Abstract
HM1.24 antigen is preferentially overexpressed in multiple myeloma (MM) cells but not in normal cells. To explore the potential of HM1.24 as a target for cellular immunotherapy, we selected 4 HM1.24-derived peptides that possess binding motifs for HLA-A2 or HLA-A24 by using 2 computer-based algorithms. The ability of these peptides to generate cytotoxic T lymphocytes (CTLs) was examined in 20 healthy donors and 6 patients with MM by a reverse immunologic approach. Dendritic cells (DCs) were induced from peripheral-blood mononuclear cells of healthy donors or peripheral-blood stem-cell (PBSC) harvests from patients with MM, and autologous CD8+ T cells were stimulated with HM1.24 peptide–pulsed DCs. Both interferon-γ–producing and cytotoxic responses were observed after stimulation with either HM1.24-126 or HM1.24-165 peptides in HLA-A2 or HLA-A24 individuals. The peptide-specific recognition of these CTLs was further confirmed by tetramer assay and cold target inhibition assay. Importantly, HM1.24-specific CTLs were also induced from PBSC harvests from patients with MM and these CTLs were able to kill MM cells in an HLA-restricted manner. These results indicate the existence of functional DCs and HM1.24-specific CTL precursors within PBSC harvests and provide the basis for cellular immunotherapy in combination with autologous PBSC transplantation in MM.
Introduction
Multiple myeloma (MM) is an incurable B-cell malignancy characterized by the accumulation of neoplastic plasma cells mainly in the bone marrow.1 High-dose chemotherapy followed by autologous peripheral blood stem cell transplantation (PBSCT) as well as molecular-targeting therapy for both MM cells and the bone marrow microenvironment have prolonged overall survival in patients with MM; however, most patients still ultimately have a relapse because of the emergence of residual disease.2-6 Because cellular immunotherapy, including allogeneic transplantation and donor lymphocyte infusion, is the only approach to induce a cure,7-9 immune-based strategies to eradicate the remaining MM cells have been studied extensively in recent years.10
Active immunotherapy based on either immunization against tumor-specific antigens or dendritic-cell (DC) vaccines generated ex vivo represents an ideal approach for inducing tumor-specific immunity.11 Specifically, immunoglobulin idiotype (Id) has been considered as the most specific tumor antigen, and clinical trials using Id-pulsed DC vaccinations have demonstrated cytotoxic T lymphocyte (CTL) responses in some patients with MM.12-14 In addition, generation of MM-specific CTLs has been achieved by using DCs pulsed with MM-cell lysates.15 Unfortunately, vaccination with Id variations or primary MM-cell lysates cannot provide shared immunotherapy for other MM patients and this problem may limit the wide clinical application of these methods. To date, several tumor-associated antigens such as MUC1, WT-1, Sp-17, NY-ESO-1, SPAN-Xb, and MAGE families have been shown to mediate immune responses against MM cells,16-21 but these antigens are not consistently expressed on MM cells.22 Therefore, it is necessary to identify relevant MM-specific antigens for developing more efficient strategies for large numbers of patients with MM.
HM1.24 was originally identified as a cell-surface protein that is preferentially overexpressed on MM cells23 and later was found to be identical to bone marrow stromal cell antigen 2 (BST-2).24,25 HM1.24/BST-2 (CD317) is also expressed at low levels in selected tissues but is specifically up-regulated in metastatic tumor cells and chemoresistant cancer cells.26,27 Subsequent studies have revealed that HM1.24 is a type II transmembrane glycoprotein with a unique topology that is present in lipid rafts via a glycosylphosphatidylinositol anchor at the C-terminus and that the rat homologue of cell-surface HM1.24 can be internalized and localized to the trans-Golgi network.28 Moreover, large-scale analysis of the human cDNA has demonstrated that the HM1.24 gene is one of the important activators of the nuclear factor κB pathway that is involved in the pathogenesis of MM.29 These findings suggest that HM1.24 provides a platform for a signaling complex at the cell membrane and plays an essential role in trafficking and signaling between the intracellular and cell surface of MM cells.
Several researchers have focused on HM1.24 as a target molecule for active immunotherapy in MM. Chiriva-Internati et al have induced HM1.24-specific CTLs using adeno-associated virus-based HM1.24 gene loading of DCs in healthy donors.30 On the other hand, Yong's group has used HM1.24 protein itself as a source of antigen and observed the induction of HM1.24-specific CTLs in patients with MM.31 In peptide-based immunotherapy, HM1.24-126 peptide has been shown to elicit CTL responses in HLA-A2+ donors.32 Based on these findings, we hypothesized that the generation of HM1.24-specific CTLs might become an attractive strategy for residual disease after PBSCT.
In this study, we constructed 4 peptides that possess HLA-A2– or HLA-A24–binding motifs in the extracellular domain of HM1.24 by using 2 computer-based algorithms and examined the potential of these peptides to induce HM1.24-specific CTLs in HLA-A2+ or HLA-A24+ individuals. Furthermore, to explore the possibility of HM1.24-targeting cellular immunotherapy in combination with PBSCT, we evaluated the ability of PBSC harvests from patients with MM as a source of DCs and CTLs.
Patients, materials, and methods
Cell lines and primary MM cells
The MM cell line U266 (HLA-A2+, A24-), the B-lymphoblastic cell lines ARH-77 and IM-9 (HLA-A2+, A24-), the breast cancer cell line MDA-MB-231 (HLA-A2+, A24-), and the proerythroblastic cell line K562 (HLA-A2-, A24-) were obtained from American Type Culture Collection (Manassas, VA). The lung cancer cell line A549 (HLA-A2-, A24-) was from Health Science Research Resources Bank (Osaka, Japan). The myeloma cell line TSPC-1 (HLA-A2-, A24+) was established in our laboratory. An Epstein-Barr virus (EBV)–transformed B-cell line, YN, was derived from a healthy donor (HLA-A2+, A24+), and was kindly provided by Dr Yasuhiko Nishioka (University of Tokushima, Tokushima, Japan). Primary MM cells were purified from bone marrow aspiration specimens from MM patients using magnetic-activated cell sorting (MACS) CD138 Microbeads (Miltenyi Biotec, Auburn, CA). All cell lines were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (FCS). Cell-surface expression of HM1.24 was analyzed by flow cytometry (Beckman Coulter, Tokyo, Japan) using fluorescein isothiocyanate (FITC)–conjugated anti-HM1.24 monoclonal antibody (MoAb) that recognizes an extracellular epitope corresponding to residues 116-127 of HM1.24 protein.23
PBSC collection
Eight patients with MM with HLA-A2+ or -A24+ type undergoing autologous PBSCT were enrolled in this study. The study was performed with the approval of the University of Tokushima Institutional Review Board and informed consent was provided according to the Declaration of Helsinki. PBSCs were collected after high-dose cyclophosphamide (100 mg/kg) and granulocyte colony-stimulating factor (G-CSF; 400 μg/m2/d) mobilization using a COBE Spectra (Gambro, Tokyo, Japan). The median percentage of CD34+ cells in the harvest product was 1.5% (range, 0.61%-13.0%). PBSCs were cryopreserved in 5% dimethyl sulfoxide (DMSO) and 6% hydroxyethyl starch until use.
Synthetic peptides
The HM1.24 sequence was scanned for potential T-cell epitopes using 2 HLA peptide-binding prediction software products such as Bioinformatics & Molecular Analysis Section (BIMAS, http://bimas.dcrt.nih.gov) and SYFPEITHI (Tübingen, Germany, http://www.syfpeithi.de/). From the extracellular region of HM1.24 protein, one peptide with binding motif for HLA-A*0201 and three 9-mer HM1.24 peptides consisting of binding motif for HLA-A*2402 were selected based on the HLA-binding scores. A peptide derived from MAGE-3 residues 195-203 was also synthesized and used as a positive control because it binds to HLA-A24 molecules and elicits the generation of peptide-specific and HLA-A24-restricted CTLs.33 These peptide sequences and predicted HLA-binding scores are listed in Table 1. All peptides were prepared by solid-phase peptide synthesis to a minimum purity of 90% using an automated peptide synthesizer (Protein Technologies, Tucson, AZ). HM1.24-126, HM1.24-136, and HM1.24-152 peptides were dissolved in milliQ water (Millipore, Bedford, MA), and HM1.24-165 and MAGE-3-195 were in DMSO at a concentration of 1 mg/mL, and were stocked at -80°C until use.
HM1.24-derived peptides and their predictive binding scores
. | . | HLA-A2 binding score . | . | HLA-A24 binding score . | . | ||
|---|---|---|---|---|---|---|---|
| Antigen position . | Sequence . | BIMAS . | SYFPEITHI . | BIMAS . | SYFPEITHI . | ||
| HM1.24 | |||||||
| 126-134 | KLQDASAEV | 998.1 | 26 | 0.4 | 2 | ||
| 136-144 | RLRRENQVL | 0.7 | 20 | 9.6 | 9 | ||
| 152-160 | KYYPSSQDS | 0.0 | 4 | 14.4 | 12 | ||
| 165-173 | APQLLIVLL | 0.3 | 19 | 7.2 | 13 | ||
| MAGE-3 | |||||||
| 195-203 | IMPKAGLLI | 12.8 | 19 | 1.5 | 15 | ||
. | . | HLA-A2 binding score . | . | HLA-A24 binding score . | . | ||
|---|---|---|---|---|---|---|---|
| Antigen position . | Sequence . | BIMAS . | SYFPEITHI . | BIMAS . | SYFPEITHI . | ||
| HM1.24 | |||||||
| 126-134 | KLQDASAEV | 998.1 | 26 | 0.4 | 2 | ||
| 136-144 | RLRRENQVL | 0.7 | 20 | 9.6 | 9 | ||
| 152-160 | KYYPSSQDS | 0.0 | 4 | 14.4 | 12 | ||
| 165-173 | APQLLIVLL | 0.3 | 19 | 7.2 | 13 | ||
| MAGE-3 | |||||||
| 195-203 | IMPKAGLLI | 12.8 | 19 | 1.5 | 15 | ||
HLA-binding peptides in HM1.24 protein were identified using 2 computer algorithms (BIMAS and SYFPEITHI). The binding scores represent an estimated half-time of dissociation or binding capacity of each peptide to HLA-A*0201 or HLA-A*2402 molecules.
Generation of DCs
DCs were generated from peripheral-blood mononuclear cells (PBMCs) of healthy donors or PBSC harvests from patients with MM as described previously.34 In brief, monocyte-enriched fractions were obtained from freshly isolated PBMCs or thawed PBSCs using a plastic adherence technique. These cells were cultured in medium containing 250 U/mL recombinant human interleukin 4 (IL-4; R&D Systems, Minneapolis, MN) and 500 U/mL granulocyte-macrophage colony-stimulating factor (GM-CSF; R&D Systems) for 6 days, and then in the presence of 50 U/mL tumor necrosis factor α (TNF-α; R&D Systems) for 24 hours. On day 7, cells were harvested and used as monocyte-derived DCs for antigen stimulation. Expression of cell-surface markers was analyzed by flow cytometry using FITC-conjugated MoAbs specific for CD1a, CD40, CD80, CD83, and CD86 (Pharmingen, San Diego, CA).
Induction of peptide-specific CTLs
Peptide-specific CTLs were generated as described previously with slight modifications.35 Autologous CD8+ T lymphocytes were isolated from PBMCs of healthy donors using MACS CD8 Microbeads (Miltenyi Biotec). DCs were pulsed with 10 μg/mL of each peptide for 4 hours at 37°C, washed, and then irradiated (25 Gy). A total of 1 × 106 CD8+ cells was cultured with 1 × 105 peptide-pulsed autologous DCs in RPMI 1640 medium supplemented with 10% heat-inactivated human AB type serum and 5 ng/mL recombinant human IL-7 (R&D Systems). CD8+ T cells were stimulated with peptide-pulsed DCs weekly and cultured with 10 U/mL IL-2 (PeproTech, Rocky Hill, NJ). Five days after the third stimulation (day 19), cytotoxic activity of these cells was assessed in a 4-hour cytotoxicity assay against either relevant peptide-loaded or unloaded target cells.
Nonattached fractions (1 × 106 cells) of PBSC harvests from MM patients were stimulated with 1 × 105 autologous DCs pulsed with peptides. Cells were stimulated weekly with the peptide-pulsed DCs in the medium containing IL-7. Due to the limited numbers of PBSCs available for this study, CD8+ T cells were enriched using Microbeads after 2 rounds of stimulation, and their cytotoxicity was examined against either peptide-loaded or unloaded target cells in enzyme-linked immunoSPOT (ELISPOT) assay for granzyme B.
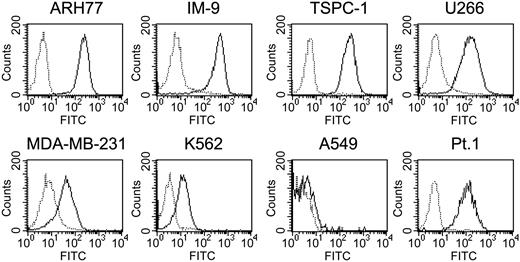
Flow cytometric analysis of HM1.24 expression on tumor cell lines and primary MM cells. Cells were stained with either FITC-labeled control mouse IgG (dotted line) or FITC-labeled anti-HM1.24 MoAb (solid line) and were analyzed by flow cytometry.
Flow cytometric analysis of HM1.24 expression on tumor cell lines and primary MM cells. Cells were stained with either FITC-labeled control mouse IgG (dotted line) or FITC-labeled anti-HM1.24 MoAb (solid line) and were analyzed by flow cytometry.
CTL expansion procedure
To obtain sufficient cells for more detailed characterization, CTLs (5 × 104) were expanded by stimulations with 5 × 106 autologous irradiated (30 Gy) peptide-pulsed PBMCs together with 1 × 106 irradiated (30 Gy) EBV-transformed YN cells as feeder cells. After 24 hours, 120 U/mL IL-2 was added to the cultures. The cultures were fed with fresh medium containing 50 U/mL IL-2 every 3 days. Two weeks later, expanded cells were harvested and used as CTL lines.
Tetramer assay
The antigen specificity of CTLs was confirmed by a tetramer assay. PBMCs or peptide-induced CTLs were stained with HM1.24-126/HLA-A*0201 phycoerythrin (PE)–labeled tetramer (MBL, Nagoya, Japan) and FITC-labeled anti-CD8 MoAb (Pharmingen) for 30 minutes, and analyzed by flow cytometry.
Detection of IFN-γ production
Culture supernatants from each CTL were harvested on day 9, 16, and 19, and the concentration of interferon γ (IFN-γ) was measured by an enzyme-linked immunosorbent assay (ELISA; R&D Systems). In some experiments, production of IFN-γ by CTL lines was measured by ELISPOT assay (R&D Systems). A total of 1 × 104 CTLs was cultured with 1 × 104 target cells in ELISPOT plates coated with anti–IFN-γ MoAb for 24 hours, and then spots were developed according to the manufacturer's instructions.
51Cr-release assay
Target cells were labeled with 100 μCi (3.7 MBq) 51Cr for 1 hour at 37°C, and placed in 96-well plates. CTLs were then added to the plates and incubated for 4 hours. 51Cr release in supernatants was measured using a γ counter. Spontaneous release and maximal release were determined in the presence of either medium alone or 5% Triton X-100, respectively. The percentage of specific cytotoxicity was calculated from the following formula: (experimental 51Cr-release - spontaneous 51Cr-release)/(maximum 51Cr-release - spontaneous 51Cr-release) × 100. For screening of primary CTL responses, peptide-loaded YN cells were prepared by incubating with each peptide (10 μg/mL) overnight at 37°C and used as target cells. For inhibition assays, target cells were incubated with either 10 μg/mL anti–HLA class I MoAb (w6/32; Dako, Carpinteria, CA) or an isotypic control mouse IgG (UPC-10; Cappel, Malvern, PA) for 45 minutes at room temperature before use. The antigen specificity of tumor-cell lysis was assessed in a cold target inhibition assay. Peptide-loaded or unloaded 51Cr-unlabeled MDA-MB-231 cells were used as cold inhibitors to block lysis of 51Cr-labeled target cells at a ratio of 20:1 (inhibitor-target ratio).
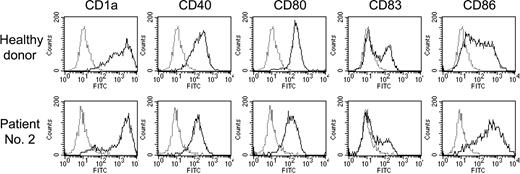
Surface phenotype of DCs derived from PBMCs from healthy donors and PBSC harvests from patients with MM. Monocyte fractions were obtained from PBMCs from healthy donors or PBSC harvests from MM patients using a plastic adherence method. These cells were cultured in the presence of IL-4 and GM-CSF for 6 days, followed by stimulation with TNF-α for 24 hours. Cells were stained with either FITC-labeled control mouse IgG (dotted line) or FITC-labeled anti-CD1a, anti-CD40, anti-CD80, anti-CD83, or anti-CD86 MoAbs (solid line) and were analyzed by flow cytometry. The data were representative of DCs from 3 healthy donors and 3 MM patients.
Surface phenotype of DCs derived from PBMCs from healthy donors and PBSC harvests from patients with MM. Monocyte fractions were obtained from PBMCs from healthy donors or PBSC harvests from MM patients using a plastic adherence method. These cells were cultured in the presence of IL-4 and GM-CSF for 6 days, followed by stimulation with TNF-α for 24 hours. Cells were stained with either FITC-labeled control mouse IgG (dotted line) or FITC-labeled anti-CD1a, anti-CD40, anti-CD80, anti-CD83, or anti-CD86 MoAbs (solid line) and were analyzed by flow cytometry. The data were representative of DCs from 3 healthy donors and 3 MM patients.
Granzyme B ELISPOT assay
Granzyme B secretion was measured using ELISPOT assay as described previously.36 Polyvinylidene difluoride (PVDF) MultiScreen plates (Millipore, Bedford, MA) were coated overnight at 4°C with anti–human granzyme B MoAb (GB-10; Cell Sciences, Canton, MA). Effector cells (1 × 104) were added to target cells (1 × 104) in the plates and incubated for 24 hours. The plates were washed, and spots were detected with biotinylated anti–human granzyme B MoAb (GB-11; Cell Sciences) followed by streptavidin-alkaline phosphatase system (Invitrogen, Carlsbad, CA).
Statistical analysis
Data are shown as mean plus or minus SD. Comparisons between experimental data were performed by one-way analysis of variance (ANOVA). P below .05 was considered statistically significant.
Results
HM1.24 expression on neoplastic cell lines
Several neoplastic cell lines were tested for expression of HM1.24 by flow cytometry. As shown in Figure 1, MM cell lines and B-lymphoblastic cell lines such as ARH-77, IM-9, TSPC-1, and U266 cells strongly expressed HM1.24 on the cell surface. Primary MM cells also expressed HM1.24. In contrast, MDA-MB-231 and K562 expressed HM1.24 at a low level, and A549 did not express HM1.24. These cells were used as targets for CTL assay.
Generation of DCs in healthy donors and patients with MM
We generated DCs by using PBMCs from healthy donors and PBSC harvests from patients with MM and investigated whether functional DCs could be induced from these cell fractions in vitro. Flow cytometric analysis revealed that DCs induced from both cell populations consistently expressed mature DC markers such as CD1a, CD40, CD80, CD83, and CD86, suggesting that DCs were inducible from PBMCs and PBSC harvests from MM patients (Figure 2). In particular, the yield of DCs from PBSC harvests was approximately 2-fold higher than that from normal PBMCs. We therefore applied this method to generate DCs in both healthy donors and patients with MM.
Induction of primary HM1.24-specific CTLs from healthy donors
To induce peptide-specific CTLs, CD8+ T cells from healthy donors were stimulated with autologous DCs pulsed with HM1.24 or MAGE-3 peptides. Culture supernatants were harvested on days 9, 16, and 19, and production of IFN-γ was measured by ELISA. The highest level of IFN-γ was observed on day 16 (1600 pg/mL) compared with that on day 9 (800 pg/mL) or day 19 (1100 pg/mL) in the supernatants of HM1.24-165–induced CTLs from a healthy donor (HLA-A2-, A24+). HM1.24-126 and MAGE-3-195 peptides also induced the highest IFN-γ production on day 16 (data not shown). Therefore, the concentration of IFN-γ was measured on day 16 in the following experiments. Among 4 HLA-A2+, A24+ donors, 2 donors showed elevated levels of IFN-γ in the culture supernatants of CD8+ cells stimulated with DCs pulsed with HM1.24-126 and HM1.24-165 as well as positive control peptide MAGE-3-195 (data not shown). However, secretion of IFN-γ was not observed in CD8+ cells stimulated with HM1.24-136 or HM1.24-152 peptide–pulsed DCs in these donors.
To further clarify the ability of these peptides for CTL induction, CD8+ T cells from 20 healthy donors were stimulated with peptide-pulsed autologous DCs. After 3 rounds of stimulation, their cytotoxic activity was tested against YN cells in the absence or presence of the relevant or irrelevant peptide as targets. The cytotoxic activity of these CTLs was only detected against YN cells that had been loaded with the relevant peptide used for the induction (Table 2). HM1.24-126 peptide–specific CTLs were successfully induced in HLA-A2+ or HLA-A24+ donors. HM1.24-165 and MAGE-3-195 also elicited CTLs in HLA-A2+ donors and more effectively in HLA-A24+ donors. In accordance with the binding scores of these peptides (Table 1), the efficacy of CTL induction by HM1.24-126 was higher in HLA-A2+ donors and those by HM1.24-165 and MAGE-3-195 were higher in HLA-24+ donors. The cytotoxic activity of these CTLs against different peptide-loaded or unloaded YN cells was not detected, suggesting that these CTLs recognize exogenous peptides presented on the target cells (data not shown). In contrast, only limited numbers of CTLs were induced in response to HM1.24-136 or HM1.24-152 peptides. These data indicate that HM1.24-126 and HM1.24-165 peptides have enough potential to induce CTL responses in HLA-A2 or -A24 populations.
Induction of primary CTLs from healthy donors after stimulation with HM1.24 peptide–pulsed DCs
. | HLA-A2+, A24+ . | . | . | . | HLA-A2+, A24- . | . | . | HLA-A2-, A24+ . | . | . | . | . | . | . | . | . | . | . | HLA-A2-, A24- . | . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | 19 . | 20 . | ||||||||||||||||
| HM1.24-126 | 0 | 35 | 0 | 29 | 0 | 25 | 38 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 30 | 0 | 26 | 0 | 0 | 0 | ||||||||||||||||
| HM1.24-136 | 1 | 6 | 3 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 33 | 1 | 0 | 0 | ||||||||||||||||
| HM1.24-152 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 5 | 0 | 0 | 0 | 3 | 44 | 0 | 8 | 2 | 0 | 0 | 0 | ||||||||||||||||
| HM1.24-165 | 31 | 25 | 3 | 0 | 26 | 0 | 20 | 29 | 35 | 1 | 0 | 1 | 13 | 7 | 36 | 8 | 16 | 1 | 0 | 0 | ||||||||||||||||
| MAGE-3-195 | 2 | 35 | 0 | 37 | 0 | 0 | 1 | 38 | 2 | 0 | 0 | 0 | 28 | 0 | 20 | 19 | 3 | 3 | 0 | 0 | ||||||||||||||||
. | HLA-A2+, A24+ . | . | . | . | HLA-A2+, A24- . | . | . | HLA-A2-, A24+ . | . | . | . | . | . | . | . | . | . | . | HLA-A2-, A24- . | . | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peptide . | 1 . | 2 . | 3 . | 4 . | 5 . | 6 . | 7 . | 8 . | 9 . | 10 . | 11 . | 12 . | 13 . | 14 . | 15 . | 16 . | 17 . | 18 . | 19 . | 20 . | ||||||||||||||||
| HM1.24-126 | 0 | 35 | 0 | 29 | 0 | 25 | 38 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 30 | 0 | 26 | 0 | 0 | 0 | ||||||||||||||||
| HM1.24-136 | 1 | 6 | 3 | 0 | 0 | 0 | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 | 2 | 33 | 1 | 0 | 0 | ||||||||||||||||
| HM1.24-152 | 0 | 0 | 0 | 0 | 0 | 0 | 24 | 0 | 5 | 0 | 0 | 0 | 3 | 44 | 0 | 8 | 2 | 0 | 0 | 0 | ||||||||||||||||
| HM1.24-165 | 31 | 25 | 3 | 0 | 26 | 0 | 20 | 29 | 35 | 1 | 0 | 1 | 13 | 7 | 36 | 8 | 16 | 1 | 0 | 0 | ||||||||||||||||
| MAGE-3-195 | 2 | 35 | 0 | 37 | 0 | 0 | 1 | 38 | 2 | 0 | 0 | 0 | 28 | 0 | 20 | 19 | 3 | 3 | 0 | 0 | ||||||||||||||||
CD8+ T cells were purified from 20 healthy donors and stimulated with autologous DCs pulsed with HM1.24 peptides or MAGE-3-195 peptide once a week. Five days after the third stimulation (day 19), cells were harvested and tested for CTL activity against YN cells that had been loaded with the relevant peptide. Cytotoxicity (%) was determined by a 4-hour 51Cr-release assay at an E/T ratio of 20. Values are shown as the mean cytotoxic activity of triplicate experiments of each individual in 4 HLA groups.
Detection of HM1.24 peptide–specific CTLs by HLA-tetramer assay
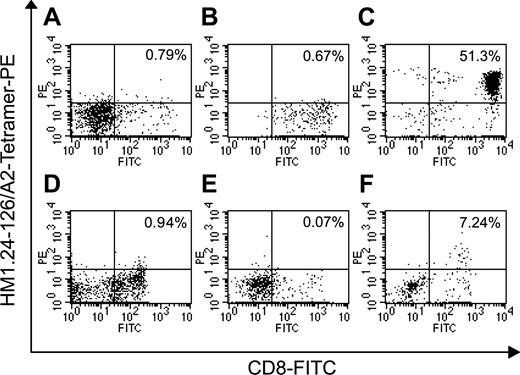
We next analyzed the frequency of CD8+ T cells specific for HM1.24 peptide by tetramer assay. After 3 rounds of stimulation with DCs pulsed with HM1.24-126 or MAGE-3-195 peptides, CTLs (HLA-A2+, A24-) were stained with FITC-labeled anti-CD8 MoAb and PE-labeled HM1.24-126/HLA-A*0201 tetramer complex and analyzed by flow cytometry. Spontaneous HM1.24-126-specific CD8+ T cells comprised less than 1% of the normal PBMC population as shown in Figure 3. CD 8+ T cells stimulated with MAGE-3-195 were also negative for HM1.24-126/A*0201 tetramer. In contrast, expanded CTLs after stimulation with HM1.24-126 peptide–pulsed DCs showed a remarkable increase in the number of CD8+ T cells specific for HM1.24-126 peptide. More importantly, HM1.24-126–specific CD8+ cells were detected in PBSC harvests from MM patients, and these peptide-specific CTLs were expanded by the stimulation with DCs pulsed with HM1.24-126 peptide. These results suggest the existence of HM1.24-specific CTLs in the peripheral blood at low levels and confirm the induction of HM1.24 peptide–specific CTLs by this approach.
Cytotoxic activity of HM1.24-specific CTL lines
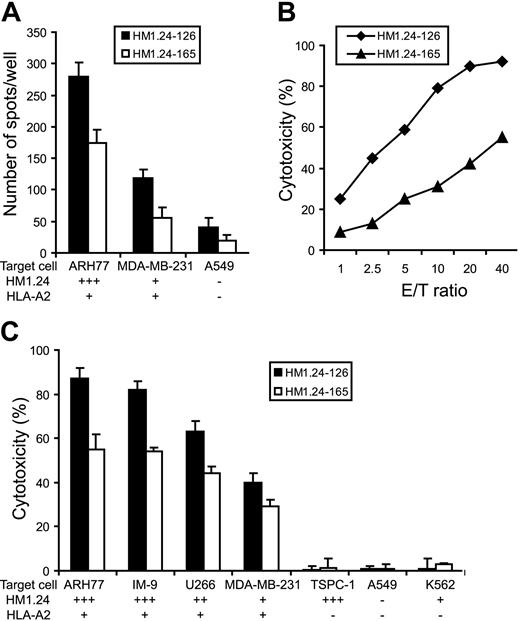
To further characterize HM1.24 peptide–induced CTLs, we expanded the primary CTLs and successfully established 2 CTL lines that react to HM1.24-126 or HM1.24-165 from a healthy donor (HLA-A2+, A24-). To clarify whether CTLs can recognize HM1.24-derived peptides naturally processed by human tumor cells, these CTL lines were cultured with HLA-A2+ tumor cell lines expressing HM1.24 antigen at different levels. Specific recognition of tumor cells by CTL lines was evaluated by IFN-γ ELISPOT assay. As shown in Figure 4A, increased numbers of cells secreting IFN-γ were observed in response to ARH-77 that strongly expresses HM1.24 antigen. In contrast, small numbers of IFN-γ–secreting cells were detected in response to MDA-MB-231 that expresses HM1.24 at a low level, suggesting that the recognition by these CTL lines is dependent on expression levels of HM1.24.
Tetramer analysis of HM1.24 peptide–specific T cells. CD8+ T cells from normal PBMCs (HLA-A2+, A24-) or PBSC harvests of patient no. 3 (HLA-A2+, A24-) were stimulated with autologous DCs pulsed with HM1.24-126 or MAGE-3-195 peptides 3 times. Normal PBMCs (A), MAGE-3–stimulated CD8+ cells (B), HM1.24-126–stimulated CD8+ cells (C), PBSC harvests (D), MAGE-3–stimulated PBSC harvests (E), and HM1.24-126–stimulated PBSC harvests (F) were stained with FITC-labeled anti-CD8 MoAb and PE-labeled HM1.24-126/A2 tetramer and were analyzed by 2-color flow cytometry. The numbers represent the percentage of CD8+/tetramer+ cells. Negative control staining showed less than 0.1% of CD8+/tetramer+ cells in each experiment.
Tetramer analysis of HM1.24 peptide–specific T cells. CD8+ T cells from normal PBMCs (HLA-A2+, A24-) or PBSC harvests of patient no. 3 (HLA-A2+, A24-) were stimulated with autologous DCs pulsed with HM1.24-126 or MAGE-3-195 peptides 3 times. Normal PBMCs (A), MAGE-3–stimulated CD8+ cells (B), HM1.24-126–stimulated CD8+ cells (C), PBSC harvests (D), MAGE-3–stimulated PBSC harvests (E), and HM1.24-126–stimulated PBSC harvests (F) were stained with FITC-labeled anti-CD8 MoAb and PE-labeled HM1.24-126/A2 tetramer and were analyzed by 2-color flow cytometry. The numbers represent the percentage of CD8+/tetramer+ cells. Negative control staining showed less than 0.1% of CD8+/tetramer+ cells in each experiment.
Specific IFN-γ production and cytotoxic activity of HM1.24-specific CTL lines against human neoplastic cell lines. Purified CD8+ T cells from a healthy donor (HLA-A2+, A24-) were cultured with autologous DCs pulsed with HM1.24-126 or HM1.24-165 peptides, and then CTL lines were established as described in “Patients, materials, and methods.” (A) Specific recognition of target cells by HM1.24 peptide–induced CTL lines. Each CTL (1 × 104 cells) was cultured with target cell lines (1 × 104 cells) for 24 hours and IFN-γ production was measured by ELISPOT assay. (B) Cytotoxic activity of these CTLs against ARH-77 cells. Cytotoxicity (%) was determined by 51Cr-release assay at different E/T ratios. (C) Cytotoxic activity of these CTLs against several tumor cell lines expressing HM1.24 at different levels. Cytotoxicity (%) was determined by 51Cr-release assay at an E/T ratio of 20. Values indicate the mean ± SD of triplicate experiments.
Specific IFN-γ production and cytotoxic activity of HM1.24-specific CTL lines against human neoplastic cell lines. Purified CD8+ T cells from a healthy donor (HLA-A2+, A24-) were cultured with autologous DCs pulsed with HM1.24-126 or HM1.24-165 peptides, and then CTL lines were established as described in “Patients, materials, and methods.” (A) Specific recognition of target cells by HM1.24 peptide–induced CTL lines. Each CTL (1 × 104 cells) was cultured with target cell lines (1 × 104 cells) for 24 hours and IFN-γ production was measured by ELISPOT assay. (B) Cytotoxic activity of these CTLs against ARH-77 cells. Cytotoxicity (%) was determined by 51Cr-release assay at different E/T ratios. (C) Cytotoxic activity of these CTLs against several tumor cell lines expressing HM1.24 at different levels. Cytotoxicity (%) was determined by 51Cr-release assay at an E/T ratio of 20. Values indicate the mean ± SD of triplicate experiments.
We next determined the cytotoxic activity of these CTL lines using ARH-77 cells as targets. These CTLs mediated the strong cytotoxicity in an effector-target (E/T) ratio-dependent manner (Figure 4B). When we tested the cytotoxic activity against several tumor cell lines, these HM1.24-specific CTL lines killed only HLA-A2+ cell lines such as ARH-77, IM-9, U266, and MDA-MB-231, but not HLA-A2- MM cell line TSPC-1 (Figure 4C). The cytotoxic activity of these CTL lines was also related to the HM1.24 expression levels on target cells. In addition, the cytotoxicity of HM1.24-126–specific CTL lines was higher than that of HM1.24-165–specific CTL lines, which was consistent with the results of IFN-γ production assay. No cytotoxic response was observed against NK-sensitive K562 cells, indicating that the cytotoxic activity was not mediated by nonspecific NK-cell activity.
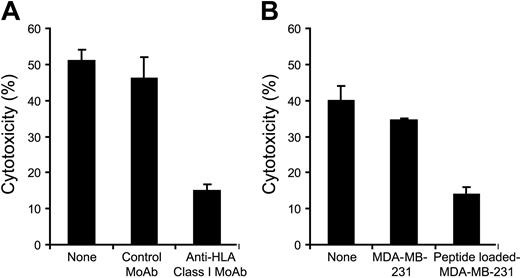
HLA restriction and peptide specificity of CTL lines
We further determined whether induced CTL lines mediate cytotoxic activity in an HLA-restricted and peptide-specific manner. To block HLA class I–mediated recognition by CTL lines, anti–HLA class I MoAb was used in the cytotoxic assay. The cytotoxic activity of HM1.24-165–specific CTL line from a healthy donor (HLA-A2+, A24-) was abolished in the presence of anti–HLA class I MoAb (Figure 5A). Peptide recognition by CTL lines was further evaluated using a cold target inhibition assay. The cytotoxic activity of HM1.24-165–specific CTL line against ARH-77 was significantly inhibited by the addition of cold MDA-MB-231 that had been loaded with HM1.24-165 peptides (Figure 5B). In addition, preincubation of target cells with anti-HM1.24 MoAb did not affect the cytotoxicity of HM1.24-specific CTLs, suggesting that cell-surface HM1.24 protein is not responsible for the recognition by HM1.24 peptide–specific CTLs (data not shown).
Induction of primary HM1.24-specific CTLs from patients with MM
Next, we determined the existence of HM1.24-specific T cells in PBSC collections from MM patients. Nonattached fractions of thawed PBSC harvests were stimulated with peptide-pulsed DCs generated also from PBSC harvests. After 2 rounds of stimulation, CD8+-cell expansion was observed in 6 of 8 products and the cytotoxic activity of these CTLs was examined by granzyme B ELISPOT assay. Although the different method for cytotoxicity assay was used, HM1.24-126 and HM1.24-165 peptide–pulsed DCs appeared to induce peptide-specific CTLs more effectively in MM patients, that is, 4 of 4 HLA-A2+ patients and 2 of 4 HLA-A24+ patients, respectively (Table 3). These data indicate the existence of functional DCs and HM1.24-specific CTL precursors in PBSC harvests as well and suggest the elevated frequency of HM1.24-specific CTLs in patients with MM.
Cytotoxic activity of primary CTLs from PBSC harvests in MM patients after stimulation with HM1.24 peptide–pulsed DCs
. | HLA-A2+, A24+ . | . | HLA-A2+, A24- . | . | HLA-A2-, A24+ . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Peptide . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | |||
| HM1.24-126 | ++ | + | ++ | ++ | – | + | |||
| HM1.24-136 | – | – | – | – | – | – | |||
| HM1.24-152 | + | – | – | – | – | – | |||
| HM1.24-165 | ++ | – | – | + | ++ | – | |||
| MAGE-3-195 | – | – | – | – | + | – | |||
. | HLA-A2+, A24+ . | . | HLA-A2+, A24- . | . | HLA-A2-, A24+ . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Peptide . | Patient 1 . | Patient 2 . | Patient 3 . | Patient 4 . | Patient 5 . | Patient 6 . | |||
| HM1.24-126 | ++ | + | ++ | ++ | – | + | |||
| HM1.24-136 | – | – | – | – | – | – | |||
| HM1.24-152 | + | – | – | – | – | – | |||
| HM1.24-165 | ++ | – | – | + | ++ | – | |||
| MAGE-3-195 | – | – | – | – | + | – | |||
Nonattached fractions of PBSC harvests were stimulated with peptide-pulsed DCs generated also from PBSC harvests. Five days after the second stimulation, CD8+ T cells were isolated using MACS beads and further cultured for 4 days. Cytotoxic activity of these CTLs was tested against YN cells that had been pulsed with relevant peptides. Cytotoxicity was determined by granzyme B ELISPOT assay and CTL responses were evaluated by the number of spots (–, < 15 spots; +, 15-30 spots; and ++, 31-60 spots).
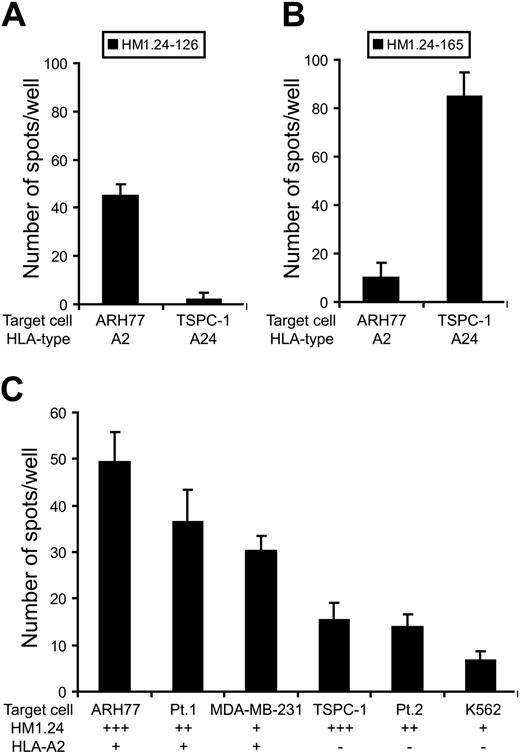
Characterization of HM1.24-specific CTLs from MM patients
Finally, we determined whether CTLs induced from PBSC harvests could recognize HM1.24-derived peptides naturally expressed on MM cell lines. CTLs were cultured with MM cell lines for 24 hours, and IFN-γ– or granzyme B-secreting cells were detected by ELISPOT assay. HM1.24-126–induced CTLs from patient no. 2 (HLA-A2+, A24+) produced a significant amount of IFN-γ in response to ARH-77 but not to TSPC-1 (Figure 6A). On the other hand, HM1.24-165–induced CTLs from patient no. 5 (HLA-A2-, A24+) showed a prominent number of IFN-γ–secreting cells against TSPC-1 but not against ARH-77 (Figure 6B). In terms of the cytotoxic activity, HM1.24-126–induced CTLs from PBSC harvests of patient no. 3 (HLA-A2+, A24-) exhibited specific killing of ARH-77 cells and primary MM cells (patient no. 1) that express both HM1.24 and HLA-A2 (Figure 6C). In contrast, these CTLs did not mediate extensive cytotoxicity against TSPC-1 and K562. From these results, it appears that CTLs generated from MM patients can also recognize and kill MM cells in an HLA-restricted and antigen-specific manner.
HLA restriction of peptide recognition by HM1.24-specific CTL line. CTL line specific for HM1.24-165 peptide was established from a healthy donor (HLA-A2+, A24-). (A) HLA restriction of HM1.24-165–induced CTLs was determined by blocking HLA class I on target cells. 51Cr-labeled target ARH-77 cells were preincubated with either control MoAb or anti–HLA class I MoAb for 45 minutes at room temperature before addition of the CTLs. Cytotoxicity (%) was measured by 51Cr-release assay (E/T ratio = 20). (B) Peptide recognition by the CTLs was determined by cold target inhibition assay. CTLs (6 × 104 cells) were incubated with 51Cr-labeled target ARH-77 cells (3 × 103 cells) in the presence of either HM1.24-165 peptide-loaded or unloaded MDA-MB-231 cells (6 × 104 cells). Cytotoxic activity was measured by 51Cr-release assay (E/T ratio = 20). Values indicate the mean ± SD of triplicate experiments.
HLA restriction of peptide recognition by HM1.24-specific CTL line. CTL line specific for HM1.24-165 peptide was established from a healthy donor (HLA-A2+, A24-). (A) HLA restriction of HM1.24-165–induced CTLs was determined by blocking HLA class I on target cells. 51Cr-labeled target ARH-77 cells were preincubated with either control MoAb or anti–HLA class I MoAb for 45 minutes at room temperature before addition of the CTLs. Cytotoxicity (%) was measured by 51Cr-release assay (E/T ratio = 20). (B) Peptide recognition by the CTLs was determined by cold target inhibition assay. CTLs (6 × 104 cells) were incubated with 51Cr-labeled target ARH-77 cells (3 × 103 cells) in the presence of either HM1.24-165 peptide-loaded or unloaded MDA-MB-231 cells (6 × 104 cells). Cytotoxic activity was measured by 51Cr-release assay (E/T ratio = 20). Values indicate the mean ± SD of triplicate experiments.
Discussion
In the present study, we investigated the ability of HM1.24-derived peptides to generate peptide-specific CTLs in healthy donors as well as patients with MM. We found that DCs pulsed with HM1.24-126 and HM1.24-165 peptides were able to induce CTLs that recognize and kill MM cell lines and primary MM cells in HLA-A2+ or HLA-A24+ individuals. More importantly, HM1.24-126– and HM1.24-165–specific CTLs were also induced from frozen PBSC collections from patients with MM, suggesting the existence of HM1.24-specific CTL precursors within the peripheral T-cell repertoire of MM patients. These CTLs were specific for each HM1.24 peptide on HLA molecules and exerted the cytotoxic activity dependent on the levels of HM1.24 expression on target cells in an HLA-restricted manner. Because MM cells express high levels of HM1.2423 and HLA-A molecules (Etsuko Sekimoto, Shuji Ozaki, Takashi Ohshima, Hironobu Shibata, Toshihiro Hashimoto, Masahiro Abe, et al; manuscript submitted), the induction of HM1.24-specific CTLs appears to be a promising strategy for the treatment of MM. Thus, our data confirm the previous observation of CTL response to HM1.24-126 peptide in HLA-A2+ donors and extend the application of the HM1.24-derived peptide-based immunotherapy to both HLA-A2+ and HLA-A24+ patients with MM.
IFN-γ production and cytotoxic activity of HM1.24-specific CTLs from patients with MM. HM1.24-126–specific CTLs (A) and HM1.24-165–specific CTLs (B) were generated from patient no. 2 (HLA-A2+, A24+) and patient no. 5 (HLA-A2-, A24+), respectively. CTLs (1 × 104 cells) were cultured with target cell lines (1 × 104 cells) for 24 hours and IFN-γ production was measured by ELISPOT assay. (C) HM1.24-126–specific CTLs (1 × 104 cells) from patient no. 3 (HLA-A2+, A24-) were cultured with 2 primary MM cells (patients no. 1 and 2) and several target cell lines (1 × 104 cells) for 24 hours and cytotoxic activity of CTLs was evaluated by granzyme B ELISPOT assay. Values indicate the mean ± SD of triplicate experiments.
IFN-γ production and cytotoxic activity of HM1.24-specific CTLs from patients with MM. HM1.24-126–specific CTLs (A) and HM1.24-165–specific CTLs (B) were generated from patient no. 2 (HLA-A2+, A24+) and patient no. 5 (HLA-A2-, A24+), respectively. CTLs (1 × 104 cells) were cultured with target cell lines (1 × 104 cells) for 24 hours and IFN-γ production was measured by ELISPOT assay. (C) HM1.24-126–specific CTLs (1 × 104 cells) from patient no. 3 (HLA-A2+, A24-) were cultured with 2 primary MM cells (patients no. 1 and 2) and several target cell lines (1 × 104 cells) for 24 hours and cytotoxic activity of CTLs was evaluated by granzyme B ELISPOT assay. Values indicate the mean ± SD of triplicate experiments.
Among 4 synthetic HM1.24 peptides used in this study, HM1.24-126 and HM1.24-165 peptides more efficiently expanded the peptide-specific CTLs than other peptides. Several reasons have been suggested for preferential induction of CTLs by peptide antigens. It has been reported that high-affinity anchor residues for HLA-A2 are leucine at position 2 and valine or leucine at position 9.37 On the other hand, these residues for HLA-A24 are tyrosine or phenylalanine at position 2, and phenylalanine, tryptophane, leucine, or isoleucine at the C-terminus.38 In fact, HM1.24-126 peptide induced a high frequency of CTL response in HLA-A2+ individuals in accordance with the prediction of the BIMAS binding score. In addition, CTL response to HM1.24-126 was also observed in HLA-A24+ individuals, even though HM1.24-126 peptide does not contain HLA-A24–binding motifs. HLA-A2 anchor motifs have been shown to represent a broad cross-reactivity with not only HLA-A2 subgroups but also with A24, A26, and A28.39,40 Although we did not examine the exact binding affinity of these peptides to HLA molecules, the induction of HM1.24-126–specific CTLs from HLA-A24+ individuals might be explained by this cross-reaction mechanism. In contrast, HM1.24-165 peptide generated specific CTLs mostly from HLA-A24+ individuals, and to a lesser extent from HLA-A2+ individuals. Because HM1.24-165 contains leucine at position 9, HM1.24-165 may have some binding affinity to HLA-A2 as well as HLA-A24 as suggested by the SYFPEITHI binding scores. Similar findings have been reported in a WT1-derived peptide, CMTWNQMNL, in which this WT-1 peptide can be presented by both HLA-A241 and HLA-A2435 and induces specific CTLs in each HLA-A type despite different values of the binding scores.
Conversely, the predictive binding scores of HM1.24-165 peptide do not seem to be sufficiently high, but a significant CTL response to this peptide was observed in healthy donors and patients with MM. Recent studies have shown that predictive algorithms cannot substitute an experimental binding assay and that the binding affinity to HLA class I molecules is not the only determining factor of immunogenicity.42,43 Moreover, because high-affinity peptides are expressed highly enough to induce T-cell tolerance, intermediate- or low-affinity peptides result in more efficient induction of CTLs.44 Furthermore, there is a possibility that the frequency of HM1.24-165–specific CTLs is elevated in the peripheral blood, even though we were not able to show this by HM1.24-165 tetramer assay because of the difficulty of generating the HM1.24-165 tetramer complex. Therefore, HM1.24-165 might be an adequate epitope for eliciting peptide-specific CTLs, at least in HLA-A24+, which is one of the most common HLA-A alleles in Japanese and white individuals.
Controversial findings have been reported with regard to DC biology in patients with MM. The development and function of circulating DCs have been shown to be inhibited by humoral factors such as IL-6 and vascular endothelial growth factor that are implicated in the pathogenesis of MM.45,46 On the other hand, several studies have described no abnormality regarding the phenotype and function of DCs in MM patients.47,48 Our results demonstrated that the use of PBSC harvests appeared to yield more numbers of functional DCs compared with normal PBMCs although there was considerable individual variability in healthy donors and MM patients. This is consistent with the previous study indicating that large numbers of DCs can be obtained from PBSC products after the hematopoietic progenitor cell mobilization by chemotherapy plus G-CSF.49
High-avidity of CTLs to tissue-specific self-antigens may cause autoimmune disease but is likely to induce a successful antitumor response.50 In general, CTLs toward self-antigens appear to be eliminated by tolerance mechanism or clonal deletion. Nevertheless, clonal expansion of CTLs in response to Id protein51 or MUC116 has been detected in MM patients, especially those with a favorable prognosis, suggesting that these CTLs may inhibit the progression of disease in vivo. Although the number of analyzed patients was small and the assay system of CTLs was different, we also observed that HM1.24 peptide–pulsed DCs induce HM1.24-specific CTLs more efficiently in patients with MM than in healthy donors. These data support the hypothesis that the frequency of functional HM1.24-specific CTLs is elevated in patients with MM and indicate the feasibility of using existing HM1.24-specific CTLs in the treatment of MM. Taken together, these results suggest the potential benefits of PBSC harvests as a source of DCs as well as CTLs for either DC-based or adoptive cellular immunotherapy in combination with autologous PBSCT.
We have previously developed an anti-HM1.24 MoAb for the treatment of MM and have shown that HM1.24 antigen is a suitable target to mediate the cytotoxicity of MM cells by antibody-dependent cellular cytotoxicity (ADCC).52-54 The present study has demonstrated that HM1.24 also plays an important role as an MM-specific target for cellular immunotherapy and that pretreatment of target cells with anti-HM1.24 MoAb does not inhibit the recognition or the cytotoxicity of HM1.24 peptide–specific CTLs. Because tumor-specific MoAb therapy is another major modality of immunotherapeutic strategies, the combined efficacy of CTLs and antibody-based immunotherapy needs to be elucidated. In this respect, HM1.24-targeting therapies provide valuable tools for use in the further understanding of the mechanisms of peptide-specific CTLs and ADCC.
In conclusion, we have identified that HM1.24-126 and HM1.24-165 peptides effectively induce CTLs in healthy donors and patients with MM. Moreover, PBSC harvests can be used as a source of DCs as well as CTLs, which provides the basis for novel cellular immunotherapy in combination with PBSCT. Therefore, these results warrant further clinical trials to evaluate this approach in patients with MM.
Prepublished online as Blood First Edition Paper, July 21, 2005; DOI 10.1182/blood-2005-04-1438.
Supported in part by a Grant-in-Aid for Scientific Research (C; S.O.) and a Grant-in-Aid for Scientific Research (B; T.M.) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
A.J. analyzed the data and wrote the paper. S.O. contributed to the study design and interpreted the data. T. Hara, H.S., T. Hashimoto, and M.A. provided patient materials and analyzed the data. Y.N. interpreted the data and provided critical comments. T.M. coordinated the study and reviewed the manuscript for important intellectual content.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Hiroe Amou and Asuka Oda for their excellent technical support and Geoff Falk for assistance in the preparation of this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal