Abstract
Background: Topotecan at modest doses has activity in multiple myeloma (MM). The combination of high-dose topotecan, melphalan, and cyclophosphamide (TMC) has been used as a conditioning regimen for autologous SCT in patients with MM. We report the results of a phase II trial with this combination and perform a retrospective analysis comparing TMC to a standard preparative regimen of melphalan 200 mg/m2 (MEL200).
Patients and Methods: Between 10/99 and 3/04, 55 patients with newly diagnosed MM were treated on a Phase II trial of topotecan 3.5 mg/m2 daily for 5 days in combination with cyclophoshamide 1 gm/m2 daily for 3 days, and melphalan 70 mg/m2 daily for 2 days followed by autologous SCT. Patient characteristics are summarized in table 1. In brief, median age was 55 (range, 37–67), median time to transplant was 6 months (range, 2-59); median B2M @diagnosis 3.3 (range, 1.4–17.2); 76% of patients had chemosensitive disease prior to transplant.
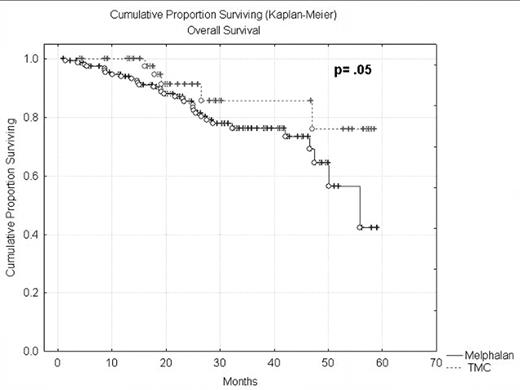
Results: No treatment related deaths were observed, the regimen was well tolerated with grade 2 Bearman mucositis and diarrhea the most common toxicities, and no grade 3 or 4 toxicities observed. When compared to a group of patients who received MEL 200 the overall response rate was lower 75% vs 82% and a lower CR rate 11% vs 36%, but a similar median time to disease progression (14 m vs 13 m), with a trend towards a better 3 yr overall survival (87% vs 77%; p=.05) (Table 2 and Figure 1).
Conclusion: TMC is a well tolerated conditioning regimen for myeloma, our results suggests that although no more effective than MEL200 as a single preparative regimen for MM, it warrants exploring as part of a tandem transplant program.
Patient Characteristics
| . | TMC . | MEL200 . |
|---|---|---|
| N | 55 | 157 |
| Median Age (range) | 55(37-67) | 56(29-75) |
| Median B2M @Dx | 3.3 (1.4 - 17.2) | 4 (0.5 - 49.8) |
| % Abn Cytogenetics | 15% | 22% |
| CR/PR @ SCT | 4%/73% | 10%/89% |
| % 1ry Refractory | 24% | 27% |
| . | TMC . | MEL200 . |
|---|---|---|
| N | 55 | 157 |
| Median Age (range) | 55(37-67) | 56(29-75) |
| Median B2M @Dx | 3.3 (1.4 - 17.2) | 4 (0.5 - 49.8) |
| % Abn Cytogenetics | 15% | 22% |
| CR/PR @ SCT | 4%/73% | 10%/89% |
| % 1ry Refractory | 24% | 27% |
Transplant Outcomes
| . | TMC . | MEL200 . | p . |
|---|---|---|---|
| ORR/CR conversion | 75%/11% | 82%/36% | |
| Non Relapse Mortality | 0 | 2 | |
| Median OS | NR | 52 months | .05 |
| . | TMC . | MEL200 . | p . |
|---|---|---|---|
| ORR/CR conversion | 75%/11% | 82%/36% | |
| Non Relapse Mortality | 0 | 2 | |
| Median OS | NR | 52 months | .05 |
Author notes
Corresponding author

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal