Abstract
MDS are heterogeneous hematopoietic disorders characterized by dyspoiesis and cytopenias. Most patients with high-risk MDS die within 1 year from infection or hemorrhage usually attributable to bone marrow failure or progression to AML. In a phase 3, open-label, multicenter, randomized study comparing azacitidine (Vidaza®) plus supportive care (N=99) versus supportive care alone (N=91) in patients with MDS (
JCO 2002;20:2429
), treatment with azacitidine was associated with worsening of pre-existing cytopenias in up to 78% (115/147) of patients with pre-existing cytopenias. The median time to first occurrence of any worsening cytopenia was 8 days (interquartile range: 6 to 15 days). All patients were treated with the same initial dose of azacitidine (75 mg/m2/day); no dose adjustments were made for pre-existing cytopenias despite the severity. We examined rates of infection and bleeding associated with azacitidine therapy in comparison with the rates seen with the underlying disease (supportive care). Our results are based on re-collected data from patients described in the published report; our analyses were conducted separately and independently. Total exposure for azacitidine was the cumulative time from the first dose to the end of study (30 days after last dose), and for supportive care was the cumulative time from randomization to withdrawal from study or day prior to crossover. The azacitidine group included all patients exposed to azacitidine (N=150), which included patients randomized to azacitidine (N=99) and patients after crossing over from supportive care (N=51). Similar to the rate of infections previously reported in patients with MDS (AJM 1991; 90:338
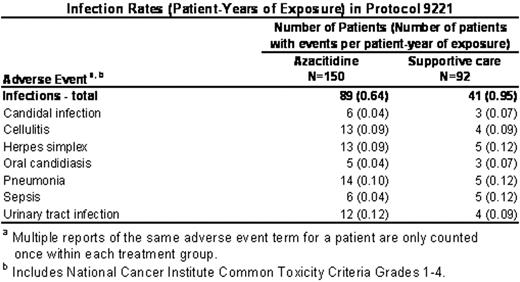
), infections occurred in patients in the supportive care group at nearly one per patient-year. However, infections occurred at an overall lower rate (patient-years) in the azacitidine group (0.64) compared with the supportive care group (0.95). (Table). Similarly, the overall rate (patient-years) of bleeding events was comparable between azacitidine (0.56) and supportive care (0.60). In particular, bleeding events in the gastrointestinal system occurred at a similar rate between the azacitidine group (0.26) and the supportive care group (0.25). This was also true for bleeding events in the central nervous system (azacitidine: 0.01, supportive care: 0.02). The rates of infection and bleeding were 3 to 4 times higher during cycles 1 and 2 in both treatment groups, reflecting the severity of the underlying disease at study entry. In summary, despite the potential to exacerbate pre-existing cytopenias early in therapy, treatment with azacitidine did not increase the rate of infection and bleeding above the rate due to the underlying disease.Author notes
Corresponding author
2005, The American Society of Hematology
2005

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal