Abstract
The IgVH mutation status is an independent predictor of time to treatment and survival in CLL. However, the biological basis of this difference is unknown. Clinical use of the IgVH mutation status as a prognostic marker is limited due to the technical difficulties of assessing this feature. As such, we probed for differences in protein expression by CLL cells with unmutated (UM) vs mutated (M) IgVH using phage peptide display libraries (PDPL).
Methods: PDPL consists of phages engineered to express random peptides (7-mer) on the coat protein. From approximately 2x1011 phages displaying all possible permutations of 7-mer peptide, we selected phages binding to UM cells by incubation with 106 UM cells and elution of bound phages. Then, from numerous phages initially binding to UM cells, we subtracted phages binding to M cells by multiple incubations with M cells (106). Thus, the phages that bound only to UM cells were selected. The procedure was repeated multiple times to increase selection specificity. The peptide sequences displayed by selected phages were evaluated. We identified phage targets on UM cells by immunoprecipitation (IP) of CLL protein with specific phage and by sequencing of precipitated protein. The expression of target protein in CLL cells was confirmed by immunobloting and flow cytometry.
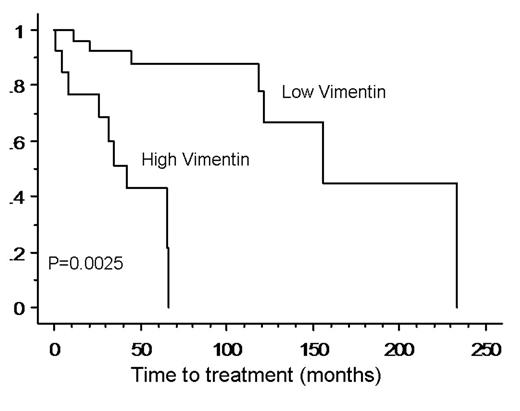
Results: Sequencing of phages from the 3rd round of selection (UM v. M) revealed several peptides, with the most predominant being FPSAHFL. IP identified the cellular target for FPSAHFL as vimentin, an intermediate filament protein involved in cell motility and activation. The level of vimentin expressed by CLL cells varied between patient samples (n=15). UM CLL cases were found to express higher levels of vimentin (the mean MFI was 1002; SD 381), than cases of M CLL (mean MFI 547; SD 109), p=0.0128. To evaluate the prognostic significance of vimentin, we assessed vimentin expression by flow cytometry in a separate subgroup of 40 CLL patients. Archived cell samples collected between 6/2000 – 6/2001 were used. The median follow up was 81 months (40–233). Vimentin expression (MFI) as a continuous variable was a risk factor for a shortened time to treatment (TTT) with a proportional hazard ratio of 4.152 (95% CI 1.33–15.220, p=0.0317). Using MFI closest to the median (and rounded to 2 decimal places) as a cutoff, we stratified patients in high (n=21) and low (n=19) vimentin expression groups. The median times to treatment (TTT) in patients with high vs low level expression of vimentin were 2.8 and 12.9 years respectively, p=0.0025 (Fig.).
Conclusion: Using novel proteomic technology, we found that CLL cells with unmutated IgVH expressed higher levels of vimentin than CLL cells with mutated IgVH. A high-level of vimentin expression defined by flow cytometry appears to be associated with high-risk disease. Validation of these findings could allow for the vimentin level to be used as a surrogate marker for expression of unmutated IgVH in CLL.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal