Abstract
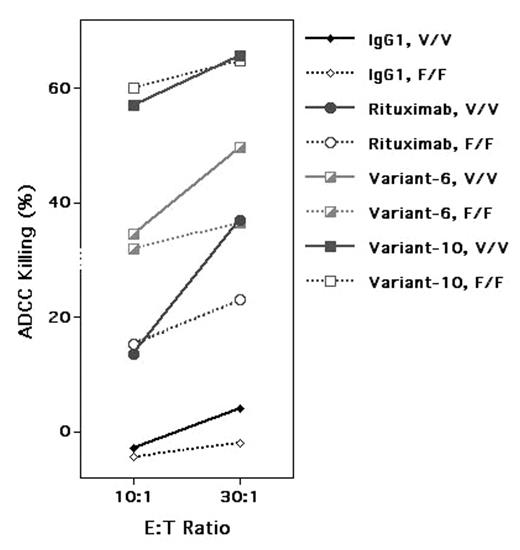
We and others have found that IgG Fc receptor, Fcγ RIIIa Valine/Valine (V/V) and Fcγ RIIa Histidine/Histidine (H/H) genotypes predicted the response to rituximab in follicular lymphoma patients probably due to their higher affinity to the constant region (Fc) of rituximab during antibody-dependent cellular cytotoxicity (ADCC). Patients with low affinity Fcγ RIIIa Phenylalanine (F) carrier (V/F and F/F) and Fcγ RIIa Arginine (R) carrier (H/R and R/R) have much lower chance to respond to rituximab and have a brief remission when they responded. This observation implicates the interaction between the Fc of rituximab and Fcγ R on effectors (NK cells and macrophages) as a determinant for clinical response. Therefore, it may be possible to re-engineer the Fc of rituximab to increase its affinity to Fcγ R and thereby to enhance the antibody’s ability to mediate ADCC and to improve its clinical efficacy. Rituximab variants with re-engineered Fc were generated by MacroGenics. Single amino acid substitutions with increased affinity to different Fcγ R alleles were identified by screening a yeast library containing randomly mutated Fc. Further re-engineering was accomplished by combining multiple amino acid substitutions within both the Fcγ R-binding CH2 and the conformational CH3 domains of the Fc to further improve their affinity. We have tested 12 such variants for their ability to mediate ADCC against primary follicular lymphoma targets. Three variants (4, 10, 12) showed significant enhancement in ADCC compared to rituximab, using effector cells of V/V genotype. We then tested whether the enhancement of ADCC applies to effectors of different Fcγ RIIIa genotypes. In one experiment, at a 30:1 effector/target ratio, specific ADCC lysis for rituximab, Variant 6 (a modest enhancer) and Variant 10 (a strong enhancer) were 37%, 50% and 66%, respectively, with V/V effectors and 23%, 36% and 65%, respectively, with F/F effectors. Thus, enhancement of ADCC by these variants applied to effectors of both high (V/V) and low (F/F) affinity Fcγ Rs. In one case, Variant 10, enhancement of ADCC with effctors of low affinity Fcγ R, brought its activity up to that of effectors with the high affinity Fcγ R. We further examined the ability of these variants to engage and internalize the Fcγ R on NK cells as a measure of primary engagement of Fcγ R. Rituximab-coated tumor cells reduced the surface Fcγ RIIIa on NK cells of V/V genotype by 78% determined by flow cytometric analysis. By comparison, Variant 6 down-regulated surface Fcγ RIIIa by 84% and Variant 10 by 88%. For NK cells of F/F genotype, rituximab down-regulated surface Fcγ RIIIa by 32%, Variant 6 by 64% and Variant 10 by 68%. This result confirmed that two rituximab variants were more effective in interacting with effectors of both high and low affinity Fcγ Rs. This study has demonstrated that several rituximab variants with re-engineered Fc showed increased interaction with Fcγ R on effectors and mediated ADCC more effectively than rituximab even with effectors of low affinity Fcγ R genotypes. These rituximab variants may prove to be more effective therapeutic anti-CD20 antibodies than rituximab, especially for patients with low affinity Fcγ Rs.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal