Abstract
Molecular monitoring of minimal residual disease (MRD) has become an independent and prognostically significant parameter in assessing outcome in adult and childhood acute lymphoblastic leukaemia (ALL). The aim of our study was to evaluate the impact of MRD in a standard risk group of adult B cell ALL patients who had achieved morphological remission following induction phase 1 therapy as part of the MRC UKALL12 protocol.
Patients and Methodology. MRD tests were evaluated in fourty-seven patients with adult B cell ALL. They were negative for the t(9;22) or t(4;11) translocation and had received chemotherapy based treatment or auto stem cell transplant (A-SCT) only. Median age was 23 yrs (range 15.5–54.6 yrs); median WBC was 9.6 (range 1.1–163×109/l) with a predominance of common (28 pts) and pre B ALL (12 pts).
All patients had at least one molecular marker which was tested by quantitative or semi-quantitative PCR with sensitivity ≥1E4. End points were either clinical relapse or disease free survival in complete remission with follow up ≥12 months. Time point for molecular evaluation were post induction phase 1, TP1 (median 0.9 mo; range: 0.6–1.8 mo) and post induction phase 2 (median 2.79 mo; range 1.8–3.5 mo).
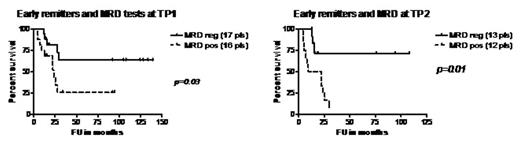
Results. Thirty three pts were tested at TP1 following morphological remission. Relapse free survival (RFS) analysis showed a statistically significant association between MRD positive tests (in 16 pts) and relapse, and MRD negative tests (in 17 pts) and CCR (p=0.03) (Figure 1, left diagram).
Twenty five pts were analysed at TP2 following their early morphological remission. At this time point we observed the strongest association between MRD negative tests (in 13 pts) and CCR (in 10 pts) and MRD positive (12 pts) and relapse (in 11 pts) (p=0.01)(Figure 1, right diagram).
Conclusions. Molecular monitoring of MRD shows that even among early morphological remitters a group of MRD positive patients can be identified that have poor overall outcome and may benefit from tailored therapies. Molecular assessment of residual disease should be used to stratify treatment in future adult ALL trials.
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal