Previous studies have implicated the immunoglobulin (Ig)–immunoreceptor tyrosine–based inhibitory motif (ITIM) superfamily member platelet endothelial cell adhesion molecule-1 (PECAM-1) in the regulation of integrin function. While PECAM-1 has been demonstrated to play a role as an inhibitory coreceptor of immunoreceptor tyrosine–based activation motif (ITAM)–associated Fcγ receptor IIa (FcγRIIa) and glycoprotein VI (GPVI)/FcR γ-chain signaling pathways in platelets, its physiologic role in integrin αIIbβ3–mediated platelet function is unclear. In this study, we investigate the functional importance of PECAM-1 in murine platelets. Using PECAM-1–deficient mice, we show that the platelets have impaired “outside-in” integrin αIIbβ3 signaling with impaired platelet spreading on fibrinogen, failure to retract fibrin clots in vitro, and reduced tyrosine phosphorylation of focal adhesion kinase p125 (125FAK) following integrin αIIbβ3–mediated platelet aggregation. This functional integrin αIIbβ3 defect could not be attributed to altered expression of integrin αIIbβ3. PECAM-1–/– platelets displayed normal platelet alpha granule secretion, normal platelet aggregation to protease-activated receptor-4 (PAR-4), adenosine diphosphate (ADP), and calcium ionophore, and static platelet adhesion. In addition, PECAM-1–/– platelets displayed normal “inside-out” integrin αIIbβ3 signaling properties as demonstrated by normal agonist-induced binding of soluble fluoroscein isothiocyanate (FITC)–fibrinogen, JON/A antibody binding, and increases in cytosolic-free calcium and inositol (1,4,5)P3 triphosphate (IP3) levels. This study provides direct evidence that PECAM-1 is essential for normal integrin αIIbβ3–mediated platelet function and that disruption of PECAM-1 induced a moderate “outsidein” integrin αIIbβ3 signaling defect.
Introduction
Platelet adhesion and aggregation at sites of injured vascular surfaces is essential for both hemostasis and thrombogenesis.1 Under normal physiologic conditions, platelet accrual at sites of vascular injury leads to formation of a haemostatic plug, while in vascular disease states such as atherosclerotic lesions, plaque rupture leads to platelet accrual that can produce pathologic thrombi. In vivo, platelets do not normally interact with nonactivated endothelium, but will roll, adhere to, translocate, and/or detach from stimulated or injured vascular surfaces.
Platelet adhesion and aggregation to injured vascular surfaces is a complex process involving multiple substrates (von Willebrand factor [VWF] and collagen) and receptors, glycoprotein Ib/IX/V (GPIb/IX/V) complex, integrins αIIbβ3, integrin α2β1 and collagen GPVI depending upon the flow conditions. The initial tethering of platelets involves VWF interacting with the amino-terminal domain of the GPIbα subunit under conditions of high shear stress. This interaction is an important step for the activation of integrin αIIbβ3, which mediates irreversible stable adhesion, cytoskeletal reorganization, platelet spreading, platelet aggregation, and thrombus growth.2 The interaction of fibrinogen with the integrin αIIbβ3 plays a crucial role in platelet adhesion and platelet activation, leading to the generation of intracellular signals that nucleate the reorganization of the cytoskeleton. Agonist-induced activation of integrin αIIbβ3 leads to a conformational change of the receptor that converts the integrin from its resting low-affinity state to its active high-affinity state, allowing binding of its soluble ligand, fibrinogen.
Integrin αIIbβ3 has the ability to transmit bidirectional signaling via “inside-out” (agonist-induced activation of integrin αIIbβ3) or “outside-in” (fibrinogen-occupied integrin αIIbβ3) signaling events. Postligand binding events of integrin αIIbβ3 lead to irreversible stable platelet adhesion, cytoskeletal reorganization required for platelet spreading, clot retraction, microvesicle formation, platelet aggregation, and subsequent thrombus growth.
Several lines of evidence suggest that platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31) may regulate integrin activation, although the precise mechanism(s) of how PECAM-1 modulates integrin function is not known. Much of these data have been derived from artificial models examining engagement of PECAM-1 by cross-linking with bivalent anti–PECAM-1 antibodies or by drug-induced multimerization of engineered chimeric PECAM-1:FK506 receptors. Several groups have shown that engagement of PECAM-1 by bivalent anti–PECAM-1 monoclonal antibodies (mAbs) on the cell surface leads to up-regulation of β1, β2, and β3 integrin function, suggesting cross talk between PECAM-1 and integrin-mediated signaling.3-9 However, it is unclear if these effects of engagement of PECAM-1 were modulating the conformation of the integrin (affinity) or integrin clustering (avidity). Similarly, the forced AP1510-induced oligomers of chimeric PECAM-1:FKBP-2 resulted in increased adherence and spreading of PECAM-1:FK506-binding protein-2 (FKBP-2)–transfected cells on integrin α5β1–dependent immobilized fibronectin.10 However, it is unclear whether this artificial model represents a physiologically relevant event, where the dynamic equilibrium of PECAM-1 monomers is converted to lateral oligomers within the plane of the plasma membrane. Recent studies found that the addition of anti–PECAM-1 mAbs to Jurkat T cells induced selective activation of a small guanosine triphosphatase (GTPase), repressor activator protein 1 (Rap1), that is implicated in integrin activation.11
At present, there is no evidence of an integrin abnormality described in PECAM-1 knockout mice. Therefore, in this study we investigated whether PECAM-1 serves to regulate the major platelet integrin αIIbβ3 by examining the effect of deletion of PECAM-1 in modulating the conformation and clustering of the integrin αIIbβ3 in murine platelets. Our studies demonstrate that PECAM-1 serves to regulate the “outside-in” signaling properties of integrin αIIbβ3 in murine platelets.
Materials and methods
Abs and FITC-fibrinogen
Antimouse integrin β3 mAb, fluorescein isothiocyanate (FITC)–conjugated anti–mouse P-selectin mAb, FITC-conjugated anti–mouse CD3 mAb, and phycoerythrin (PE)–conjugated antirat Ab were purchased from BD Pharmingen (San Diego, CA). FITC-conjugated anti–mouse CD44 mAb was obtained from Beckman Coulter (Brea, CA), while rat anti–mouse CD9 mAb and JON/A-PE mAb were from Cemfret Analytics (Würzburg, Germany). Antimouse PECAM-1 antibody was generated as culture supernatant from rat hybridoma 390 (kindly provided by Dr Steven Albelda, University of Pennsylvania, Philadelphia). Antiphosphotyrosine monoclonal antibody 4G10 was from Upstate Biotechnology (Lake Placid, NY), while a monoclonal antibody directed against total focal adhesion kinase (FAK) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). FITC-conjugated fibrinogen was generated by incubating 20 mg human fibrinogen with 5 mg FITC (Sigma-Aldrich, St Louis, MO) in 0.15 M carbonate buffer, pH 9.0, for 1 hour at room temperature. Labeled fibrinogen was then purified by separation over a PD10 column (Amersham; Piscataway, NJ).
Mice
The construction of PECAM-1–/– mice on a C57BL/6 background has been described.12 Mice were housed in a pathogen-free facility at the Austin Research Institute Animal House Facility (Heidelberg, Victoria, Australia). All mouse work was approved by the Austin Campus Animal Ethics Committee.
Preparation of washed platelets and PRP
Blood was collected by cardiac puncture from anaesthesized mice using a 26-gauge needle with a 1-mL syringe and transferred to a microfuge tube containing 100 μL 3.8% (wt/vol) trisodium citrate. Platelet-rich plasma (PRP) was obtained by centrifugation at 115g for 8 minutes at room temperature without brake. Washed platelets were generated by centrifuging PRP in the presence of 50 ng/mL prostaglandin E1 (PGE1) at 640g for 10 minutes at room temperature without brake and resuspending the pellet gently in Ringers citrate dextrose (RCD) buffer (Sigma Chemical, St Louis, MO), pH 6.5 (108 mM NaCl, 38 mM KCl, 1.7 mM NaHCO3, 21.2 mM sodium citrate, 27.8 mM glucose, and 1.1 mM MgCl2 · 6H2O). After washing, platelets were finally resuspended in RCD, pH 7.4.13
Platelet aggregation studies
Platelet aggregation was monitored by measuring light transmission using a lumi-aggregometer (Chronolog; Havertown, PA). PRP was diluted in RCD buffer, pH 7.4, to generate platelet counts of 100 × 109/L. Reactions were performed in glass cuvettes in a 250-μL volume in the presence of 100 μg/mL fibrinogen and 1 mM CaCl2 at 37°C with constant stirring (1000 rpm). The baseline was set using mouse plasma diluted 1:2. Agonist prostate apoptosis response-4 (PAR-4), agonist peptide AYPGFK (Mimotopes; Clayton, Victoria, Australia), adenosinen diphosphate (ADP; Sigma-Aldrich), calcium ionophore A23187 (Sigma-Aldrich) and cross-linked collagen-related peptide (CRP) (Richard Farndale, Cambridge, United Kingdom) were added at varying concentrations to initiate platelet aggregation.
Clot retraction assay
Clot retraction studies were performed essentially as described.14 Briefly, 1 mL platelet suspensions containing 100 × 109 platelets (derived from platelet-rich plasma and diluted in RCD buffer, pH 7.4) were placed in siliconized glass tubes with 6 μL mouse erythrocytes added for color contrast. Thrombin (2.5 U) was added to each tube to initiate clotting, and clot retraction was allowed to proceed at 37°C. At appropriate time points, both serum volume and photographic images of retracting clots were recorded.
Static platelet adhesion assays
Static platelet adhesion assays were performed as previously described.15
Platelet spreading on fibrinogen
Washed platelets (100 × 109/L) were added to 100 μg/mL fibrinogencoated coverslips and incubated over time (0-60 minutes) at 37°C. Nonadherent platelets were removed and adherent platelets were fixed with 2.5% (vol/vol) glutaraldehyde (Sigma-Aldrich) in 0.1 M sodium cacodylate buffer, pH 7.3 (Sigma-Aldrich), for 15 minutes at room temperature. The fixed platelets were washed and processed for gold labeling. Scanning electron microscope (EM) images were captured using a Philips 515 scanning electron microscope (5 kV; Philips, Andover, MA) equipped with a 3100 ×/10 μm numeric aperture objective.
Flow cytometry (FACS) assay
Washed platelets (50 μL containing 40 × 105 platelets in RCD, pH 6.5), were incubated with appropriate Abs in the presence or absence of agonists, for 0.5 hour (or 1 hour for JON/A staining experiments only) at 37°C in the dark. Incubation with secondary antibody, where necessary, was carried out for a further 30 minutes at room temperature, in the dark. Washing steps were carried out using RCD buffer, pH 6.5, containing 0.2% (wt/vol) bovine serum albumin (BSA). Analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, North Ryde, New South Wales, Australia) formatted for a platelet-specific FITC/PE protocol with forward scatter (FSC), side scatter (SSC), and fluorescent parameters in log scale. The main platelet population was selected prior to the acquisition of data. Each experiment was set up in triplicates.
Anti–integrin β3, anti-CD3, anti-CD44, and anti-CD9 Abs were used at 50 μg/mL; mAb390 culture supernatant was used at 50 μL reaction, while JON/A-PE was used at 1:50 dilution and PE-conjugated antirat Ab was used at 1:200 dilution. FITC-conjugated antibody to P-selectin was used at 10 μg/mL, while FITC-labeled fibrinogen was used at 1.2 μg/mL.
Immunoprecipitation and Western blotting
Following platelet stimulation, reactions were terminated by the addition of an equal volume of Triton lysis buffer (15 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.4, containing 145 mM NaCl, 0.1 mM MgCl2, 10 mM EGTA [ethylene glycol tetraacetic acid], 2 mM sodium orthovanadate, 0.2 mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, and 1% [vol/vol] Triton X-100). Platelet lysates were mixed on a nutator for 1 hour at 4°C, then centrifuged at 15 000 g, 15 minutes at 4°C. Triton-soluble supernatants were separated and precleared with 50 μL 50% Protein G–Sepharose beads by mixing for 15 minutes at 4°C. Precleared platelet lysates were incubated with 10 μg of anti–mouse FAK antibody overnight at 4°C with constant mixing followed by the addition of 50 μL 50% Protein G–Sepharose beads for 1 hour at 4°C. Protein-antibody complexes were washed 5 times in immunoprecipitation buffer (50 mM Tris [tris(hydroxymethyl)aminomethane], pH 7.4, containing 150 mM NaCl and 1% [vol/vol] Triton X-100) eluted in 30 μL sodium dodecyl sulfate (SDS) reducing buffer and boiled for 10 minutes. Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis (PAGE) and then transferred to polyvinylidene difluoride membranes. Membranes were blocked in 20 mM Tris, pH 7.4, containing 3% (wt/vol) BSA and 0.05% (vol/vol) Tween 20, then probed with either horseradish peroxidase (HRP)–conjugated 4G10 antiphosphotyrosine antibody (1 μg/mL) or monoclonal anti-FAK antibody (1 μg/mL) for 2 hours at room temperature. Membranes were washed for 1 hour with Tris-buffered saline (TBS; 20 mM Tris, pH 7.4, containing 150 mM NaCl and 0.05% [vol/vol] Tween 20) and then incubated with HRP-conjugated secondary antibody diluted 1:10 000 in TBS. Membranes were washed for 1 more hour, then developed by enhanced chemiluminescence.
Calcium mobilization and inositol 1,4,5 triphosphate (IP3) measurement
For calcium mobilization experiments, washed platelets (150 × 109/L) were labeled with fura-2-pentaacetoxymethylester (FURA-2) am (Molecular Probes, Eugene, OR) at 6 μM for 1 hour in RCD buffer, pH 6.5, at room temperature. Platelets were washed and resuspended in fresh RCD buffer, pH 6.5, to the same concentration. Platelets (450 μL) were stimulated with agonist in a cuvette with stirring, and intracellular calcium levels were determined by monitoring the fluorescence intensity at 510 nm following excitation at 340 and 380 nm using a luminescence spectrometer (Perkin Elmer Instruments, Boston, MA). Data are presented as 340:380 fluorescence ratio.
For IP3 measurement, washed platelets (2 × 108 in 100 μL) were stimulated with thrombin (3 U/mL) for 5 and 10 seconds in the presence of 1.8 mM CaCl2.16 The reaction was terminated by adding 20 μL ice-cold 20% (vol/vol) perchloric acid, and IP3 levels were determined using a radio-immunoassay system according to the manufacturer's (Amersham, Piscataway, NJ) recommendations.
Statistics
The statistical significance of differences between means was evaluated using Student t test for paired samples, and P values less than .05 (denoted with an asterisk in the figures) and .005 (double asterisks in the figures) were considered to be significant. Results are expressed as mean ± standard error of the mean (SEM).
Results
PECAM-1–/– platelets show abnormalities in integrin αIIbβ3–mediated clot retraction
The initial characterization of PECAM-1–deficient mice revealed that the mice are healthy and viable with normal Mendelian inheritance ratios.12 Homozygous PECAM-1 knockout mice had a tendency to fail to thrive as demonstrated by low body weight during the first few months of life compared with age- and sex-matched wild-type mice.17 PECAM-1–deficient mice were hematologically normal, including in regard to their platelet production.12 Studies by Mahooti et al18 reported that PECAM-–/– mice have an in vivo bleeding defect demonstrated by prolonged tail bleeding times, while Vollmar et al19 indicated that PECAM-1–/– mice had normal tail bleeding times. This apparent discrepancy may reflect differences in experimental protocols used. However, this tail bleeding defect was further investigated and attributed to an underlying endothelial rather than a platelet defect, as hematopoietic reconstitution of irradiated PECAM-1 knockout mice showed no correction of their prolonged tail bleeding times.18
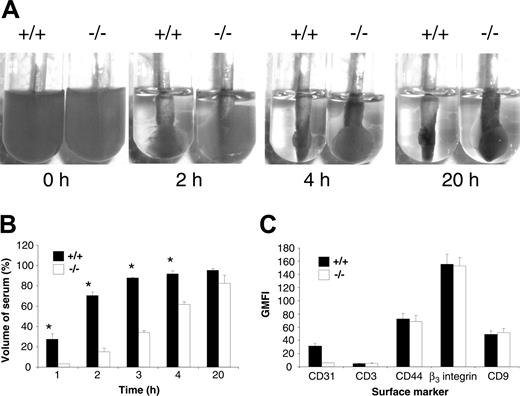
Based upon in vitro evidence that engagement of PECAM-1 leads to modulation of integrin β3–mediated functions, we wanted to investigate the possibility that PECAM-1 knockout platelets have an underlying functional platelet abnormality involving integrin αIIbβ3. As integrin αIIbβ3 is absolutely required in the processes of clot retraction, platelet aggregation, and platelet spreading, we initially tested the kinetics of clot retraction of wild-type versus PECAM-1–/– platelets. In this assay, wild-type and PECAM-1–/– PRP (normalized platelet counts) were initiated to form clots at 37°C by the addition of 2.5 U/mL thrombin. These clots were observed every 10 minutes for the first hour and then over a 20-hour period, and digital images were captured at different time points over the 20-hour period. As shown in Figure 1A, a delay in the kinetics of clot retraction was observed in PECAM-1 knockout platelets compared with wild-type platelets. Wild-type platelets started retracting at 20 minutes and were completely retracted at 4 hours, while PECAM-1–deficient platelets showed no sign of clot retraction at 20 minutes, only partial retraction by 4 hours, and almost complete retraction by 20 hours. Quantitation of the remaining serum volume after removal of fibrin clots, as described in “Materials and methods,” revealed a 3- to 5-fold reduction in the percentage of serum volume for PECAM-1–/– platelets compared with wild-type platelets during the first 4 hours of clot retraction (P < .05, n = 3). This defect was not attributable to reduced expression of integrin αIIbβ3 on the surface of PECAM-1–deficient platelets as demonstrated by flow cytometry (Figure 1C). In addition, CD44 and CD9 expression were also normal on PECAM-1–deficient platelets. Therefore, these results suggest that PECAM-1 is required for potentiating integrin αIIbβ3–mediated signaling events in murine platelets.
PECAM-1–/– platelets display normal platelet aggregation to a range of physiologic agonists
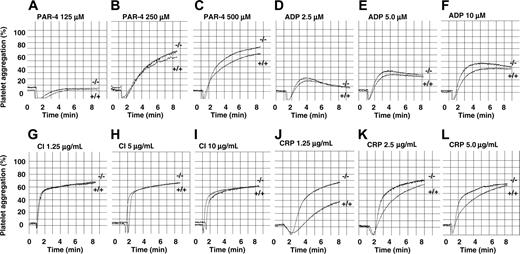
As integrin αIIbβ3 is required in the process of platelet aggregation, we next examined the effect of PECAM-1 deficiency on platelet aggregation responses using a range of physiologic agonists. As murine platelets predominantly express PAR-4 but not PAR-1, we chose the G-protein–coupled agonists, PAR-4 agonist peptide (125-500 μM) and ADP (2.5-10 μM). In addition, we included collagen-related peptide (1.25-5.00 μg/mL) and calcium ionophore (A23187; 1.25-10 μg/mL) as agonists. PRP (platelet count adjusted to 100 × 109/L) was preincubated with 1 mM CaCl2 and 100 μg/mL human fibrinogen for 5 minutes prior to stimulation with various agonists. Representative wild-type and PECAM-1 knockout platelet aggregation profiles are shown in Figure 2. PECAM-1 deficiency did not affect platelet aggregation responses to PAR-4 (125-500 μM), calcium ionophore (1.25-10 μg/mL), and ADP (2.5-10 μM). In contrast, PECAM-1 deficiency led to a 75% increase of collagen-related peptide (1.25 μg/mL)–induced platelet aggregation consistent with previous studies.13,20 Therefore, based upon these results, PECAM-1–/– platelets display normal amplitude and slope of platelet aggregation responses to PAR-4, ADP, and calcium ionophore, suggesting normal “inside-out” integrin αIIbβ3 signaling properties.
Delayed kinetics of clot retraction for PECAM-1–deficient platelets in the presence of normal integrin αIIbβ3 expression. (A) Photographs showing in vitro kinetics of clot retraction over a 20-hour time frame using platelet-rich plasma (PRP; normalized platelet counts) from wild-type and PECAM-1–/– mice. Samples were treated with 2.5 U thrombin. Each photograph is representative of at least 3 experiments. (B) Serum volume was determined from wild-type and PECAM-1–/– platelets undergoing clot retraction at 1, 2, 3, 4, and 20 hours following the addition of 2.5 U thrombin. Data are expressed as mean percentage serum volume at given time points. Results are representative of 3 independent experiments. *P < .05, n = 2. (C) The expression of surface markers on platelets was determined by staining with an isotype control (FITC-CD3), mAb 390 followed by anti–rat FITC for CD31, positive control FITC-CD44 mAb, FITC-CD9, and FITC-integrin β3 mAb for both wild-type and PECAM-1–/– platelets. FITC-labeled samples were analyzed on a FACSCalibur analyzer. Results are cumulative data derived from 3 independent experiments and presented as geometric mean fluorescence intensity (GMFI) ± SEM.
Delayed kinetics of clot retraction for PECAM-1–deficient platelets in the presence of normal integrin αIIbβ3 expression. (A) Photographs showing in vitro kinetics of clot retraction over a 20-hour time frame using platelet-rich plasma (PRP; normalized platelet counts) from wild-type and PECAM-1–/– mice. Samples were treated with 2.5 U thrombin. Each photograph is representative of at least 3 experiments. (B) Serum volume was determined from wild-type and PECAM-1–/– platelets undergoing clot retraction at 1, 2, 3, 4, and 20 hours following the addition of 2.5 U thrombin. Data are expressed as mean percentage serum volume at given time points. Results are representative of 3 independent experiments. *P < .05, n = 2. (C) The expression of surface markers on platelets was determined by staining with an isotype control (FITC-CD3), mAb 390 followed by anti–rat FITC for CD31, positive control FITC-CD44 mAb, FITC-CD9, and FITC-integrin β3 mAb for both wild-type and PECAM-1–/– platelets. FITC-labeled samples were analyzed on a FACSCalibur analyzer. Results are cumulative data derived from 3 independent experiments and presented as geometric mean fluorescence intensity (GMFI) ± SEM.
PECAM-1–deficient platelets display normal platelet aggregation responses to G-protein–coupled agonists, PAR-4 peptide, ADP, and calcium ionophore, but are hyperresponsive to GPVI-specific agonist collagen-related peptide (CRP). Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and PECAM-1–/– mice were determined following activation with different concentrations of various agonists. (A-C) PAR-4 agonist peptide used at 125, 250, and 500 μM, respectively. (D-F) ADP used at 2.5, 5, and 10 μM, respectively. (G-I) Calcium ionophore (CI) A23198 used at 1.25, 5, and 10 μg/mL, respectively. (J-L) CRP used at 1.25, 2.5, and 5 μg/mL, respectively.
PECAM-1–deficient platelets display normal platelet aggregation responses to G-protein–coupled agonists, PAR-4 peptide, ADP, and calcium ionophore, but are hyperresponsive to GPVI-specific agonist collagen-related peptide (CRP). Aggregation responses of PRP (platelet count adjusted to 100 × 109/L) for wild-type and PECAM-1–/– mice were determined following activation with different concentrations of various agonists. (A-C) PAR-4 agonist peptide used at 125, 250, and 500 μM, respectively. (D-F) ADP used at 2.5, 5, and 10 μM, respectively. (G-I) Calcium ionophore (CI) A23198 used at 1.25, 5, and 10 μg/mL, respectively. (J-L) CRP used at 1.25, 2.5, and 5 μg/mL, respectively.
PECAM-1–/– platelets display normal static platelet adhesion to integrin αIIbβ3–dependent matrices
In order to assess whether PECAM-1 deficiency modulates integrin αIIbβ3–mediated adhesive events, we examined the static platelet adhesion responses of DiOC6 fluorescently labeled wild-type and PECAM-1–/– platelets to immobilized extracellular matrix (ECM) proteins, including fibrinogen (100 μg/mL), bovine VWF (20 μg/mL) in the presence of botrocetin (1 μg/mL), and type I fibrillar collagen (20 μg/mL) over time (0-60 minutes). Nonspecific binding was assessed by binding to immobilized BSA (100 μg/mL). Following washing, fluorescently labeled platelets bound to various matrices were examined and quantitated for evidence of adhesion by fluorescence microscopy. As shown in Figure 3, wild-type and PECAM-1–/– platelets showed similar kinetics of adhesion to fibrinogen and bovine VWF under static conditions. In contrast, an increased kinetics of platelet adhesion to type I fibrillar collagen was observed for PECAM-1–deficient platelets consistent with previous studies.20 Taken together, these static platelet adhesion responses suggest that the absence of PECAM-1 does not affect the platelet adhesive properties involving inactive integrin αIIbβ3 binding to immobilized ligands, fibrinogen, and bovine VWF.
PECAM-1–deficient platelets display normal static platelet adhesion on fibrinogen and bovine VWF matrices. Anticoagulated whole blood from PECAM-1+/+ and PECAM-1–/– mice was labeled with DiOC6 (50 μg/mL) and washed platelets were isolated. Labeled platelets (1 × 109/mL) were then allowed to adhere to bovine serum albumin (BSA)–coated (100 μg/mL) coverslips, collagen-coated (2.5 mg/mL) coverslips, fibrinogen-coated (100 μg/mL) coverslips, or bovine VWF–coated (20 μg/mL) coverslips in the presence of botrocetin (1 μg/mL) for 0 to 60 minutes at 37°C. Adherent platelets were fixed at different time points and visualized using fluorescence microscopy. Quantitation of adherent PECAM-1+/+ and PECAM-1–/– platelets was determined by analysis of images acquired using a × 100 objective. These data represent the mean ± SEM from 3 independent experiments. *P < .05 (n = 3).
PECAM-1–deficient platelets display normal static platelet adhesion on fibrinogen and bovine VWF matrices. Anticoagulated whole blood from PECAM-1+/+ and PECAM-1–/– mice was labeled with DiOC6 (50 μg/mL) and washed platelets were isolated. Labeled platelets (1 × 109/mL) were then allowed to adhere to bovine serum albumin (BSA)–coated (100 μg/mL) coverslips, collagen-coated (2.5 mg/mL) coverslips, fibrinogen-coated (100 μg/mL) coverslips, or bovine VWF–coated (20 μg/mL) coverslips in the presence of botrocetin (1 μg/mL) for 0 to 60 minutes at 37°C. Adherent platelets were fixed at different time points and visualized using fluorescence microscopy. Quantitation of adherent PECAM-1+/+ and PECAM-1–/– platelets was determined by analysis of images acquired using a × 100 objective. These data represent the mean ± SEM from 3 independent experiments. *P < .05 (n = 3).
Restricted cytoskeletal reorganization of PECAM-1–/– platelets
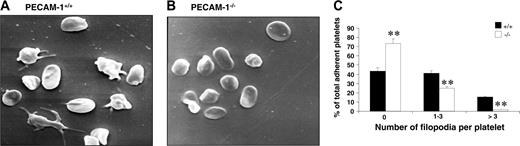
Platelet adhesion to fibrinogen results in platelets becoming activated and undergoing substantial cytoskeletal reorganization, leading to platelet shape change and spreading. This postligand occupancy event involves integrin αIIbβ3 complex being clustered on the platelet membrane, initiating downstream “outside-in” integrin αIIbβ3–mediated signaling events. In order to test whether the “outside-in” signaling properties of integrin αIIbβ3 are defective in PECAM-1–deficient platelets, we first examined the formation of platelet filopodia and spreading on fibrinogen over time with scanning EM. As shown in Figure 4A-B, PECAM-1–/– platelets adhered as efficiently to fibrinogen as wild-type mouse platelets; however, PECAM-1–/– platelets exhibit a significant reduction in their capacity to extend filopodia. Specifically, PECAM-1–/– platelets were characterized by a lack of filopodia, with fewer having 1 to 3 filopodia and fewer having an excess of 3 filopodia compared with wild-type platelets (**P < .005, n = 4; Figure 4C). These results indicate that PECAM-1–/– platelets have restricted cytoskeletal reorganization when adhered to immobilized fibrinogen.
PECAM-1–/– platelets show normal alpha granule platelet secretion
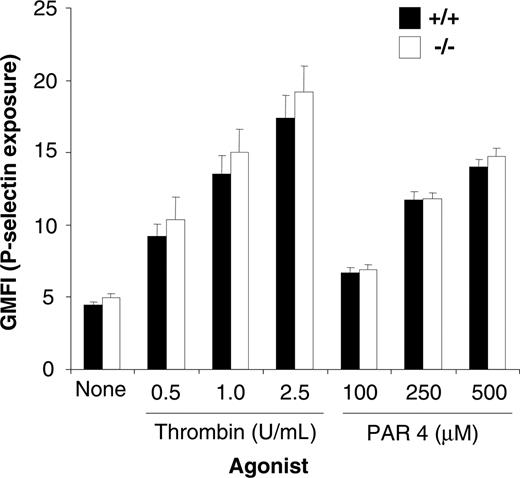
Because PECAM-1–/– platelets have a defect in cytoskeletal reorganization and clot retraction, we wanted to test the possibility that platelet secretion may also be altered. In order to test the possibility, we examined the exposure of P-selectin as an indicator of alpha granule secretion. As shown in Figure 5, the surface expression of P-selectin on thrombin- and PAR-4–stimulated platelets derived from wild-type and PECAM-1–/– platelets was equivalent over a range of thrombin (0.5-2.5 U/mL) and PAR-4 (100-500 μM) concentrations. Therefore, based upon these results, it would appear that, in contrast to their response to GPVI agonists, PECAM-1–/– platelets have normal alpha granule secretion in response to engagement of G-protein–coupled receptors.
PECAM-1–/– platelets show normal “inside-out” integrin αIIbβ3–mediated signaling properties
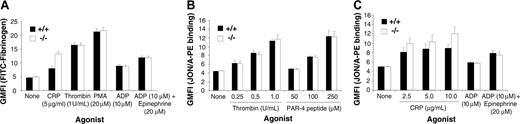
Becuse PECAM-1–/– platelets primarily show defects associated with postligand occupancy events, we wanted to confirm that PECAM-1–/– platelets showed normal “inside-out” integrin αIIbβ3–mediated signaling properties. In order to test integrin αIIbβ3 activation on PECAM-1–/– platelets, we examined the ability of wild-type and PECAM-1–/– platelets to bind to soluble FITC-fibrinogen under resting conditions and following CRP, thrombin stimulation, PMA (phorbol 12-myristate 13-acetate), ADP, and ADP + epinephrine. The binding of soluble FITC-fibrinogen was assessed by flow cytometry. As shown in Figure 6, agonist-induced stimulation of wild-type and PECAM-1–/– platelets demonstrated similar ability to bind soluble FITC-fibrinogen and to bind JON/A mAb that recognizes the active conformation of integrin αIIbβ3 complex on murine platelets. In addition, we examined cytosolic-free calcium and IP3 levels following agonist-induced stimulation, including CRP (2.5-5.0 μg/mL), thrombin (0.5-1 U/mL), and PAR-4 agonist peptide (125-250 μM). As shown in Figure 7A-B, agonist-induced stimulation of wild-type and PECAM-1–/– platelets demonstrated equivalent ability to increase cytosolic-free calcium and IP3 levels. This effect was also observed at 1.25 μg/mL CRP concentration (data not shown). These results indicate that PECAM-1–/– platelets display normal “inside-out” integrin αIIbβ3 signaling properties.
PECAM-1–deficient platelets display restricted cytoskeletal reorganization upon spreading on immobilized fibrinogen. (A-B) Washed PECAM-1+/+ and PECAM-1–/– platelets were allowed to adhere for 60 minutes at 37°C to a fibrinogen matrix. Adherent platelets were fixed and examined by scanning electron microscopy. Representative PECAM-1+/+ and PECAM-1–/– platelet scanning EM images are shown. (C) The number of filopodia per platelet was analyzed on scanning EM images from 8 random fields encompassing 100 platelets counted and expressed as a percentage of the total adherent platelets from PECAM-1+/+ and PECAM-1–/– platelets. Platelet filopodia were classified into 3 categories including 0, 1 to 3, and more than 3 filopodia. **P < .005; n = 4.
PECAM-1–deficient platelets display restricted cytoskeletal reorganization upon spreading on immobilized fibrinogen. (A-B) Washed PECAM-1+/+ and PECAM-1–/– platelets were allowed to adhere for 60 minutes at 37°C to a fibrinogen matrix. Adherent platelets were fixed and examined by scanning electron microscopy. Representative PECAM-1+/+ and PECAM-1–/– platelet scanning EM images are shown. (C) The number of filopodia per platelet was analyzed on scanning EM images from 8 random fields encompassing 100 platelets counted and expressed as a percentage of the total adherent platelets from PECAM-1+/+ and PECAM-1–/– platelets. Platelet filopodia were classified into 3 categories including 0, 1 to 3, and more than 3 filopodia. **P < .005; n = 4.
PECAM-1–deficient platelets display normal alpha secretion. Surface expression of P-selectin was determined for washed platelets stimulated by 0 to 2.5 U/mL thrombin or 100 to 500 μM PAR-4 agonist peptide and then stained with either a buffer control and FITC–P-selectin mAb for both wild-type and PECAM-1–/– platelets. FITC-labeled samples were analyzed on a FACSCalibur analyzer. Results are cumulative data from 3 independent experiments and are presented as geometric mean fluorescence intensity (GMFI) ± SEM.
PECAM-1–deficient platelets display normal alpha secretion. Surface expression of P-selectin was determined for washed platelets stimulated by 0 to 2.5 U/mL thrombin or 100 to 500 μM PAR-4 agonist peptide and then stained with either a buffer control and FITC–P-selectin mAb for both wild-type and PECAM-1–/– platelets. FITC-labeled samples were analyzed on a FACSCalibur analyzer. Results are cumulative data from 3 independent experiments and are presented as geometric mean fluorescence intensity (GMFI) ± SEM.
PECAM-1–/– platelets display hypophosphorylated 125FAK following induction of integrin αIIbβ3–mediated platelet aggregation
Induction of tyrosine phosphorylation of 125FAK appears to be an early event in “outside-in” integrin αIIbβ3–mediated signaling. In platelets, 125FAK is phosphorylated following stimulation by thrombin, provided that platelet suspensions are stirred and fully aggregated (integrin αIIbβ3–mediated clustering event). In order to test the possibility that PECAM-1 is an important regulator of integrin αIIbβ3–mediated signaling events, we examined the induction of tyrosine phosphorylation of 125FAK following integrin αIIbβ3–mediated aggregation over time of wild-type and PECAM-1–/– platelets. As shown in Figure 7C, PECAM-1–/– platelets showed a 2-fold reduction in tyrosine phosphorylation of 125FAK compared with wild-type platelets evident at 30-second and 1-minute time points of integrin αIIbβ3–mediated platelet aggregation. These results suggest that PECAM-1 serves as an important regulator of “outside-in” integrin αIIbβ3–mediated signaling events.
PECAM-1–deficient platelets display normal soluble FITC-fibrinogen binding and JON/A PE binding following treatment with thrombin, PAR-4 peptide, ADP, ADP in synergy with epinephrine, and CRP. (A) FACS analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PMA (20 μM), ADP (10 μM), ADP (10 μM) + epinephrine (20 μM), or unstimulated (control). Results are cumulative data from 3 independent assays and are presented as geometric mean fluorescence intensity (GMFI) ± SEM. (B-C) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0 to 1.0 U/mL thrombin, 0 to 250 μM PAR-4 agonist peptide, 2.5 to 10 μg/mL CRP, 10 μM ADP with or without 20 μM epinephrine, or unstimulated (control). Results are cumulative data from 3 independent experiments and are presented as geometric mean fluorescence intensity (GMFI) ± SEM.
PECAM-1–deficient platelets display normal soluble FITC-fibrinogen binding and JON/A PE binding following treatment with thrombin, PAR-4 peptide, ADP, ADP in synergy with epinephrine, and CRP. (A) FACS analysis of FITC-conjugated fibrinogen binding to platelets stimulated with thrombin (1 U/mL), PMA (20 μM), ADP (10 μM), ADP (10 μM) + epinephrine (20 μM), or unstimulated (control). Results are cumulative data from 3 independent assays and are presented as geometric mean fluorescence intensity (GMFI) ± SEM. (B-C) Flow cytometric analysis of JON/A-PE mAb binding to platelets stimulated with 0 to 1.0 U/mL thrombin, 0 to 250 μM PAR-4 agonist peptide, 2.5 to 10 μg/mL CRP, 10 μM ADP with or without 20 μM epinephrine, or unstimulated (control). Results are cumulative data from 3 independent experiments and are presented as geometric mean fluorescence intensity (GMFI) ± SEM.
Discussion
Using platelets from PECAM-1–/– mice, we have identified a role for PECAM-1 in modulating “outside-in” integrin αIIbβ3–mediated signaling. More specifically, the absence of PECAM-1 led to defects in postligand occupancy events, including delayed clot retraction, restricted platelet spreading on immobilized fibrinogen, and reduced tyrosine phosphorylation of 125FAK following integrin αIIbβ3–mediated platelet aggregation. Furthermore, normal integrin αIIbβ3 activation, alpha granule secretion, platelet aggregation to PAR-4, ADP, and calcium ionophore, and agonist-induced cytosolic-free calcium and IP3 levels argue against a role for PECAM-1 in regulating “inside-out” integrin αIIbβ3–mediated signaling in murine platelets. Taken together, these data suggest that PECAM-1 performs an essential role in regulating lateral integrin clustering (avidity) events in cell adhesion rather than modulating the conformation of the integrin activation (affinity).
Cytoplasmic calcium, IP3 levels, and 125FAK phosphorylation following agonist stimulation with either CRP, thrombin, or PAR-4. (A) Fura-2 am loaded washed wild-type or PECAM-1–/– platelets were stimulated with the indicated agonists ([i,ii] PAR-4 agonist peptide at 125 and 250 μM, respectively; [iii,iv] thrombin at 0.5 and 1.0 U/mL, respectively; and [v,vi] CRP at 2.5 and 5 μg/mL, respectively) and cytoplasmic-free calcium was determined by measuring fluorescence emission spectra following excitation at 340 and 380 nm. Data are presented as 340:380 nm ratio. Arrow indicates addition of agonist. (B) Intracellular IP3 levels are presented for wild-type (▪) and PECAM-1–/– (□) platelets at 5 and 10 seconds following stimulation with thrombin. Data are presented as the mean ± SEM from 3 independent experiments. Wild-type platelets are assigned a value of 100% for each experiment. (C) Tyrosine phosphorylation of 125FAK was induced following stimulation of wild-type or PECAM-1–/– platelets with thrombin (1 U/mL) and stirring at different time points in the platelet aggregometer (0-1 minute). Platelet suspensions were lysed and immunoprecipitated with anti-FAK antibody. Immune complexes were separated by 10% SDS-PAGE and transferred by Western blotting. Top row: 4G10; bottom row: total FAK antigen.
Cytoplasmic calcium, IP3 levels, and 125FAK phosphorylation following agonist stimulation with either CRP, thrombin, or PAR-4. (A) Fura-2 am loaded washed wild-type or PECAM-1–/– platelets were stimulated with the indicated agonists ([i,ii] PAR-4 agonist peptide at 125 and 250 μM, respectively; [iii,iv] thrombin at 0.5 and 1.0 U/mL, respectively; and [v,vi] CRP at 2.5 and 5 μg/mL, respectively) and cytoplasmic-free calcium was determined by measuring fluorescence emission spectra following excitation at 340 and 380 nm. Data are presented as 340:380 nm ratio. Arrow indicates addition of agonist. (B) Intracellular IP3 levels are presented for wild-type (▪) and PECAM-1–/– (□) platelets at 5 and 10 seconds following stimulation with thrombin. Data are presented as the mean ± SEM from 3 independent experiments. Wild-type platelets are assigned a value of 100% for each experiment. (C) Tyrosine phosphorylation of 125FAK was induced following stimulation of wild-type or PECAM-1–/– platelets with thrombin (1 U/mL) and stirring at different time points in the platelet aggregometer (0-1 minute). Platelet suspensions were lysed and immunoprecipitated with anti-FAK antibody. Immune complexes were separated by 10% SDS-PAGE and transferred by Western blotting. Top row: 4G10; bottom row: total FAK antigen.
Although it has been known for more than 10 years that PECAM-1 acts as an integrin-function modulator, the underlying mechanism of regulation of integrin function has not been understood. Much of the original studies had been based upon using artificial models of antibody-mediated engagement of PECAM-1 or drug-induced chimeric PECAM-1/FKBP-2 receptor to modulate integrin function. To our knowledge, no integrin-functional abnormality or activation defect has been described in PECAM-1 knockout mice; therefore, this study gives a direct insight into how deletion of PECAM-1 influences the signaling properties of integrin αIIbβ3 in murine platelets. In this study, we have chosen terminally differentiated anucleate platelets as the model system because they offer a unique opportunity to dissect mechanisms of integrin αIIbβ3 clustering (“outside-in”) versus integrin αIIbβ3 conformational changes (“inside-out”) without the complications of nuclear signaling events. To our surprise, there was no evidence of an integrin activation defect in PECAM-1–deficient platelets. The primary defect in PECAM-1–deficient platelets observed involves “outside-in” signaling events of integrin αIIbβ3 including restricted filopodia extensions and spreading on integrin αIIbβ3–dependent matrix, fibrinogen, and delayed clot retraction that relies on the retractile forces of an active integrin αIIbβ3 complex coupled to actin-myosin cytoskeletal proteins.
Several studies have provided experimental evidence that engagement or dimerization of PECAM-1 on the surface of lymphocytes, neutrophils, natural killer cells, and platelets results in up-regulation of integrin adhesive function. Dimerization of PECAM-1 was induced by the addition of bivalent anti–PECAM-1 mAb fragments to enhance the binding of T lymphocytes to immobilized β1 integrin substrates including fibronectin (α5β1) and vascular cell adhesion molecule 1 (VCAM-1; α4β1), up-regulation of β2 integrin function on neutrophils, and ligand-induced binding site (LIBS) exposure and increased integrin β3 platelet adhesion.3,6,8 The addition of anti–PECAM-1 mAbs to human natural killer (NK) cells has been associated with actin rearrangement and recruitment of talin to membrane ruffles, indicating that engagement of cellular PECAM-1 results in transmission of signals that culminates in cytoskeletal re-organization.21
Previous studies, including our own, have provided evidence that PECAM-1 serves as an immunoglobulin (Ig)–immunoreceptor tyrosine–based inhibitory motif (ITIM) inhibitory receptor to negatively modulate immunoreceptor tyrosine–based activation motif (ITAM)–associated signaling pathways in platelets. These include collagen GPVI/Fc receptor (FcR) γ-chain–mediated signaling, the low-affinity IgG receptor, FcγRIIa-mediated signaling and GPIb-IX-V–mediated signaling pathways (hereafter referred to as Model 1).13,20,22-24 Platelets from PECAM-1–/– mice form larger thrombi over time when perfused over a type I collagen matrix under in vitro physiologic conditions of flow compared with wild-type mice.13 Consistent with this finding, PECAM-1 knockout mice form larger thrombi in vivo following induction of mild laser-induced vascular injury.25 In addition, PECAM-1–/– platelets are hyperaggregable to VWF, display enhanced spreading, and form larger thrombi on immobilized VWF under conditions of arterial flow compared with wild-type mice.24
Apart from ITAM-associated pathways, Cicmil et al have proposed that PECAM-1 may negatively modulate non-ITAM signaling pathways in platelets involving G-protein–coupled receptor (GPCR)–mediated platelet responses (hereafter referred to as Model 2).23 This study demonstrated that cross-linking of anti–PECAM-1 antibody fragments would inhibit only low-dose GPCR agonist–stimulated human platelet aggregation (0.05 U/mL thrombin). This effect on GPCR-mediated platelet aggregation has not been borne out on our study with PECAM-1–/– platelets. This apparent discrepancy may be explained by differences observed in composition of PAR receptor expression and GPCR-mediated signaling in human (PAR-1, PAR-3, and PAR-4) versus mouse (PAR-3 and PAR-4 but not PAR-1) platelets. In humans, G-protein–coupled signaling works primarily through PAR-1 thrombin receptor and P2Y1 ADP receptor. This is in contrast to mice, where G-protein–coupled signaling works primarily through PAR-4 thrombin receptor and P2Y1 ADP receptor that are linked to Gαq but not Gi signaling.
Engagement and clustering of integrin αIIbβ3 activates downstream signaling pathways, including multiple protein-tyrosine kinases (PTKs) such as Src, Syk, and focal adhesion kinase, 125FAK. The coordination of Src PTK and FAK function is critical in integrin αIIbβ3–signaling events and formation of focal adhesions.26 However, it has remained controversial as to the order of activation of Src and 125FAK. Some reports have indicated that 125FAK activation precedes Src activation, whereas others indicate that Src PTKs are acting upstream of 125FAK.27-30 In platelets, a pool of Src is constitutively associated with integrin αIIbβ3 through interaction of its Src homology 3 (SH3) domain with the β3 cytoplasmic domain. Upon integrin αIIbβ3 clustering, Src is dephosphorylated at its inhibitory C-terminal tyrosine 529 residue by an unidentified protein-tyrosine phosphatase(s) (PTP) and autophosphorylation of Tyr418 occurs.31 Candidate PTPs include PTP1B, Src homology tyrosine phosphatase 2 (SHP-2), and PTP-PEST. Under these conditions, autophosphorylation of FAK creates binding sites for SH2 domains of Src, creating the opportunity for full phosphorylation and activation of Src and FAK involving a FAK-Src positive-feedback loop.
In this study, we observed that PECAM-1–/– platelets displayed reduced tyrosine phosphorylation of 125FAK following clustering of integrin αIIbβ3 (Figure 7C), indicating that PECAM-1 modulates integrin αIIbβ3–mediated signaling via FAK-Src positive-feedback loop with integrin β3 tyrosine phosphorylation pathways. This effect is bidirectional as integrin αIIbβ3–mediated clustering and recruitment of Src family kinases results in induction of the tyrosine phosphorylation of PECAM-1.32 Under these conditions, PECAM-1 can recruit SH2-domain–containing protein-tyrosine phosphatases including SHP-2 and, to a lesser extent, SHP-1, that may sequester PECAM-1 to locations where it can regulate activatory adhesive receptors. The fact that PECAM-1–/– platelets display a defect in integrin αIIbβ3–mediated cytoskeletal reorganization, clot retraction, and integrin αIIbβ3–mediated tyrosine phosphorylation of 125FAK suggests that PECAM-1 signaling complexes may play an important role in regulating the formation and stabilization of integrin αIIbβ3–mediated focal adhesions.33 A recent study by O'Brien et al34 suggested that SHP-2 recruited by PECAM-1 ITIMs was essential for turnover of focal adhesions and motility of endothelial-like REN cells. This study suggested that the phosphatase activity of SHP-2 was important for regulating components of focal adhesions, including paxillin. If this scenario applied to platelets, then we would expect 125FAK to be hyperphosphorylated in the absence of PECAM-1 and SHP-2 recruitment. This does not appear to be the case, suggesting that the avidity of lateral clustering of integrin αIIbβ3 is essential for the strength and duration of downstream signaling and tyrosine phosphorylation events involving 125FAK.
Based upon this study, we propose a third model of how PECAM-1 regulates platelet function. Our study shows that PECAM-1 acts as a positive component in integrin αIIbβ3 engagement and clustering that is normally associated with sequestering SHP-2 to PECAM-1 to form signaling scaffolds in platelets. We suggest a model in which SHP-2–PECAM-1 complexes promote a positive-feedback loop leading to Src and FAK activation during integrin αIIbβ3–mediated clustering and signaling. This integrin αIIbβ3–mediated positive-feedback loop would be essential for regulation of biologic processes, including cytoskeletal changes that influence cell spreading and clot retraction. In the absence of PECAM-1/SHP-2 localization to regulate the adhesive integrin αIIbβ3–mediated clustering events, dephosphorylation of Src and optimal tyrosine phosphorylation of FAK would not occur leading to defects in downstream signaling events and delays in kinetics of cell spreading, clot retraction, and possibly focal adhesions. This model is consistent with the action of other immunoreceptors such as SHP substrate 1 (SHPS-1)/SHP-2 scaffold complexes in regulating integrin-mediated signaling events and cell motility.35
The modest “outside-in” integrin αIIbβ3–mediated signaling defect observed in PECAM-1–deficient platelets is reminiscent of the phenotype observed in Y747,759F integrin β3 mutant mice.36 These Y747,759F mutant mice display a selectively impaired “outside-in” integrin αIIbβ3–mediated signaling defect with defective platelet aggregation (secondary phase), clot retraction responses in vitro, and an in vivo bleeding defect characterized by a tendency to rebleed. This study highlighted the importance of the integrin β3 tyrosine phosphorylation signaling mechanism in “outside-in” integrin signaling events, as tyrosine phosphorylation of integrin β3 cytoplasmic domain induces recruitment of cytosolic proteins myosin (required in clot retraction) and adaptor protein Src homology collagen (Shc; required in platelet stimulation). In addition, one of the early events in integrin β3 tyrosine phosphorylation and recruitment of Src family kinase members is the induction of tyrosine phosphorylation of 125FAK following integrin αIIbβ3 clustering (via platelet aggregation). In our study, we observed that PECAM-1–/– platelets displayed reduced tyrosine phosphorylation of 125FAK following clustering of integrin αIIbβ3 (Figure 7C), indicating that PECAM-1 modulates integrin αIIbβ3–mediated signaling via cross talk with integrin β3 tyrosine phosphorylation pathways. This study provides the first example of an underlying integrin αIIbβ3 signaling defect in PECAM-1–/– murine platelets, indicating that PECAM-1 modulates integrin αIIbβ3 avidity and is associated with positive regulation of integrin αIIbβ3–mediated platelet functions. Future studies will examine the signaling components required for cross talk between PECAM-1 and integrin αIIbβ3.
Prepublished online as Blood First Edition Paper, August 4, 2005; DOI 10.1182/blood-2005-03-0911.
Supported by grants from the National Heart Foundation of Australia and National Health and Medical Research Council (NHMRC) of Australia (D.E.J.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


![Figure 7. Cytoplasmic calcium, IP3 levels, and 125FAK phosphorylation following agonist stimulation with either CRP, thrombin, or PAR-4. (A) Fura-2 am loaded washed wild-type or PECAM-1–/– platelets were stimulated with the indicated agonists ([i,ii] PAR-4 agonist peptide at 125 and 250 μM, respectively; [iii,iv] thrombin at 0.5 and 1.0 U/mL, respectively; and [v,vi] CRP at 2.5 and 5 μg/mL, respectively) and cytoplasmic-free calcium was determined by measuring fluorescence emission spectra following excitation at 340 and 380 nm. Data are presented as 340:380 nm ratio. Arrow indicates addition of agonist. (B) Intracellular IP3 levels are presented for wild-type (▪) and PECAM-1–/– (□) platelets at 5 and 10 seconds following stimulation with thrombin. Data are presented as the mean ± SEM from 3 independent experiments. Wild-type platelets are assigned a value of 100% for each experiment. (C) Tyrosine phosphorylation of 125FAK was induced following stimulation of wild-type or PECAM-1–/– platelets with thrombin (1 U/mL) and stirring at different time points in the platelet aggregometer (0-1 minute). Platelet suspensions were lysed and immunoprecipitated with anti-FAK antibody. Immune complexes were separated by 10% SDS-PAGE and transferred by Western blotting. Top row: 4G10; bottom row: total FAK antigen.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/12/10.1182_blood-2005-03-0911/2/m_zh80240587310007.jpeg?Expires=1769111566&Signature=3GjsKgkU0dCRrGZTpxkjiVsYbh3AjsnjK00G81tQxCUCepAzwvB2Wd~WBlDC4g~P4iGEe7DSMR7SRr5tTIj0pLhq~KrR0vST9cQG238ahjnCFSpuiSVV~37nqiZbnHvHeZtq~DexiyH7XUDknLHWMzJGgrP5G6YVJM7pXxlekIw9J0r54SE3q47VJKF0X8DGTqhwbT7g1QT2UcnM-0FYmd4XvfypM1fBrAQg5hEtjsG4nHdBb8pjPucYsO0U6eRCIh~lucY5d4j0bn~~shyG38rynQltqO-1MykPnPYRrXs~uIQqZM4VMyeDXjWeIVWYhjVFaAvkOv0HXNcFg3ZTXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal