Abstract
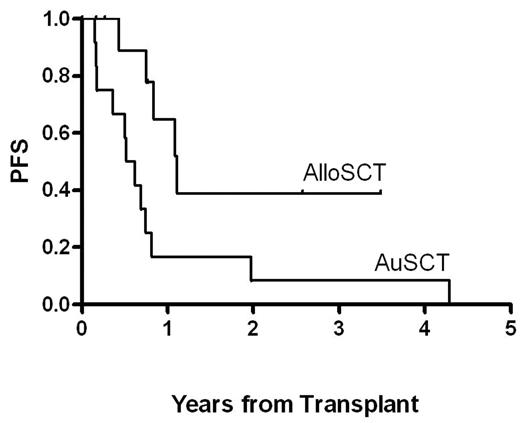
Twelve patients with relapsed or refractory HL after autologous stem cell transplant (AuSCT), all nodular sclerosis subtype, underwent allogeneic stem cell transplant (AlloSCT) from either an HLA identical sibling (n = 5) or a matched unrelated donor (n = 7) between January 2002 and May 2005. The median number of chemotherapy regimens received prior to AlloSCT was four (range: 4–5). Eleven patients received reduced intensity conditioning with melphalan 100 mg/m2 only and the final patient was conditioned with busulfan 130 mg/m2 days −6 to −3 and fludarabine 40 mg/m2 days −6 to −3. All 11 patients who received melphalan conditioning were treated with calcineurin inhibitor (cyclosporin or tacrolimus) and Cellcept for graft vs host-disease (GVHD) prophylaxis. The patient who received busulfan and fludarabine conditioning received methotrexate and tacrolimus for GVHD prophylaxis. Median age at transplantation was 39 years (range: 22.5–60.8). Five patients were female. The treatment related mortality was 0/12 (0%). Median time to progression after AuSCT was 6.1 months (range: 1.8 – 50.2). Patients received a median of 5.5 x 106 CD34+ peripheral blood progenitor cells/kg (range: 4.1–8.3). Median time to ANC engraftment (> 500/μL) was 13 days (range: 11–20) and median time to platelet engraftment (platelet count over 20,000/μL for 3 consecutive days with no platelet transfusions for the preceeding 7 days) was 20 days (range: 13–93). Median follow-up for patients after AlloSCT is 15.8 months (range: 2– 41). Median progression-free survival (PFS) after AlloSCT is 9.5 months (range: 2–41+) with median overall survival of 15.8 months (range: 2–41+). Common toxicities included fever and mucositis. There was one instance each of presumed fungal pneumonia, streptococcal mitis bacteremia, acute renal failure, and hemolysis from ABO mismatch. Three of twelve (25%) patients developed TTP secondary to calcineurin inhibitors for GVHD prophylaxis, and it resolved in all cases. One patient developed ARDS that required intubation and subsequently resolved. Four patients who had extensive cGVHD had PFS of 5.1 to 41+ months with a median of 19.6 months, compared to median PFS of 9.5 months for all patients, suggesting an association between severe cGVHD and disease control. One of these patients developed GVHD after progression at 8.4 months, followed by immunosuppression withdrawal, stabilization of disease and survival to 30.7 months. For patients who have been followed post AlloSCT for longer than their time to relapse post AuSCT, in nine of ten cases, the response duration post AlloSCT exceeded the time post AuSCT. As shown in the figure below, there is a trend toward greater PFS with AlloSCT than with the preceding AuSCT. These data support the concept of a GVL effect. Further study will better define the population of HL patients most likely to benefit from reduced intensity AlloSCT.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal