Thrombocytes are the nucleated equivalent of platelets in nonmammalian vertebrates such as the zebrafish, Danio rerio. We have cloned zebrafish CD41 cDNA (αIIb, glycoprotein IIb [GPIIb]) and its promoter and have generated transgenic zebrafish lines with green fluorescent protein (GFP)–tagged thrombocytes. CD41 mRNA transcripts appeared 42 hours after fertilization (hpf) by reverse-transcriptase–polymerase chain reaction (RT-PCR) and at 48 hpf in circulating hematopoietic cells. Flow sorting of thrombocytes from the mesonephros of adult CD41-GFP zebrafish showed a GFPhigh subset, which had the morphologic appearance of mature thrombocytes, and a GFPlow subset with an immature appearance, suggesting that they may be thrombocyte precursors. Confocal laser microscopy of embryos 40 and 48 hpf also showed a nonmobile population of GFP+ cells in a discrete area between the dorsal aorta and caudal vein. Production of circulating thrombocytes was inhibited by the injection of antisense morpholinos for the stem-cell transcription factor scl and c-mpl, the receptor for thrombopoietin. The nonmobile pool of GFP+ cells was abolished by scl knockdown and partially inhibited by c-mpl knockdown. These studies have shown that it is possible to identify thrombocytes, thrombocyte precursors, and, possibly, early hematopoietic stem cells in zebrafish embryos and track their proliferation and maturation.
Introduction
Platelets play an important role in primary hemostasis by adhering to the damaged vessel wall, forming intravascular aggregates, secreting vaso-active mediators, and serving as a nidus for coagulation reactions. The zebrafish thrombocyte is the functional equivalent of the mammalian platelet, although thrombocyte formation differs from platelet generation as the nucleus is retained and each cell remains diploid throughout differentiation.1 In mammals, platelets are derived from megakaryocytes by a complex process of proplatelet extension from the parent megakaryocyte.2 The relatively low number of platelet precursors in human bone marrow has been a major hindrance to the study of megakaryocyte and platelet development. They represent less than 0.1% of the nucleated cells in human or murine bone marrow and are fragile and somewhat difficult to purify. In a recent study, however, highly purified megakaryocyte precursors, which generated mature megakaryocytes when placed in culture, were successfully isolated from murine bone marrow.3
One additional impediment to the study of megakaryocyte development is the paucity of platelet-specific proteins that can be used to identify the earliest megakaryocyte/platelet precursors. Many proteins that were formerly thought to be “platelet specific,” such as P-selectin, are now known to be expressed in either resting or stimulated vascular endothelial cells.4 Platelet factor 4 (PF-4), a heparin-neutralizing protein, which is a member of the CXC family of chemokines,5 is one of a relatively small number of truly platelet-specific proteins. However, PF-4 is expressed relatively late in the megakaryocyte/platelet developmental sequence. CD41, the α subunit of the platelet integrin CD41/CD61 (αIIb/β3, glycoprotein IIb [GPIIb]/GPIIIa) complex, another candidate platelet-specific protein, is clearly expressed on the surface of early megakaryocyte/platelet progenitors as well as on mature platelets. In addition, there are several recent studies showing that CD41 expression is not restricted to the platelet lineage. During murine embryonic development, CD41 is expressed on early hematopoietic progenitors.6-8 In fact, CD41 is expressed on the earliest definitive hematopoietic stem cells (HSCs) identified in the aorta-gonad-mesonephric region (AGM) in day-10.5 murine embryos.8
Although the most popular animal model for studying vertebrate hematopoiesis has been the mouse, zebrafish provide another attractive system to study the maturation of hematopoietic progenitors. The transparency of zebrafish embryos and larvae, coupled with the ease of their genetic manipulation, has made it relatively easy to both generate and propagate mutant phenotypes and recover mutant alleles.9,10 The utility of the zebrafish model is nicely illustrated by the increasing number of erythroid as well as early hematopoietic mutants that have been identified and characterized.11-15 Cardiac function and blood circulation are readily observed 24 hours after fertilization (hpf), and anemic mutants can be identified in developing embryos by the absence or paleness of circulating erythrocytes. Defects in the other hematopoietic lineages are more difficult to detect, as they are not visible in the circulation of a developing embryo without specific staining or tagging. Whole-mount in situ hybridization with lineage-specific transcripts has been used to study lymphoid and myeloid development but is time consuming and labor intensive. Recently, transgenic technology has been used to produce zebrafish with cells or tissues that are marked by lineage-specific expression of the green fluorescent protein (GFP). This has facilitated the conduct of large-scale genetic screens.16-18
To date, there have been no genetic studies of zebrafish thrombopoiesis. In order to use zebrafish to study thrombocyte development, we have cloned the zebrafish ortholog of mammalian CD41, mapped and analyzed the CD41 gene, and cloned its promoter elements. Of particular importance is the development of transgenic zebrafish lines that have fluorescent (GFP+) thrombocytes and prothrombocyte precursors. These transgenic zebrafish strains should make it possible to study thrombocyte development and function in intact animals, to better identify thrombocyte precursors, and to carry out the chemical and mutagenic screens for which zebrafish are ideally suited. We report here on the use of these tools to analyze the ontogeny of the thrombocyte lineage.
Materials and methods
Cloning of the zebrafish CD41 cDNA
Initially, a partial CD41 cDNA was cloned from a zebrafish kidney cDNA library by low-stringency polymerase chain reaction (PCR). The degenerate primers 5′-GGYCCNAAYGGYAGCTAYTTYGGYTT-3′ and 5′-TTC-CARTGYTGCCANGGNGCRCA-3′ were deduced based on the aligned protein sequences from human, mouse, rat, and catfish CD41 (GenBank NM_000419, NM_010575, A60163, and A58533, respectively). The resulting cDNA probe was then P32-labeled using a random labeling kit (Roche Diagnostics, Indianapolis, IN) and was used to screen the zebrafish kidney cDNA phage library. Positive clones were isolated as phagemids using the vector pBK-CMV (Stratagene, La Jolla, CA) and a full-length cDNA was retrieved and sequenced.
For reverse-transcriptase (RT)–PCR analysis, we isolated total RNA from embryos staged 24, 34, 42, and 48 hpf and in larvae 3 and 5 days after fertilization (dpf) using TRIZOL reagent (Invitrogen, Philadelphia, PA). We reverse transcribed approximately 2 to 5 μg total RNA to make first-strand cDNA using a Superscript RT-PCR kit (Invitrogen). After first-strand cDNA synthesis, we amplified CD41 sequences by PCR using the CD41-specific primers 5′-TGTGCCCCATTGTTTCACTGG-3′ (forward) and 5′-CTGTCCAGCTGAAGCTCT-3′ (reverse). For an internal control, a portion of the EF-1α gene, corresponding to nucleotides 651 to 870 of the cDNA sequence (GenBank L23807), was probed using 5′-CGGTGACAACATGCTGGAGG-3′ (forward) and 5′-ACCAGTCTCCACACGACCCA-3′ (reverse) oligonucleotides (oligos).
Cloning of the zebrafish CD41 promoter
Using the zebrafish CD41 cDNA as a probe, 5 phage artificial chromosome (PAC) clones containing the CD41 gene were obtained from Dr Bruce Barut (Children's Hospital, Boston, MA). They were restricted with BamHI and BglII and subcloned into the pGEM4 vector (Promega, Madison, WI). We used PCR, with primers 5′-ACTCTAACAAGCATCTTGGA-3′ and 5′-GAAACCAAAGTAGCTGTC-3′ that are located 155–base pair (bp) apart in exon 1 of the CD41 gene to detect clones likely to encode the promoter region. Initially, a 1.7-kb segment 5′ to the CD41 coding region was isolated. This 1.7-kb fragment provided the information for designing the primers 5-′TGGTTATGAATTCTATGTTATG-3′ and 5′-AGGGCATCTGACCTCCAG-3′ that were used to clone additional 5′ sequence. A 6-kb DNA sequence containing putative CD41 promoter elements was identified and cloned and placed upstream of the GFP cDNA in the expression vector pEGFP-1 (Clontech, Palo Alto, CA) to form the CD41-GFP construct.
Injection of CD41-GFP into zebrafish embryos
The CD41-GFP plasmid was double-digested with XhoI and NaeI and the resulting restriction fragment separated on an 0.7% agarose gel. The DNA fragment was purified using the Compass DNA purification kit (American Bioanalytical, Natick, MA) and the recovered DNA extracted once with phenol/chloroform, ethanol precipitated, and resuspended in 150 mM KCl with 0.1% phenol red at a concentration of 50 to 100 ng/μL. Approximately 30 to 50 pg DNA was microinjected into multiple zebrafish embryos at the one-cell stage using standard microinjection techniques.
Identification of founders with germ-line expression of the transgene
DNA-injected embryos were raised to adulthood. When sexually mature, they were crossed with wild-type AB strain fish to produce F1 embryos. At 24 hpf, 50 F1 embryos were collected in a microcentrifuge tube with 100 μL DNA extraction buffer containing 200 μg/mL Proteinase K (Roche Diagnostic, Penzberg, Germany).19 The mixture was sheared to accelerate cell lysis by aspiration into a 1-mL syringe fitted with a 25-gauge, 5/8-inch needle. The sheared lysate was then incubated at 55°C for 1 hour and extracted with phenol/chloroform. Supernatant (1 μL) was used to identify founders by PCR with the primers 5′-GGTGAACTTCAAGATCCG-3′ and 5′-CGCCTTAAGATACATTGATG-3′ derived from the GFP cDNA sequence. Offspring of founder fish were examined under a fluorescent microscope for GFP expression, and GFP+ embryos were used to establish homozygous transgenic lines.
Whole-mount in situ hybridization and photomicrographs
Antisense mRNA probes were made for zebrafish CD41 and Clontech GFP cDNAs using the Riboprobe Combination System (Promega). Embryos were either treated with 0.003% 1-phenyl-2-thiourea (PTU; Sigma, St Louis, MO) or bleached after fixation in 4% paraformaldehyde (PFA). Whole-mount in situ hybridization was carried out as previously described20 with a few modifications. Posthybridization washes were carried out for 10 minutes with 3:1, 1:1, and 1:3 dilutions of hybridization buffer with 2 × SSCT (sodium chloride sodium citrate SSC plus 0.1% Tween-20 [T-20]), followed by two 30-minute washes with 0.2 × SSCT at 65 to 68°C with shaking. This was followed by two 5-minute washes with PBST (phosphate-buffered saline plus 0.1% T-20) at room temperature followed by incubation in blocking buffer (blocking buffer contained 5 mg/mL bovine serum albumin [BSA] and 5% lamb serum in PBST). Embryos were suspended in 90% glycerol in PBST before photomicrographs were taken with a Leica DC 500 camera attached to a dissecting microscope (Leica, Heidelberg, Germany). Photomicrographs of embryos expressing GFP were taken with the same camera attached to a mercury lamp excited dissecting/fluorescent microscope (Leica MZ FLIII). For the 2-color in situ hybridizations, the purple color was developed first using NBT (nitro blue tetrazolium chloride)/BCIP (5-bromo-4-chloro-3-indolyl phosphate toluidine salt) (Roche Diagnostics) as substrate. Pictures were taken and then the red color was developed using INT (2-[4-iodophenyl]-3-[4-nitrophenyl]-5-phenyl-tetrazolium chloride)/BCIP (Roche Diagnostics) and a second set of photographs obtained.
Confocal microscopy
LMO2-DsRed transgenic fish were generated using a 2.5-kb LMO2 promoter to drive the expression of the DsRed reporter. In the LMO2-DsRed transgenic embryos, the major blood vessels, including the dorsal aorta, axial vein, and intersomitic vessels, emitted red fluorescence. LMO2-dsRed transgenic adults were mated with CD41-GFP+ fish and doubly positive (LMO2-dsRed and CD41-GFP) embryos were selected. The embryos were anesthetized in 0.02% tricaine and embedded in 2% low-melting agarose made in E3 embryo medium for microscopy.
The images were captured using a Zeiss LSM 510 laser scanning confocal microscope with a Zeiss 40 ×/0.6NA LD-Archropan objective lens (Carl Zeiss, Heidelberg, Germany). A multitrack configuration was used to collect each channel (color) independently. Pixels (512 × 512) per frame were collected using the mean pixel intensities of 2 laser passes per line to reduce noise. Z-stacks were captured by marking the top and bottom parameters of the area that we chose to image and then imaging with an optic-slice thickness of less than 4 μm and an interval of 3 μm in all 3 channels automatically. Zeiss Advanced Imaging Microscopy software (Carl Zeiss) was used to render the 3-dimensional (3D) images and export them as Quicktime movies (Apple, Cupertino, CA).
Cell collection
Wild-type and transgenic adult zebrafish were anaesthetized with 0.02% tricaine prior to blood, kidney, and spleen collection. The spleen and kidney were dissected following a ventral, midline incision and placed into ice-cold 0.9% PBS containing 5% fetal calf serum and 1000 U/mL heparin (staining medium, SM) to prevent thrombocyte aggregation. Single-cell suspensions were generated by aspiration followed by gentle teasing of each organ atop a 40-μm nylon mesh filter using a plunger from a 1-cc syringe. Blood was obtained by cardiac puncture using micropipette tips coated with heparin and immediately placed into SM.
Flow cytometry
Hematopoietic cells isolated from transgenic zebrafish were processed as described in “Cell collection,” and propidium iodide (PI; Sigma) was added as a marker (1 μg/mL) to exclude dead cells and debris. Fluorescence-activated cell sorter (FACS) analysis and sorting were performed based on PI exclusion, forward scatter, and side scatter, and GFP fluorescence was performed using a FACS Vantage flow cytometer (Becton Dickinson, San Jose, CA). All cell populations were sorted twice to optimize cell purity. Percentages of GFP+ cells were determined by analyzing at least 50 000 cells per sample. Mature erythrocytes were also purified from whole kidney marrow (WKM) as previously described for comparison with the purified thrombocytes.21
Cytology
Cytospin preparations were performed using double-sorted GFP+ prothrombocytes and thrombocytes cytocentrifuged at 300 rpm for 3 minutes onto glass slides (Shandon, Pittsburgh, PA). Cytospin preparations were processed through May-Grünwald and Giemsa stains (Fluka; Sigma) for morphologic analyses.
Cloning of the zebrafish c-mpl cDNA
We designed primers encoding a segment of the c-mpl gene from a database compiled in Dr Leonard Zon's laboratory at Children's Hospital, Boston, MA (http://134.174.23.160/compGenomics/keyWordsRetrieve.htm). Primers 5′-CACTTGCTTTTGGGATGC-3′ and 5′-TGACCAGTCACTCCAGTT-3′ were used for the amplification by PCR of a reverse-transcribed cDNA mixture generated from flow-sorted CD41-GFP+ cells as previously described. In this case, CD41-GFP embryos were used rather than adult fish kidneys. The 3′ end of the cDNA was recovered using Smart RACE (Clontech) and the primer 5′-CAGCGGAAACTGGAGTGACTGGTC-3′ derived from the initial incomplete c-mpl cDNA.
Morpholino knockdown of zebrafish c-mpl and scl
Antisense morpholino oligo (Gene Tools, Corvalis, OR), 5′-CAGAACTCTCACCCTTCAATTATAT-3′, targeting a sequence from the exon/intron boundary of exon 2 was made for the zc-mpl gene. The inverted morpholino oligo 5′-TATATTAACTTCCCACTCTCAAGAC-3′ served as a control. For scl knockdown, oligos targeting the exon/intron boundary sequences of exons 1 and 2 of the zebrafish scl gene are described elsewhere.22 Morpholinos were resuspended in Danieau solution (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca(NO3)2, 5.0 mM Hepes [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], pH 7.6). Embryos were microinjected at the 1- to 4-cell stage with 1 nL morpholino solutions (containing both SCL E1/I and SCL E2/I morpholinos) at a final total concentration of 0.5 mM or with c-mpl MO at a concentration of 0.75 mM. Phenol red was coinjected as a tracer.
Results
Molecular cloning of CD41 cDNA, detection of CD41 mRNA, and CD41+ cells in developing embryos
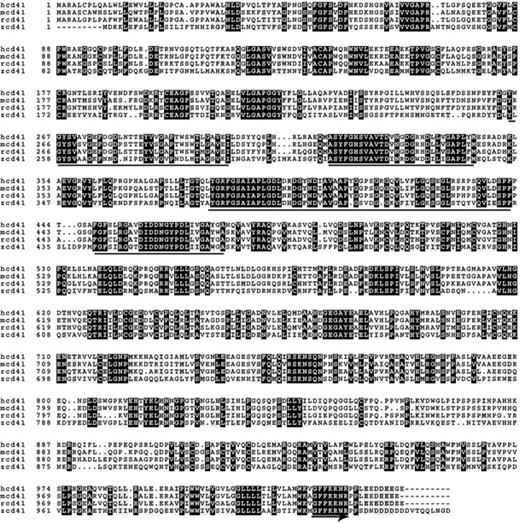
We compared the predicted protein sequence of zebrafish CD41 cDNA to the human, mouse, and rat sequences using the online box-shade program (http://www.ch.embnet.org/software/BOX_form.html). The zebrafish CD41 sequence was published as GenBank Locus AY319286. As shown in Figure 1, the sequence similarity between the 3 mammalian species is striking, sharing at least 70% identical amino acids. The zebrafish sequence is clearly more divergent from mammals, with only 40% of the sequence identical to the mammalian CD41 sequences. There are, however, still some highly conserved sequences that have been preserved among all of the species. In particular, the location of critical cysteines involved in disulfide bond formation and the location of the 4 potential calcium binding domains are conserved in all the species examined.
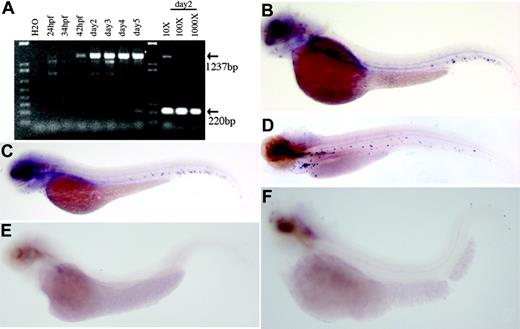
The time course of zebrafish CD41 mRNA production was assessed by RT-PCR, using zebrafish CD41 primers to detect zebrafish embryo RNA. As shown in Figure 2A, the CD41 transcript was first detected 42 hpf. Punctate staining within the area between the dorsal aorta and caudal vein was first detected 48 hpf by whole-mount in situ hybridization using an antisense CD41 cRNA probe (Figure 2B). The number of CD41+ cells increased as embryogenesis proceeded, and circulating thrombocytes were readily seen 3 to 4 days after fertilization (dpf) and in embryos 5 dpf (Figure 2C-D). In order to be certain that the punctate staining observed with the CD41 cRNA probe was within thrombocytes and thrombocyte precursors, we studied embryos with mutations known to abolish all evidence of hematopoiesis. The 2 mutants chosen were cloche and spadetail.23,24 After staining these embryos at 3 dpf, no circulating CD41+ cells were detected (Figure 2E-F). In contrast, in the blood mutant Retsina with defective erythropoiesis,25 embryos 3 and 5 dpf express CD41 normally, indicating unaltered thrombopoiesis (data not shown).
The CD41 promoter, injection, and examination of founder fish
We initially cloned a 1.7-kb DNA fragment that contained the proximal portion of the CD41 promoter. However, in the transgenic lines made with this promoter, GFP expression was not restricted to thrombocytes and there was strong expression in the skin along with weaker expression in thrombocytes (data not shown). We subsequently cloned a 6-kb fragment containing additional upstream sequences and used it to drive the GFP reporter. The zebrafish CD41 promoter sequence has little similarity to the human or mouse CD41 promoters, although it does contain several potential GATA-1 and ETS-1 binding sites, which have been previously noted in the human and mouse CD41 promoters.26-28 The sequence of the 1.7 kb of genomic DNA containing the promoter can be found in the Sanger Center Ensemble Data Assembly in contig Zv4_NA6523, as nucleotides 4257 to 5907.
We injected approximately 500 one-cell embryos. For injection, the CD41 promoter–GFP construct was restriction digested and the insert purified from the vector backbone, as vector sequences could potentially diminish or inhibit promoter activity. Of the injected embryos, 119 (23.8%) survived to adulthood and were fertile. We identified 7 founders (6%) of the 119 adults by PCR and confirmed the presence of a functional transgene by GFP expression. Three of these founders gave rise to progeny with similarly strong and specific GFP expression in their thrombocytes. We chose to focus on one of the lines and refer to it as the CD41-GFP zebrafish strain in all subsequent studies.
Pattern of GFP expression in CD41-GFP fish
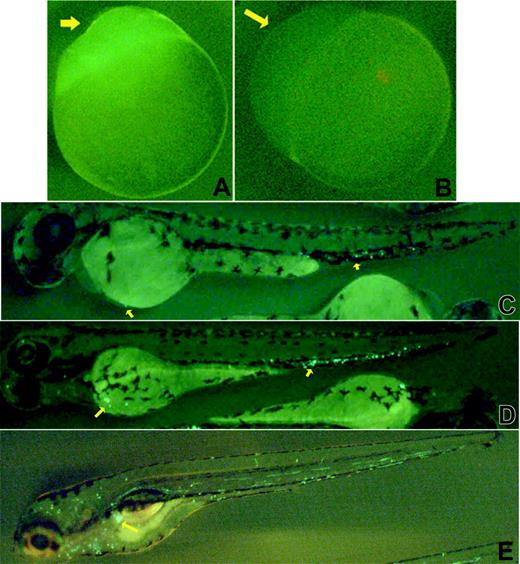
GFP fluorescence was noted in the animal poles of eggs derived from homozygous CD41-GFP females. This GFP expression was, presumably, maternally derived, as expression was also observed in unfertilized eggs carrying the transgene (Figure 3A), but not in the eggs of wild-type embryos (Figure 3B). GFP expression was also observed in the skin of early embryos (< 24 hpf), especially at the junction between the cardiac sinus and forehead and around the eye chamber (data not shown).
Alignment of deduced CD41 protein sequence. Alignment of the deduced amino acid sequences of the human (hcd41), mouse (mcd41), rat (rcd41), and zebrafish (zcd41) CD41 is shown. Forty percent of the zebrafish sequence is identical to the sequences of the other three species. There are some conserved features, including the location of cysteine residues, the four potential calcium-binding domains (underlined), and the C-terminal GFFKR sequence (underlined with arrow). The regions of sequence identity in the four species are shaded.
Alignment of deduced CD41 protein sequence. Alignment of the deduced amino acid sequences of the human (hcd41), mouse (mcd41), rat (rcd41), and zebrafish (zcd41) CD41 is shown. Forty percent of the zebrafish sequence is identical to the sequences of the other three species. There are some conserved features, including the location of cysteine residues, the four potential calcium-binding domains (underlined), and the C-terminal GFFKR sequence (underlined with arrow). The regions of sequence identity in the four species are shaded.
By 48 hpf, we observed brightly fluorescent, nonmobile GFP+ cells in the caudal region of the embryo between the dorsal aorta and the caudal vein. Cells were also seen occasionally in the cardiac sinus region (Figure 3C) at that time. By 3 dpf, there was an increased number of GFP+ cells in the caudal region and GFP+ cells had begun to circulate (Figure 3D). There were also some weakly fluorescent, nonmobile cells located between the dorsal aorta and the axial vein. By 5 dpf, the majority of the GFP+ cells were circulating, with small groups of nonmobile GFP+ cells now clustered anteriorly near the site of the developing mesonephros (Figure 3E, arrow). The collections of nonmobile GFP+ cells ventral to the dorsal aorta disappeared as the embryos matured further.
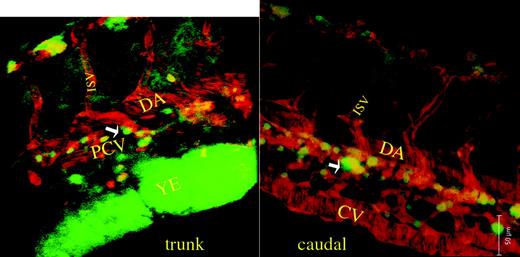
In order to better assess the anatomic location of the nonmobile GFP+ cells noted in embryos 2 to 3 dpf, we examined the embryos with a confocal laser microscope with computerized 3D reconstruction. An LMO2 (LIM domain only 2)–dsRed transgenic zebrafish line, which allows one to observe blood vessels, including the dorsal aorta, axial vein, and intersomitic vessels, was crossed with the CD41-GFP fish and doubly positive (dsRed and GFP) embryos were selected for confocal microscopy. We observed the nonmobile GFP+ cells in embryos 40 and 48 hpf. Two-dimensional photomicrographs in the trunk and caudal area of an embryo 48 hpf are shown in Figure 4. The corresponding movies of the 3D reconstruction are available as supplemental materials on the Blood website; click on the Supplemental Movies link at the top of the online article. The 3D reconstruction clearly shows that the GFP+ cells are located between the dorsal aorta and axial vein in a region that extends along both the subsidiary posterior cardinal and caudal veins.
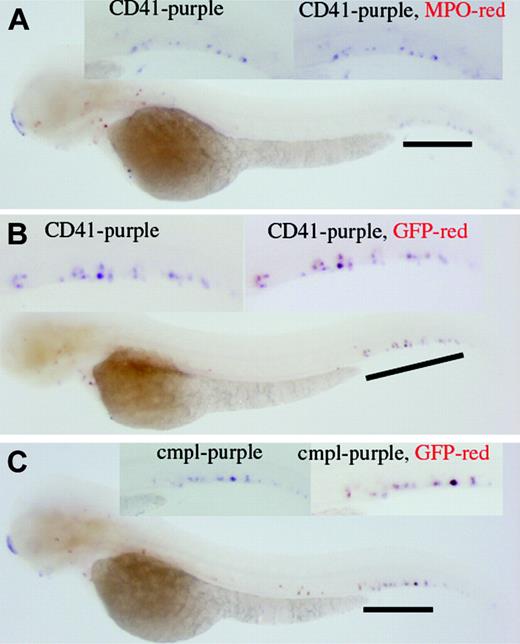
Results of whole-mount 2-color in situ hybridization indicate that the CD41-expressing cells do not express myeloperoxidase (MPO; Figure 5A) but express GFP (Figure 5B). c-mpl–expressing cells also express GFP (Figure 5C). Altogether, the in situ data support that the established transgenic line can serve as an in vivo tool to monitor the production of the CD41-expressing cells.
Flow sorting of GFP+ thrombocytes
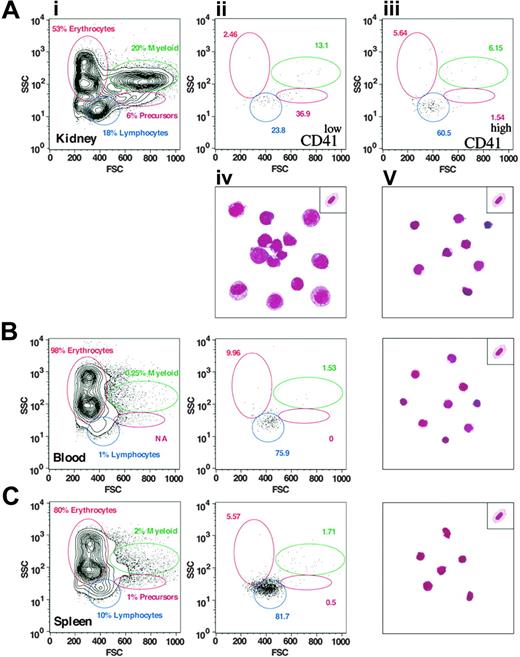
To assess expression of the CD41-GFP transgene in adult fish, we analyzed adult kidney, spleen, and peripheral blood by flow cytometry (FACS). Previous characterization of adult hematopoietic and lymphoid tissues by FACS has shown that each of the major blood lineages—erythroid, myeloid, and lymphoid—could be resolved by their light-scattering characteristics, along with another population of cells in the kidney that contains all immature precursors.21 As shown in Figure 6A and Table 1, 2 populations of CD41+ (GFP+) cells were found in the kidney. CD41low cells (0.81% of WKM) were found in both the precursor and lymphoid fractions, whereas CD41high cells (0.79% of WKM) were predominantly found within the lymphoid fraction. Isolation of each population to purity by 2 rounds of cell sorting showed that the CD41low fraction was composed primarily of large cells with basophilic cytoplasm and nuclei with open chromatin structure (Figure 6Aiv). These features are characteristic of hematopoietic progenitors, and we assume that these CD41low cells are prothrombocytes. In accordance with their lower forward scatter, sorted CD41high cells were small cells with scant cytoplasm and basophilic, condensed nuclei (Figure 6Av). The CD41high population contains only mature thrombocytes.
Percentage of GFP+ cells as demonstrated by FACS analysis
Tissue . | Cells, mean ± standard deviation (total no.) . |
|---|---|
| Kidney | |
| GFPhigh | 0.81 ± 0.41 (27) |
| GFPlow | 0.79 ± 0.30 (27) |
| Blood, GFP+ | 0.64 ± 0.12 (11) |
| Spleen, GFP+ | 4.21 (pooled) |
Tissue . | Cells, mean ± standard deviation (total no.) . |
|---|---|
| Kidney | |
| GFPhigh | 0.81 ± 0.41 (27) |
| GFPlow | 0.79 ± 0.30 (27) |
| Blood, GFP+ | 0.64 ± 0.12 (11) |
| Spleen, GFP+ | 4.21 (pooled) |
Results of RT-PCR and whole-mount in situ hybridization of CD41. (A) Shown is the analysis of the expression of CD41 mRNA in developing zebrafish embryos by RT-PCR. The CD41 transcript is first detected at 42 hpf. Dilutions (10 ×, 100 ×, and 1000 ×) of the cDNA preparation 2 dpf, with EF-1α as a control, indicated that CD41 transcript is at least 1000 × lower than EF-1α transcript. The expected PCR product for CD41 is 1237 bp and for EF-1α is 220 bp. (B-D) Shown also are results of whole-mount in situ hybridization of wild-type zebrafish embryos with zebrafish CD41 antisense cRNA. (B) In embryos 2 dpf, punctate purple CD41+ cells are present in the ventral region of the dorsal aorta. (C) In embryos 3 dpf, the number of CD41+ cells has increased and they appear in the circulation. (D) In embryos 5 dpf, more CD41+ cells are present in the circulation. The CD41 transcript is not detected in the spadetail (E) and cloche (F) embryos 3 dpf.
Results of RT-PCR and whole-mount in situ hybridization of CD41. (A) Shown is the analysis of the expression of CD41 mRNA in developing zebrafish embryos by RT-PCR. The CD41 transcript is first detected at 42 hpf. Dilutions (10 ×, 100 ×, and 1000 ×) of the cDNA preparation 2 dpf, with EF-1α as a control, indicated that CD41 transcript is at least 1000 × lower than EF-1α transcript. The expected PCR product for CD41 is 1237 bp and for EF-1α is 220 bp. (B-D) Shown also are results of whole-mount in situ hybridization of wild-type zebrafish embryos with zebrafish CD41 antisense cRNA. (B) In embryos 2 dpf, punctate purple CD41+ cells are present in the ventral region of the dorsal aorta. (C) In embryos 3 dpf, the number of CD41+ cells has increased and they appear in the circulation. (D) In embryos 5 dpf, more CD41+ cells are present in the circulation. The CD41 transcript is not detected in the spadetail (E) and cloche (F) embryos 3 dpf.
Expression of GFP in the CD41-GFP embryos. Photomicrographs showing CD41-GFP+ expression in transgenic embryos are presented. Unfertilized eggs from homozygous (A) and wild-type (B) females demonstrate the maternally derived GFP. At 48 hpf, bright CD41-GFP+ cells are detected in the region between dorsal aorta and caudal vein (arrows, C) and cardiac sinus/yolk sac (arrow near yolk). By 3 dpf, increasing numbers of CD41-GFP+ cells are detected in a similar region (arrows near tail, D), and the cardiac sinus/yolk sac (arrow near yolk), as well as in the circulation (D). By 5 dpf, the majority of the CD41-GFP+ cells are circulating, and a collection of CD41-GFP+ cells appears near the developing mesonephros (arrow, E).
Expression of GFP in the CD41-GFP embryos. Photomicrographs showing CD41-GFP+ expression in transgenic embryos are presented. Unfertilized eggs from homozygous (A) and wild-type (B) females demonstrate the maternally derived GFP. At 48 hpf, bright CD41-GFP+ cells are detected in the region between dorsal aorta and caudal vein (arrows, C) and cardiac sinus/yolk sac (arrow near yolk). By 3 dpf, increasing numbers of CD41-GFP+ cells are detected in a similar region (arrows near tail, D), and the cardiac sinus/yolk sac (arrow near yolk), as well as in the circulation (D). By 5 dpf, the majority of the CD41-GFP+ cells are circulating, and a collection of CD41-GFP+ cells appears near the developing mesonephros (arrow, E).
Similar analyses of peripheral blood (Figure 6B) and splenocytes (Figure 6C) showed only the presence of CD41high cells. GFP+ cells were a minor subset of peripheral blood (0.64%), and the purified cells all showed the morphology of mature thrombocytes (Figure 6B, right panel). The spleen contained a relatively high proportion of CD41high cells (4.21%), and all purified cells were found to be mature thrombocytes (Figure 6C, right panel). Taken together, the FACS and cellular morphology data suggest that thrombocytes are born in the kidney via prothrombocytic intermediates that then circulate through the peripheral blood and spleen.
Molecular cloning and functional analysis of zebrafish c-mpl
In order to initiate studies of the control of thrombopoiesis in zebrafish and to further characterize the CD41-GFP cells, we sought to identify the zebrafish ortholog of c-mpl in flow-sorted GFP+ cells. Thrombopoietin binding to c-mpl, the sole known receptor for thrombopoietin, plays an important role in thrombocyte production by increasing progenitor maturation along the thrombocyte lineage.29,30 We obtained putative zebrafish c-mpl cDNA by PCR using reverse-transcribed cDNA made from embryonic preparations and from GFP+ cells purified from adult transgenic zebrafish. cDNA samples made from embryos at 50% epiboly or embryos 4 dpf yielded relatively little PCR product compared with PCR performed on the FACS GFP+ cells, indicating enrichment in thrombocyte-specific transcripts in the GFP+ cells. Two partial zc-mpl cDNAs were recovered and the full-length cDNA was deduced by combining the data from 2 cDNAs and the contig ctg13356 (http://www.ensembl.org/Danio_rerio). The deduced sequence was submitted as GenBank Locus AY319287. The coding sequence is 30% identical to those of human and mouse (NM_005373 and NM_010823, respectively) at the protein level. The splicing patterns of the intron-exon boundaries are also conserved when compared with the human sequence.
Confocal scanning microscopy of CD41-GFP/LMO2-dsRed embryos. Shown are 2-dimensional photomicrographs of confocal microscopy near the trunk and caudal portion of an embryo 48 hpf. Two corresponding movies of 3-dimensional reconstructions of the same embryo are available as supplemental material on the Blood website; click on the Supplemental Movies link at the top of the online article. Of note is that the major vessels, whose endothelial cells are tagged with LMO2-dsRed, emit red fluorescence, and the nonmobile CD41-GFP+ cells emit green fluorescence. The GFP+ cells are extravascular and are clearly located in the region between the dorsal aorta and the posterior cardinal vein (trunk view, arrow) and caudal portion between the caudal dorsal aorta and caudal vein (caudal view, arrow). DA indicates dorsal aorta; PCV, posterior cardinal vein; ISV, intersomitic vessel; YE, yolk extension; and CV, caudal vein.
Confocal scanning microscopy of CD41-GFP/LMO2-dsRed embryos. Shown are 2-dimensional photomicrographs of confocal microscopy near the trunk and caudal portion of an embryo 48 hpf. Two corresponding movies of 3-dimensional reconstructions of the same embryo are available as supplemental material on the Blood website; click on the Supplemental Movies link at the top of the online article. Of note is that the major vessels, whose endothelial cells are tagged with LMO2-dsRed, emit red fluorescence, and the nonmobile CD41-GFP+ cells emit green fluorescence. The GFP+ cells are extravascular and are clearly located in the region between the dorsal aorta and the posterior cardinal vein (trunk view, arrow) and caudal portion between the caudal dorsal aorta and caudal vein (caudal view, arrow). DA indicates dorsal aorta; PCV, posterior cardinal vein; ISV, intersomitic vessel; YE, yolk extension; and CV, caudal vein.
The staining pattern of c-mpl+ cells by whole-mount in situ hybridization is similar to that previously observed after staining with CD41 cRNA probes (Figure 7A-B). Injection of an antisense c-mpl morpholino into one-cell embryos effectively eliminated the production of circulating GFP+ cells for at least the first 5 days of embryogenesis, providing additional evidence that the GFP+ cells are thrombocytes (Figure 7, MOc 3 dpf and 5 dpf). Injection of a control (inverted sequence) morpholino did not reduce the number of circulating GFP+ cells (Figure 7, MOinv). These experiments reproduced the murine c-mpl knock-out phenotype, where platelet production was profoundly reduced, but not completely eliminated.31
Some nonmobile GFP+ cells located between the dorsal aorta and the caudal vein were still visible after injection of the c-mpl morpholino, raising the possibility that they were either nonhematopoietic cells or that they were sufficiently immature that they did not yet require c-mpl/thrombopoietin for their growth. In order to evaluate these 2 possibilities, we performed a transient scl (T-cell acute lymphocytic leukemia 1, also known as scl and scl/tal1) knockdown. In contrast to the results obtained with c-mpl knockdown, the injection of a scl antisense morpholino into CD41-GFP embryos markedly reduced the number of both the circulating and the nonmobile GFP+ cells observed at 4 dpf (Figure 7, MOs). This demonstrates that these cells are hematopoietic, but raises the intriguing possibility that some of the nonmobile cells may be either very early thrombocyte progenitors or even earlier hematopoietic progenitors.
Two-color whole-mount in situ hybridization. (A) CD41 expression does not colocalize with MPO, as judged by the CD41 cells (purple) that do not turn red in the in situ result. (B) On the contrary, the coexpression of CD41 and GFP is supported by the red color (GFP) outcome of originally purple cells (CD41). (C) In addition, coexpression of c-mpl and GFP in the same cells is supported.
Two-color whole-mount in situ hybridization. (A) CD41 expression does not colocalize with MPO, as judged by the CD41 cells (purple) that do not turn red in the in situ result. (B) On the contrary, the coexpression of CD41 and GFP is supported by the red color (GFP) outcome of originally purple cells (CD41). (C) In addition, coexpression of c-mpl and GFP in the same cells is supported.
Discussion
In the present study, we report on the use of a platelet-specific marker, the α subunit of the integrin CD41/CD61, to track thrombocyte proliferation and maturation during zebrafish embryogenesis. Zebrafish CD41 cDNA was cloned and used to generate zebrafish cRNA probes for whole-mount in situ hybridization. We subsequently developed a transgenic zebrafish strain with fluorescent thrombocytes by fusing CD41 promoter elements to the GFP cDNA and used these fish to assess thrombocyte development, in real time, in living embryos.
Results of FACS analyses. (A) Panel i shows 2 populations of GFP+ cells (CD41low and CD41high representing 0.81% and 0.79% of WKM, respectively) that are detected in the kidney. CD41low cells (ii) are predominantly large prothrombocytic cells and CD41high cells (iii) are differentiated thrombocytes as demonstrated by the corresponding Wright-Giemsa staining of flow-sorted cells shown in panels iv and v (see “Flow sorting of GFP+ thrombocytes”). A representative erythrocyte is placed at the top right corner for comparison. By contrast, the vast majority of cells isolated from peripheral blood (B) and spleen (C) are CD41high (0.64% and 4.21%, respectively) and display the mature thrombocyte morphology.
Results of FACS analyses. (A) Panel i shows 2 populations of GFP+ cells (CD41low and CD41high representing 0.81% and 0.79% of WKM, respectively) that are detected in the kidney. CD41low cells (ii) are predominantly large prothrombocytic cells and CD41high cells (iii) are differentiated thrombocytes as demonstrated by the corresponding Wright-Giemsa staining of flow-sorted cells shown in panels iv and v (see “Flow sorting of GFP+ thrombocytes”). A representative erythrocyte is placed at the top right corner for comparison. By contrast, the vast majority of cells isolated from peripheral blood (B) and spleen (C) are CD41high (0.64% and 4.21%, respectively) and display the mature thrombocyte morphology.
In the zebrafish, yolk sac or primitive hematopoiesis is well established by 24 hpf,23 and several previous studies have shown the production of erythroid and myeloid cells in the yolk sac.23,32,33 Our studies clearly demonstrate that thrombocytes are not produced during this very early phase of hematopoiesis. As noted, CD41 mRNA transcripts first appeared 42 hpf, and circulating cells that stain with CD41 antisense cRNA probes were detected 2 to 3 dpf. The number of CD41+ cells steadily increased during the later stages of embryogenesis and into the larval stage (5-7 dpf).
Results of whole-mount in situ hybridization of c-mpl and c-mpl and scl morpholino injection. Shown are the results of whole-mount in situ hybridization with antisense c-mpl cRNA (A; high power, B) at the embryo 3 dpf. c-mpl+ cells are detected in the same location as CD41+ and GFP+ cells detected in previous studies. Injection of a zebrafish c-mpl antisense morpholino (MOc) reduced the number of GFP+ cells 3 and 5 dpf. c-mpl morphants have some residual GFP+ cells. Injection of an inverted morpholino (MOinv) did not decrease the number of circulating GFP+ cells. Injection of 2 scl morpholinos (MOs) also reduced the number of GFP+ cells in the morphants at 4 dpf, in addition to causing tail deformation. For comparison, uninjected siblings of the same developmental stages are posted in the right corner of each picture.
Results of whole-mount in situ hybridization of c-mpl and c-mpl and scl morpholino injection. Shown are the results of whole-mount in situ hybridization with antisense c-mpl cRNA (A; high power, B) at the embryo 3 dpf. c-mpl+ cells are detected in the same location as CD41+ and GFP+ cells detected in previous studies. Injection of a zebrafish c-mpl antisense morpholino (MOc) reduced the number of GFP+ cells 3 and 5 dpf. c-mpl morphants have some residual GFP+ cells. Injection of an inverted morpholino (MOinv) did not decrease the number of circulating GFP+ cells. Injection of 2 scl morpholinos (MOs) also reduced the number of GFP+ cells in the morphants at 4 dpf, in addition to causing tail deformation. For comparison, uninjected siblings of the same developmental stages are posted in the right corner of each picture.
Although whole-mount in situ hybridization can be used to detect circulating thrombocytes, the signal obtained with the CD41 cRNA probe was relatively weak. For example, it was not easy to stain the mesonephric area, where definitive hematopoiesis, including thrombopoiesis, is known to occur. In addition, with whole-mount in situ hybridization, it was not possible to observe thrombocytes in real time or in living embryos. For these reasons, we generated GFP transgenic fish by fusing zebrafish CD41 promoter elements to GFP cDNA. We anticipated that with this transgene, GFP expression would be restricted to thrombocytes and thrombocyte precursors. While this was largely accurate, we did observe some extrahematopoietic GFP expression during the first 24 hpf.
In our study, cells expressing the CD41-GFP transgene were first noted 48 hpf in the ventral region of the dorsal aorta. This site superficially resembles the AGM region where hematopoietic progenitors have been noted to localize in day-10.5 murine embryos34,35 and in zebrafish embryos 24 to 48 hpf.36 However, there are several important differences. First, the CD41-GFP+ cells are not visible until just after the classic zebrafish AGM cells are no longer detected. Second, the collection of cells is more caudal than the classic zebrafish AGM. We do not yet know whether these nonmobile caudal CD41-GFP+ cells are multipotent hematopoietic progenitors or thrombocyte precursors, although the fact that some of the cells persist after injection of a c-mpl antisense morpholino raises the possibility that they could be early hematopoietic cells that do not yet express c-mpl. One intriguing possibility is that this caudal area is a previously unrecognized site of early hematopoiesis that appears after dissolution of the AGM but before definitive hematopoiesis is established in the interstitium of the developing mesonephros. Additional studies including transplantation of these cells into irradiated hosts will be needed to establish the hematopoietic potential of this collection of CD41-GFP+ cells.
The FACS analysis provided some additional useful information about the nature of the GFP+ cells. Analysis by FACS detected 2 distinct populations of GFP-expressing cells. The brightly fluorescent CD41high subset looked like typical thrombocytes after Wright-Giemsa staining and is presumed to be the more mature and well-differentiated thrombocyte subset. A smaller population of cells that was weakly fluorescent, the CD41low subset, had a more undifferentiated morphology. They were larger than the CD41high subset, round rather than oval, and had larger nuclei with basophilic cytoplasm. Although additional studies are necessary to be definitive, we speculate that these cells might be thrombocyte progenitors or prothrombocytes. This is the first time, to our knowledge, that it has been possible to differentiate thrombocytes and possible thrombocyte precursors in nonmammalian vertebrates.
Marking cells of the thrombocyte lineage with GFP provides a way to sort cells for gene-expression studies or to create cDNA libraries enriched in thrombocyte mRNA transcripts. Thrombocyte and thrombocyte precursor cDNA libraries can then be used to identify genes that regulate thrombocyte specification and differentiation as well as to study the expression of genes that play a role in normal hemostasis or thrombosis. Zebrafish are particularly useful for these studies, as the pattern of expression of a gene of interest can then be assessed by whole-mount in situ hybridization. Gene function can also be tested during embryogenesis by inhibiting gene expression with morpholino knockdowns or by rescuing mutant embryos with mRNA injection. Finally, the transgenic fish could be used to study thrombocyte interactions with the vessel wall or with each other during the process of hemostasis.
Prepublished online as Blood First Edition Paper, August 11, 2005; DOI 10.1182/blood-2005-01-0179.
Supported by the Jock and Bunny Adams Research & Education Fund, a 50th Anniversary Fellowship from Harvard Medical School, the Malcolm Hecht Family Fellowship, and National Institutes of Health (NIH) National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) KO1 DK066254-01.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Matt Salanga for assistance with confocal microscopy, Chris Lawrence for his help with fish husbandry, and the many members of the Zon laboratory who provided informal assistance, consultation, and tutoring.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal