Abstract
The CALGB conducted a series of clinical trials with azacitidine (Vidaza®) administered subcutaneously or intravenously in patients with MDS using the FAB classification (
Best Response using IWG Response Criteria for MDS in Studies 8421, 8921, and 9221
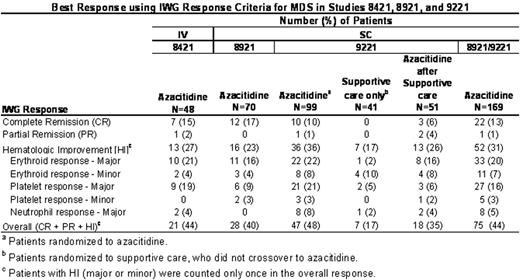
Median duration of any response (CR, PR or HI) in the 114 azacitidine-treated responders was 366 days (range: 56 to 4641+ days). The median duration of CR in the 32 azacitidine-treated responders was 379 days (range: 92 to 4412+ days). The median number of cycles from first treatment with azacitidine to any response (CR, PR or HI) was 3 cycles (range: 1 to 17 cycles). Although 75% of the responders achieved a response by cycle 4, the other 25% achieved a response as late as cycle 17. The majority (90%) of responders achieved a response by cycle 6. Best response was observed, on average, 2 cycles after the initial response. In summary, reanalysis of the response rates using IWG criteria demonstrate consistent results across three sequential studies and further validate the superiority of azacitidine over supportive care alone.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal