t test). IFN-γ and IL-10 levels have been quantified by ELISA in these experiments. When T CD4+ lymphocytes were incubated with supernatants from ATP-treated DCs, Th1 and Th2 cytokine production was abolished (Figure 3F-G). Control experiments were also performed using supernatants of ATP-treated or untreated DCs preincubated for 30 minutes at room temperature with apyrase (50 U/mL) and/or adenosine deaminase (5 U/mL) to avoid any effect of residual ATP or degradation products such as ADP and adenosine. The use of these enzymes separately or in combination had no effect on T CD4+ proliferation and did not affect inhibition of proliferation observed with ATP-treated DC supernatants (data not shown).
Effect of supernatants from ATP-treated DCs on naive T CD4+ cells. Naive T CD4+ cells were partially activated (A) with coated anti-CD3 or (C) fully activated with coated anti-CD3 and soluble anti-CD28 in the presence of supernatant from nontreated DCs (CTRL) or ATP-treated DCs (ATP) for 48 hours. Cell suspensions were then additionally incubated for 16 hours in the presence of [3H]-thymidine (0.037 MBq/well [1 μCi/well]). The effect of human recombinant TSP-1 (7 μg/mL) was also tested on thymidine incorporation of partially activated (B) or fully activated (D) naive T CD4+ cells in fresh medium. (E) Supernatants from nontreated DCs (left) or ATP-treated DCs (right) were previously incubated for 2 hours at room temperature in the presence (▪) or the absence (□) of neutralizing anti–TSP-1 mAb at 25 μg/mL (clone C6.7, which selectively interferes with TSP-1/CD47 interaction). Fully activated T CD4+ cells were incubated in the presence of these DC supernatants for thymidine incorporation experiments. (F-G) Fully activated T CD4+ cells were incubated in the presence of supernatants from nontreated or ATP-treated DCs. After 48 hours, IFN-γ (F) or IL-10 (G) levels were measured by ELISA. Data represent the mean ± SD of triplicate experimental points obtained in 1 representative experiment out of 5 (A-D) or 2 (E-G).
Effect of supernatants from ATP-treated DCs on naive T CD4+ cells. Naive T CD4+ cells were partially activated (A) with coated anti-CD3 or (C) fully activated with coated anti-CD3 and soluble anti-CD28 in the presence of supernatant from nontreated DCs (CTRL) or ATP-treated DCs (ATP) for 48 hours. Cell suspensions were then additionally incubated for 16 hours in the presence of [3H]-thymidine (0.037 MBq/well [1 μCi/well]). The effect of human recombinant TSP-1 (7 μg/mL) was also tested on thymidine incorporation of partially activated (B) or fully activated (D) naive T CD4+ cells in fresh medium. (E) Supernatants from nontreated DCs (left) or ATP-treated DCs (right) were previously incubated for 2 hours at room temperature in the presence (▪) or the absence (□) of neutralizing anti–TSP-1 mAb at 25 μg/mL (clone C6.7, which selectively interferes with TSP-1/CD47 interaction). Fully activated T CD4+ cells were incubated in the presence of these DC supernatants for thymidine incorporation experiments. (F-G) Fully activated T CD4+ cells were incubated in the presence of supernatants from nontreated or ATP-treated DCs. After 48 hours, IFN-γ (F) or IL-10 (G) levels were measured by ELISA. Data represent the mean ± SD of triplicate experimental points obtained in 1 representative experiment out of 5 (A-D) or 2 (E-G).
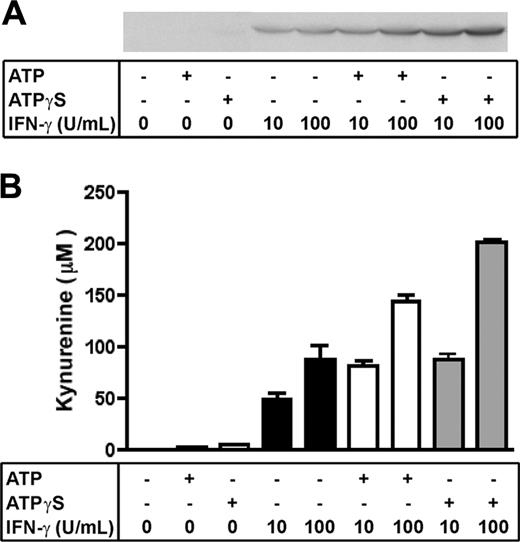
ATP regulates IDO expression and activity in human DCs
IDO, an enzyme involved in the catabolism of tryptophan, was shown to be a target gene of ATPγS in our microarray experiments. We have confirmed by reverse transcriptase–polymerase chain reaction (RT-PCR) experiments that ATPγS and ATP—but not PGE2—were able to up-regulate IDO mRNA in monocyte-derived DCs (data not shown). To confirm that DCs treated with ATPγS (100 μM) express IDO protein, we performed Western blotting experiments with a rabbit polyclonal antibody against human IDO. Figure 4A shows that no detectable signal was observed with ATPγS or ATP alone, whereas a signal was already detectable with 10 U/mL IFN-γ. Strikingly, 100 μM ATPγS and 300 μM ATP synergize with IFN-γ, at both 10 U/mL and 100 U/mL, to up-regulate IDO expression, ATPγS being more efficient.
Synergy between ATP/ATPγS and IFN-γ on the functional expression of IDO. (A) DCs were either untreated or treated with ATP (300 μM) or ATPγS (100 μM) alone or in the presence of IFN-γ (10 or 100 U/mL) for 24 hours in complete medium. The same amounts of total protein were analyzed by Western blotting with an anti–human IDO rabbit polyclonal antibody. (B) After a similar treatment with ATP (300 μM) or ATPγS (100 μM), DCs were washed and incubated for 5 additional hours in red-phenol–free RPMI supplemented with 300 μM L-tryptophan. Kynurenine levels were determined in each supernatant by HPLC. Data (mean ± range) were obtained in an experiment performed in duplicate and representative of 3 independent experiments.
Synergy between ATP/ATPγS and IFN-γ on the functional expression of IDO. (A) DCs were either untreated or treated with ATP (300 μM) or ATPγS (100 μM) alone or in the presence of IFN-γ (10 or 100 U/mL) for 24 hours in complete medium. The same amounts of total protein were analyzed by Western blotting with an anti–human IDO rabbit polyclonal antibody. (B) After a similar treatment with ATP (300 μM) or ATPγS (100 μM), DCs were washed and incubated for 5 additional hours in red-phenol–free RPMI supplemented with 300 μM L-tryptophan. Kynurenine levels were determined in each supernatant by HPLC. Data (mean ± range) were obtained in an experiment performed in duplicate and representative of 3 independent experiments.
As previously shown by other groups, expression of IDO is not always correlated with full enzymatic activity. To check its activity we performed an HPLC-based assay of kynurenine quantification, the main product of tryptophan degradation by IDO.22 Figure 4B shows that ATP or ATPγS alone does not induce a significant production of kynurenine, whereas IFN-γ does. Of interest, ATP and ATPγS potentiate the effect of IFN-γ and leads to an increase of kynurenine production of 168.8% ± 5.4% and 201.4% ± 3.0%, respectively, compared with 100 U/mL IFN-γ alone (mean ± SD of 3 independent experiments; P < .01 according to the Student t test).
Discussion
Gene profiling experiments allowed us to select relevant target genes of extracellular ATP in human DCs. Among the regulated genes, several regulations associated with DC maturation were observed, such as up-regulation of CXCR4, CCR7, and CD83, as well as down-regulation of CCR5, CCR1, and DC-STAMP, which are known to be expressed on immature DCs.9 It was previously shown that DCs exposed to ATP up-regulate costimulatory molecules such as CD83 and CD86.6-8 ATP is also able to up-regulate receptors for lymphoid chemokines and down-regulate receptors for inflammatory chemokines:9 this pattern of regulations favors a preferential lymph node localization of ATP-treated DCs. More particularly, down-regulation of CXCL10/IP-10 chemokine in response to ATP observed in this study was correlated previously with reduced attraction of Th1 lymphocytes using ATP-treated DC supernatants.9 Up-regulation of interleukins such as IL-10 and IL-23 by ATP in DCs was also reported6-8,11,12 and was confirmed by our microarray experiments.
Until now, understanding of the role of ATP in DC physiology was essentially focused on its capacity to trigger a Th2-polarized response. On the other hand, la Sala et al26 proposed that the ATP-induced alternative maturation of DCs leads to reduced inflammation and control of the immune response. In this context, we decided to explore a possible link between this semimature state and immune tolerance by focusing further analysis of our gene-profiling experiments on genes associated with tolerance. More particularly, TSP-1 and IDO are 2 major target genes strongly up-regulated by ATPγS and involved in T-cell immunosuppression.
First, our data demonstrate that stimulation of monocyte-derived DCs with extracellular ATP induces a strong and early expression and secretion of TSP-1 using microarray, ELISA, and immunodetection. Additional mass spectrometry analysis revealed that different forms of intact TSP-1 were up-regulated by ATPγS. Indeed, posttranscriptional modifications such as C-mannosylation and O-fucosylation have been reported for human TSP-1.27,28 The pharmacologic profile supports the involvement of P2Y11 receptor—which is the only P2 receptor coupled to cAMP pathway29,30 —in ATP-mediated TSP-1 production. Indeed, ATPγS was more potent than ATP, and we have observed comparable effects of other ATP derivatives such as AR-C67085MX and BzATP, known agonists of P2Y11 receptor.29 Moreover, the importance of cAMP increase in TSP-1 production was highlighted by the effect of forskolin and PGE2, which elevates cAMP levels through activation E-prostanoid 2 (EP2) receptors. Calcium-elevating agents were not able to induce any TSP-1 production or to potentiate PGE2-mediated TSP-1 production. The stronger effect of ATPγS and ATP on TSP-1 production could be explained by the activation of additional specific signal transduction proteins.
Cocultures of ATP-treated monocyte-derived DCs and naive T CD4+ lymphocytes have revealed positive effects of nucleotides on T lymphocyte proliferation6 reflecting DC maturation. An important point in the setup of these experiments is that ATP-treated DCs are washed before coculture with lymphocytes, and any potential effect of soluble factors released by DCs during nucleotide stimulation is lost. To circumvent this problem we designed a proliferation assay using supernatants from DCs treated with ATP or untreated. These experiments show that the high TSP-1 secretion by DCs in response to ATP leads to inhibition of T CD4+ cell proliferation through TSP-1/CD47 interaction as demonstrated by the use of an antibody blocking this interaction. The observed IFN-γ and IL-10 down-regulations suggest that ATP/DC-conditioned medium affects proliferation of both Th1 and Th2 lymphocytes.
TSP-1 is also reported to be an autocrine negative regulator of human DCs21 and to activate in vivo latent TGF-β1,31 a major component of the immunosuppressive response. We can conclude that ATP is able to inhibit T-cell proliferation through a direct effect on lymphocytes24 and indirectly through the activation of P2Y11 receptors expressed on immature DCs and subsequent TSP-1 secretion.
Among the ATP-regulated sequences, another major gene involved in immunosuppression has been identified that encodes IDO protein: an intracellular enzyme involved in the catabolism of tryptophan.22,23 Recent studies support that the tolerogenic capacity of DCs could be dependent on their expression of IDO, which consequently inhibits expansion of activated T lymphocytes.22,23 In humans, IDO has been shown to play a crucial role in tumoral immune resistance by depleting tryptophan locally and blocking the proliferation of alloreactive T lymphocytes.32 We have observed IDO up-regulation at the mRNA level in response to ATP alone. However, the presence of IFN-γ was necessary to observe the regulation of IDO protein and kynurenine production by ATP and ATPγS. Still, the mechanisms and the molecules that trigger IDO expression in human cells need to be clarified. In this context, the ability of ATP to potentiate kynurenine production in IFN-γ–treated DCs could be correlated with studies suggesting that costimulatory signals must be present to activate tolerogenic DCs.33-35 Previous studies showed that ATP can act as an important regulatory cofactor on the production of several cytokines (IL-12, IL-10, IL-23, and TNF-α)6-8,11,12 triggered by various stimuli such as Toll-like receptor (TLR) ligands. Our data show that this concept can be extended to the regulation of other molecules than cytokines such as the intracellular enzyme IDO. Of interest, tryptophan metabolites in the kynurenine pathway were shown to have immunosuppressive effects on Th1 but not on Th2 mouse lymphocytes.36 In our experiments, the high production of kynurenine (up to 150 μM) in response to ATP in combination with IFN-γ is sufficient to induce human T-cell immunosuppression through tryptophan metabolites as previously reported. Indeed, Terness et al37 have demonstrated that kynurenine inhibits T-cell proliferation induced by allogenic DCs with an inhibitory concentration at 50% (IC50) of 157 μM.
In conclusion, the present study demonstrates that ATP—concomitantly with its previously described action on DCs maturation—potentiates IDO regulation and induces high TSP-1 release from human DCs that consequently affects T CD4+ cell proliferation. These findings offer perspectives to manipulate pharmacologically the immune tolerance at the level of DCs highlighting P2Y11 as a preferential target to generate tolerogenic DCs using extracellular nucleotides.
Prepublished online as Blood First Edition Paper, August 23, 2005; DOI 10.1182/blood-2005-05-1843.
Supported by an Action de Recherche Concertée of the Communauté Française de Belgique; the Belgian Programme on Interuniversity Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Federal Service for Science, Technology, and Culture; and grants from the Fonds de la Recherche Scientifique Médicale and the Fonds Emile DEFAY. F.M. is supported by the Fonds National de la Recherche Scientifique/Fonds pour la Formation à la Recherche dans l'Industrie et dans l'Agriculture (FRIA), Belgium; Didier C. and David C. are Research Associates of the Fonds National de la Recherche Scientifique; and N.S.G. is supported by Euroscreen.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Prof B. van den Eynde from Université Catholique de Louvain, Belgium (UCL) for the generous gift of anti-IDO antibody. We thank Drs M. Moser, O. Leo, and P. Van Hummelen for helpful discussions.

![Figure 3. Effect of supernatants from ATP-treated DCs on naive T CD4+ cells. Naive T CD4+ cells were partially activated (A) with coated anti-CD3 or (C) fully activated with coated anti-CD3 and soluble anti-CD28 in the presence of supernatant from nontreated DCs (CTRL) or ATP-treated DCs (ATP) for 48 hours. Cell suspensions were then additionally incubated for 16 hours in the presence of [3H]-thymidine (0.037 MBq/well [1 μCi/well]). The effect of human recombinant TSP-1 (7 μg/mL) was also tested on thymidine incorporation of partially activated (B) or fully activated (D) naive T CD4+ cells in fresh medium. (E) Supernatants from nontreated DCs (left) or ATP-treated DCs (right) were previously incubated for 2 hours at room temperature in the presence (▪) or the absence (□) of neutralizing anti–TSP-1 mAb at 25 μg/mL (clone C6.7, which selectively interferes with TSP-1/CD47 interaction). Fully activated T CD4+ cells were incubated in the presence of these DC supernatants for thymidine incorporation experiments. (F-G) Fully activated T CD4+ cells were incubated in the presence of supernatants from nontreated or ATP-treated DCs. After 48 hours, IFN-γ (F) or IL-10 (G) levels were measured by ELISA. Data represent the mean ± SD of triplicate experimental points obtained in 1 representative experiment out of 5 (A-D) or 2 (E-G).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-05-1843/2/m_zh80240587260003.jpeg?Expires=1765004557&Signature=SKiR2xsbA5El4yCowk4I-5k05BP6j3yL2Io~5fSTvpdnvTqZ9n8z38Qr6oDQP-XJ0YIyBxN9JP8aSEvXluCiGM4pMOWU73YxCOEapeGv67NLiodAzZSfIxFPyJLqlxvZ2SrzZCppft9PbctG2IBzCMx~ENOLrtY6TmkUREhQ8Br2w35MW9jIINmmNvAc0mWOzPL3p5n4JZzHtQoXmjbJM1YSrZ8CfEW9DbzfWEt1J4sPJY6V-kIiCUXdnoWLplwmUTIpj9sty4VBF4QCqvhnC1f3zGVMA-qHKikYfzRlWTqNW~E7TASulBuZzhYTfHRl0WnOR3CFtIk6c6EnIeKr3g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal