Abstract
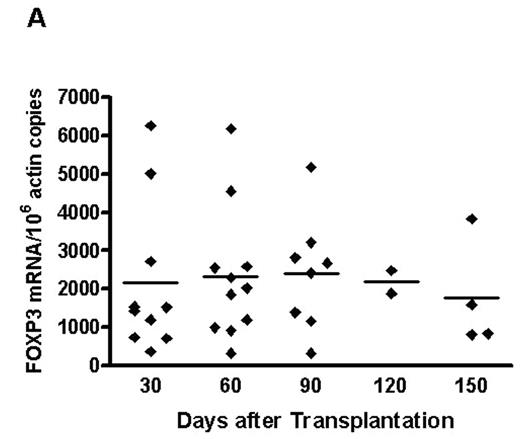
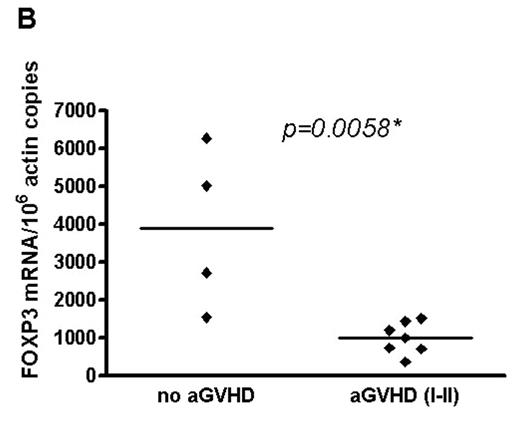
Ex-vivo selective depletion (SD) of alloreactive donor lymphocytes by anti-CD25 immunotoxin is a novel method to reduce the severity of graft-versus-host-disease (GVHD) in elderly patients undergoing matched-sibling transplantation (Blood 2005, 106 (3), 1123–9). Nevertheless, there is concern that the concurrent removal of CD4+CD25+ regulatory T cells (Tregs) with the alloactivated CD25-expressing T cells could precipitate more GVHD if depletion of alloreacting cells were incomplete. We, therefore, studied the recovery of Tregs in 11 patients within the first 150 days after SD transplantation by surface phenotyping for CD4 and CD25 and real time PCR for forkhead protein3 (foxp3) gene, shown to be specific for characterisation of Tregs. At transplant patients received a median of 1.0 (0.5–1.5) x108/kg selectively depleted (CD25−) CD3+ T cells and a stem cell product containing 0.25 x104/kg (0.1–5.0) residual unselected CD3+ T cells. Three patients received SD-DLIs on day +60 or day +100. Low average levels of foxp3 gene expression appeared around day +30 post-transplant and remained almost stable until day +150 (Figure A). GVHD occurred in 7 patients (2 grade I and 5 grade II). Acute grade I-II GVHD was restricted to 7 patients with significantly lower foxp3 gene expression at day +30 (for one patient the earliest available sample was from day +60) (Figure B). In contrast to the relatively steady state of foxp3 gene expression average CD4+CD25+ cell numbers were highest during the first 60 days declining thereafter and suggesting the presence of relatively more effector cells in the early post-transplantation period. Chimerism analysis on selected CD4+CD25+ cells showed them to be predominantly of donor origin. These results suggest that foxp3+ Tregs delivered with the stem cell product may undergo modest expansion post-transplant. The absence of high grade aGVHD in these 11 patients probably relates to the efficient removal of host-alloreactive T cells by the SD process. Nevertheless, the occurrence of low-grade aGVHD in patients with the lowest foxp3 gene expression indicates that Tregs are also involved in GVHD protection after SD transplants. As the SD approach is at a very early clinical stage of development this possibility remains to be explored in further studies.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal