Abstract
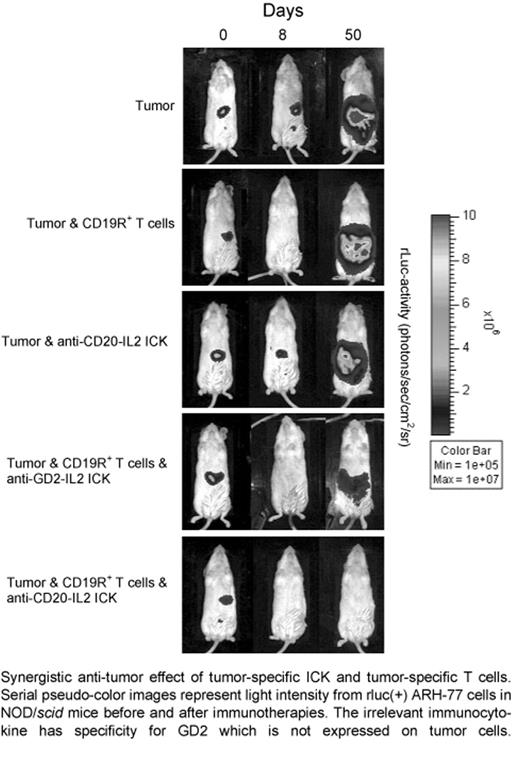
Lineage-specific cell-surface molecules, such as CD19 and CD20 on B-lineage malignancies are the targets for immunotherapies. However, the therapeutic success of CD19-specific T-cell therapy is predicted to be dependent on the continued persistence of adoptively transferred T cells. It is well-recognized that exogenous IL-2 can help sustain the in vivo persistence of ex vivo-propagated CD8+ T cells. Therefore, in order to target biologically active IL-2 to the tumor microenvironment, we have used an immunocytokine (ICK) to deliver this cytokine to binding-sites of a CD20-specific monoclonal antibody (mAb) on malignant B cells. This anti-CD20-IL2 ICK was based on the Leu16 anti-CD20 mAb that was dehumanized to remove T-helper epitopes. Flow cytometry demonstrated that anti-CD20-IL2 ICK specifically bound to CD20+ tumor and IL-2R+ T cells. Thus, we investigated the ability of this ICK to improve the persistence of adoptively transferred B-lineage lymphoma-specific T cells. To obtain CD19-specific T cells that could be non-invasively imaged in vivo, we used non-viral gene transfer to introduce a DNA plasmid to co-express both a CD19-specific immunoreceptor (designated CD19R) and firefly luciferase (ffLuc). The CD19R combines antibody recognition with T-cell effector functions mediated through CD3-ζ . The genetically modified T cells were characterized as differentiated CD8+ effector cells expressing the IL-2 receptor complex, which specifically recognize and lyse CD19+ lymphoma targets. To model the survival of adoptively transferred T cells and treatment of lymphoma in vivo, we generated a CD20+CD19+ ARH-77 tumor line expressing the Renilla luciferase (rLuc) reporter gene. Our data demonstrate that this tumor line is resistant to a CD20-specific mAb, Rituximab, in vivo. Sub-optimal doses of CD19R+ffLuc+ CD8+ T cells, which do not cause complete eradication of tumor by themselves, were infused along with anti-CD20-IL2 ICK and control ICK (with irrelevant specificity) in NOD/scid mice bearing xenografts of the rLuc+ tumor. In vivo non-invasive bioluminescent imaging (BLI) was used to longitudinally measure the persistence of ffluc+ T cells and growth of rluc+ tumor. In our mouse model, the T cells persisted significantly (p<0.05) longer in the mice treated with anti-CD20-IL2 ICK, compared to mice receiving the ICK with an irrelevant specificity. This improvement in T-cell persistence translated into augmented anti-tumor activity (Figure). These results suggest that combining tumor-specific ICK with tumor-specific T cells may improve the outcome of immunotherapy. Since Phase I trials are underway using anti-CD20-IL2 ICK and CD19-specific T cells as monotherapy, our results warrant clinical trials using combination of these immunotherapies.
Synergistic anti-tumor effect of tumor-specific ICK and tumor-specific T cells. Serial pseudo-color images represent light intensity from rluc(+) ARH-77 cells in NOD/scid mice before and after immunotherapies. The irrelevant immunocytokine has specificity for GD2 which is not expressed on tumor cells.
Synergistic anti-tumor effect of tumor-specific ICK and tumor-specific T cells. Serial pseudo-color images represent light intensity from rluc(+) ARH-77 cells in NOD/scid mice before and after immunotherapies. The irrelevant immunocytokine has specificity for GD2 which is not expressed on tumor cells.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal