Abstract
Chronic lymphocytic leukemia (CLL) is a disease of CD5+ B lymphocytes (designated as CLL cells) that are inefficient antigen-presenting cells. Their poor ability to present antigens to the T cells, largely due to an inadequate costimulatory capacity, is manifested as a failure to stimulate proliferation of both allogeneic and autologous T cells. We have investigated the ability of in vitro manipulated CLL cells, via hyperexpression of a triad of costimulatory molecules (B7-1, intercellular adhesion molecule 1 [ICAM-1], and leukocyte-function–associated antigen 3 [LFA-3], designated TRICOM), to stimulate effective antitumor T-cell responses. A recombinant modified vaccinia virus strain Ankara (MVA), which is a highly attenuated, replication-impaired virus variant, was successfully used to infect and deliver the simultaneous expression of the 3 human costimulatory molecules in TRICOM on the surface of the CLL cells. Proliferation of allogeneic and autologous T cells was observed when MVA-TRICOM–infected CLL cells were used as stimulators in proliferation assays. Cytotoxic T lymphocytes, generated in vitro by stimulation of autologous T cells with MVA-TRICOM–infected CLL cells, showed cytotoxicity against unmodified/uninfected CLL cells. Therefore, our findings suggest that the use of CLL cells infected ex vivo with MVA-TRICOM or direct injection of MVA-TRICOM in patients with CLL has potential for the immunotherapy of CLL.
Introduction
Chronic lymphocytic leukemia (CLL) is a disease characterized by accumulation of a clonal population of malignant CD5+ B cells (designated as CLL cells) that are defective in apoptosis and arrested in the G0/G1 phase of their cell cycle.1-3 With more than 7000 new cases diagnosed in the United States each year, CLL constitutes 25% of all adult leukemias.4 Although alkylating agents and/or nucleoside analogs have been used for palliation of symptoms and manifestations of the disease, new therapies are currently under investigation in an effort to achieve a curative therapy for CLL.5 Among these new interventions are the use of monoclonal antibodies against antigens on the CLL cells and allogeneic stem cell transplantation for adoptive immunotherapy.6-8
There has also been interest in developing T-cell–specific immunity against CLL cells by active immunization of patients with CLL with genetically manipulated tumor cells.9 Although CLL cells express tumor-associated antigens and molecules of the major histocompatibility complex (MHC) class I and class II, they are ineffective stimulants of both allogeneic and autologous T-cell proliferation,10,11 which is attributable to their lack of expression of T-cell costimulatory molecules on the cell surface.12 The delivery of a “signal 1” by the antigen-presenting cell (APC) to the T-cell receptor, in the absence of adequate costimulation (“signal 2”) by the APC, results in T-cell anergy and insensitivity to subsequent antigen activation. Reconstitution of productive costimulation to CLL cells has been achieved via CD40 ligand (CD40L)–induced signaling, which increases their antigen-presentation ability by up-regulating the expression of a variety of costimulatory molecules on their cell surface.9,13-18
Among the costimulatory molecules expressed by “professional” APCs, B7-1, intercellular adhesion molecule 1 (ICAM-1), and leukocyte function-associated antigen 3 (LFA-3) play a major role in T-cell activation.19-22 We have previously used an avipox (fowlpox, rF-) vector encoding for human B7-1, ICAM-1, and LFA-3 (designated as rF-TRICOM) to efficiently infect and direct the overexpression of these costimulatory molecules on human dendritic cells (DCs) and B cells. The increased antigen-presenting potency of rF-TRICOM–modified DCs and naive B cells, as compared with the uninfected counterparts, was demonstrated by the enhancement of T-cell responses.23,24 Here, we report on the ability of 4 poxvirus vectors encoding for the human TRICOM molecules to infect CLL cells in vitro, resulting in expression of the encoded transgenes. The replication-defective modified vaccinia virus strain Ankara (MVA)–TRICOM vector proved to be the most efficient of the poxvirus vectors studied here to augment the antigen-presenting ability of CLL cells.
MVA is a highly attenuated strain of vaccinia virus obtained after more than 500 passages in chicken embryo fibroblasts that has lost the ability to complete a full replication cycle in human cells.25,26 Because of its safety profile, MVA is an attractive vector for use in vaccination programs or in immunotherapeutic regimens, especially when involving immunocompromised individuals such as patients with CLL. Therefore, CLL cells infected ex vivo with MVA-TRICOM, and then irradiated, could form the basis for whole tumor cell vaccine for the immunotherapy of CLL.
Patients, materials, and methods
Patients and healthy donor PBMCs
Peripheral blood was collected from patients diagnosed with CLL after informed consent was provided in accordance with the Declaration of Helsinki, and following approval by the University of Pittsburgh Institutional Review Board. Mononuclear-cell–enriched blood fractions were obtained from healthy donors by leukapheresis. Demographics of patients included in the study are presented in Table 1. For all experiments, peripheral blood mononuclear cells (PBMCs) were isolated as previously described24 and viably cryopreserved in liquid nitrogen, in heat-inactivated fetal bovine serum (Gemini Bioproducts, Woodland, CA) containing 10% dimethyl sulfoxide (Sigma-Aldrich, St Louis, MO) until further use. In all experiments, cells were thawed and immediately reconstituted in RPMI 1640 medium (Mediatech., Herndon, VA), supplemented with 2 mM glutamine, 1X antibiotic-antimycotic solution (Mediatech), and 10% human AB serum (Gemini Bio-Products).
Characteristics of patients
Patient . | Sex . | Age, y . | Treatment . | WBC count, × 109/L . | Rai stage . | CD38 . | Interphase cytogenetics* . |
|---|---|---|---|---|---|---|---|
| 1 | M | 55 | None | 20 | 1 | – | NA |
| 2 | M | 85 | Chlorambucil† | 68 | 3/4 | NA | NA |
| 3 | M | 47 | Multiple | 40 | 1 | – | Normal |
| 4‡ | NA | NA | NA | NA | NA | NA | NA |
| 5 | F | 70 | None | 60 | 0 | NA | NA |
| 6 | M | 47 | None | 30 | 1 | – | NA |
| 7 | F | 54 | Chlorambucil§ | 16 | 0 | + | +12 13q |
| 8 | M | 57 | None | 200 | 1 | NA | 13q |
| 9 | M | 50 | None | 37 | 1 | NA | NA |
| 10 | M | 75 | Multiple∥ | 100 | 3/4 | – | 13q |
| 11 | M | 61 | None | 69 | 1 | + | 13q 11q |
| 12 | F | 79 | Multiple¶ | 34 | 1 | NA | NA |
| 13 | F | 69 | None | 107 | 1 | + | Normal |
| 14 | M | 52 | Fludarabine# | 200 | 2 | + | Normal |
| 15 | F | 52 | None | 42 | 1 | NA | NA |
| 16 | F | 57 | None | 300 | 1 | + | +12 t(1;6) |
| 17 | M | 75 | Multiple** | 21 | 2 | + | 13q |
| 18 | F | 57 | None | 219 | 1 | – | 13q 6q |
| 19 | F | 56 | None | 72 | 1 | + | +12 |
Patient . | Sex . | Age, y . | Treatment . | WBC count, × 109/L . | Rai stage . | CD38 . | Interphase cytogenetics* . |
|---|---|---|---|---|---|---|---|
| 1 | M | 55 | None | 20 | 1 | – | NA |
| 2 | M | 85 | Chlorambucil† | 68 | 3/4 | NA | NA |
| 3 | M | 47 | Multiple | 40 | 1 | – | Normal |
| 4‡ | NA | NA | NA | NA | NA | NA | NA |
| 5 | F | 70 | None | 60 | 0 | NA | NA |
| 6 | M | 47 | None | 30 | 1 | – | NA |
| 7 | F | 54 | Chlorambucil§ | 16 | 0 | + | +12 13q |
| 8 | M | 57 | None | 200 | 1 | NA | 13q |
| 9 | M | 50 | None | 37 | 1 | NA | NA |
| 10 | M | 75 | Multiple∥ | 100 | 3/4 | – | 13q |
| 11 | M | 61 | None | 69 | 1 | + | 13q 11q |
| 12 | F | 79 | Multiple¶ | 34 | 1 | NA | NA |
| 13 | F | 69 | None | 107 | 1 | + | Normal |
| 14 | M | 52 | Fludarabine# | 200 | 2 | + | Normal |
| 15 | F | 52 | None | 42 | 1 | NA | NA |
| 16 | F | 57 | None | 300 | 1 | + | +12 t(1;6) |
| 17 | M | 75 | Multiple** | 21 | 2 | + | 13q |
| 18 | F | 57 | None | 219 | 1 | – | 13q 6q |
| 19 | F | 56 | None | 72 | 1 | + | +12 |
WBC indicates white blood cell; NA, information not available.
The only patients from which variable heavy chain (VH) gene mutational status was available were patients 8 and 10, both having VH unmutated
Rituximab, cyclophosphamide, vincristine, and prednisone (2000)
Patient 4 entered the study in condition of anonymity
Treatment received in 2003 and 2004
Fludarabine/mitoxantrone (1999); cyclophosphamide, vincristine, and prednisone/rituximab (2003); alemtuzumab (2003)
Chlorambucil (1992–2000); rituximab, cyclophosphamide, vincristine, and prednisone (2002)
Treatment received in 2001
Pentostatin/cyclophosphamide/rituximab (2003 and 2004)
T cells were negatively isolated from PBMCs of patients with CLL and healthy donors by using the Pan T-cell isolation kit (Miltenyi Biotec, Auburn, CA). Isolation of CLL cells was achieved by using anti–CD19-conjugated magnetic beads (Miltenyi Biotec). Purity of all cell fractions was greater than 95% as assessed by flow cytometry analysis.
Recombinant poxviruses
Three recombinant poxviruses encoding for the human B7-1, ICAM-1, and LFA-3 costimulatory molecules (designated as TRICOM) were used in this study: (1) the avipox (fowlpox) rF-TRICOM virus, (2) the vaccinia (rV-TRICOM) virus, and (3) the MVA-TRICOM virus. All recombinants contain human CD80 under control of the SE/L promoter,27 human CD58 under control of the 30K promoter,28 and human CD54 under control of the vaccinia I3 promoter.29 Wild-type fowlpox, vaccinia, and MVA viruses (designated FP-WT, V-WT, and MVA-WT, respectively) were used as control vectors. Therion Biologics (Cambridge, MA) kindly provided all viruses, as part of an ongoing Collaborative Research and Development Agreement between the National Cancer Institute/National Institutes of Health (NIH) and Therion Biologics.
Psoralen-UV light inactivation of vaccinia virus
Photochemical DNA crosslinking of vaccinia virus preparations (V-WT and rV-TRICOM) was carried out as previously described.30,31 Each lot of psoralen-UV–inactivated vaccinia virus was analyzed for its ability to form plaques on BS-C-1 cells in a standard plaque assay before being used for infection of PBMCs. Nonreplicative viruses were kept frozen in aliquots at -80°C and subsequently used for infection of PBMCs as described in the next paragraph.
Infection of PBMCs with recombinant poxviruses
Infection of PBMCs from patients with CLL with the MVA or vaccinia vectors was conducted by incubating 2 × 106 cells/mL in Opti-MEM (Minimum Essential Media) medium (Invitrogen, Carlsbad, CA) with 5 or 10 plaque-forming units (pfu)/cell (multiplicity of infection [MOI], 5 or 10), respectively, for 1 hour at 37°C. Fowlpox vectors were used at an MOI of 40, and infection was carried out for 2 hours at 37°C. The infected cells were suspended in 10 mL prewarmed medium containing 10% human AB serum and cultured for 24 additional hours.
Flow cytometry
Twenty-four hours after infection, PBMCs were analyzed by flow cytometry following a previously described protocol.23 Antibody (Ab) combinations used in the analysis were CD5–fluorescein isothiocyanate (FITC)/CD19-phycoerythrin (PE), CD80-PE/CD19-FITC, CD54-PE/CD19-FITC, and CD58-PE/CD19-FITC (Becton Dickinson Immunocytometry System, San Jose, CA).
Mixed lymphocyte reaction (MLR)
For the allogeneic MLR, purified CD3+ T cells from healthy donors (effectors, 1 × 105 cells per well in 96-well round-bottom culture plates) were cocultured with γ-irradiated (75 Gy) allogeneic PBMCs from patients with CLL (stimulators, 1 × 104 per well), in a final volume of 0.2 mL per well. Stimulator cells were either uninfected or infected with the various recombinant viral vectors, as described in “Infection of PBMCs with recombinant poxviruses.” All assays were conducted in triplicate, in RPMI 1640 medium containing 10% human AB serum. Cocultures were incubated at 37°C in a 5% CO2 atmosphere for 5 days and pulsed with 1 μCi/well (0.037 MBq/well) 3H-thymidine (Perkin-Elmer, Boston, MA), for the final 16 hours. Cells were harvested, and the 3H-thymidine incorporation was measured by liquid scintillation spectroscopy. Results were reported as mean counts per minute (cpm) ± the standard error of the mean (SEM). For the autologous lymphocyte proliferation assays, purified CD3+ T cells from patients with CLL (1 × 105 cells per well) were incubated with γ-irradiated (75 Gy) autologous PBMCs that were either uninfected or infected with the MVA-WT and MVA-TRICOM vectors, at a ratio of stimulators to effectors equal to 1:2.5 for 5 days.
Ab-blocking experiments were conducted by incubating stimulator cells for 1 hour in the presence of purified mouse monoclonal Abs directed to the human B7-1, ICAM-1, LFA-3, MHC class I, and MHC class II molecules and control Abs (mouse purified immunoglobulin G [IgG]). All blocking Abs were sodium azide free (Serotec, Oxford, United Kingdom).
Cytokine assay
Lymphocyte culture supernatants (48 hours after stimulation) were assayed for various cytokines by using a human CBA (cytometric bead assay) kit, following the instructions of the manufacturer (Becton Dickinson Immunocytometry System).
Generation of CLL-specific T cells
Purified CD3+ T cells from patients with CLL were stimulated in the presence of irradiated (75 Gy) autologous CLL cells that were infected with the MVA-TRICOM vector, at a stimulator-effector ratio equal to 1:2.5. Cultures were maintained for 3 initial days in medium containing 10% human AB serum and 4 additional days in the same medium supplemented with 20 U/mL recombinant human interleukin 2 (IL-2). After a 7-day culture period, designated as an in vitro stimulation (IVS) cycle, cells were restimulated as described in the same way as for a total of 3 IVS.
Cytotoxic assay
A 5-hour 111In release assay was used to determine T-cell–mediated killing of CLL target cells. Uninfected autologous CLL cells were labeled with 111In oxine (Amersham Health, Silver Spring, MD) for 15 minutes at room temperature and used as target cells at 3 × 103 cells per well, in 96-well round-bottom culture plates. CD8+ T cells negatively isolated (IVS 3, at day 5 of the restimulation cycle) or CD4+ T cells positively isolated by using anti–CD4-conjugated magnetic beads (IVS 3, at day 6 of the stimulation cycle) were used as effector cells, at various effector-target (E/T) cell ratios. After 5 hours of incubation, supernatants were harvested, and the 111In released was measured by γ counting. Spontaneous release was determined by incubating the target cells with medium alone, and complete lysis by incubating the target cells with 2.5% Triton X-100. Specific lysis (%) = [(observed release - spontaneous release) / (complete release - spontaneous release)] × 100.
Results
Phenotypic and functional modification of CLL cells via infection with TRICOM-encoding poxvirus vectors
Various TRICOM-encoding poxvirus vectors were compared (1) on their ability to infect and direct expression of the human B7-1, ICAM-1, and LFA-3 molecules on the surface of CLL cells and (2) on their ability to enhance the antigen-presenting potency of CLL cells. Characteristics of patients from which PBMCs were obtained for this study are listed in Table 1. Age, sex, treatment, white blood cell count, Rai stage, CD38 expression, and interphase cytogenetics are described. Eleven of 19 patients included in the study had no prior therapy, and 4 patients had multiple prior therapies. PBMCs from patients with CLL (in which greater than 95% were CD19+ cells) were first infected with various MOI (40, 60, and 80 MOI) of the avipox rF-TRICOM or the control FP-WT vector. Although no changes in expression of B7-1, ICAM-1, and LFA-3 were induced on the CLL cells 24 hours after infection with the control FP-WT vector, expression of B7-1, which was low or undetectable in uninfected CLL cells, increased after infection with rF-TRICOM in both the percentage of positive cells and the mean fluorescence intensity (MFI) value for this marker (data not shown). Expression of ICAM-1 and LFA-3 molecules, however, was enhanced little, if any, above the levels in the uninfected CLL population.
Purified CD3+ T cells from a healthy donor were incubated in the presence of irradiated (75 Gy) uninfected CLL cells or CLL cells infected with 40 MOI of rF-TRICOM. No T-cell proliferation occurred in response to uninfected or rF-TRICOM–infected CLL cells in 3 patients analyzed (data not shown). Thus, unlike results observed with human DCs, human B cells from apparently healthy individuals, and with murine normal and leukemic cells,23,24,32,33 our results indicated that rF-TRICOM is ineffective in enhancing the immunogenicity of human CLL cells.
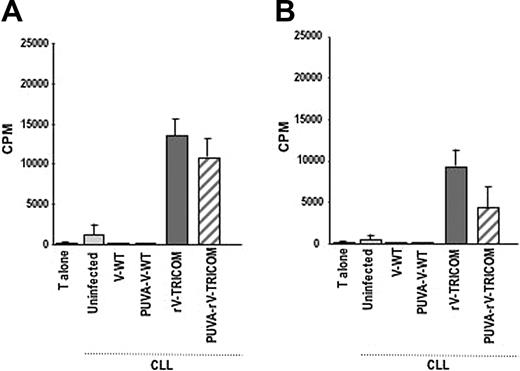
The ability of 2 replication-defective vaccinia-derived vectors, (1) a psoralen-UV–inactivated rV-TRICOM (designated as PUVA-rV-TRICOM) and (2) the replication-defective MVA-TRICOM vector, to infect CLL cells and to enhance their immunogenicity was also studied. Psoralen and UV light treatment to inactivate vaccinia has been used in vitro and in vivo as a procedure to improve safety of vaccinia virus vectors30,34,35 and was previously reported not to eliminate expression of recombinant genes driven by early promoters.36 An initial comparison using 10, 20, and 40 MOI of PUVA-rV-TRICOM for CLL infection showed similar induction of transgene expression, and all subsequent experiments used 10 MOI of PUVA-rV-TRICOM. As shown in Table 2, infection of CLL cells from 2 patients with CLL with 10 MOI PUVA-rV-TRICOM enhanced the expression of B7-1 and ICAM-1 in both percentage of positive cells and MFI; the expression of LFA-3, however, was only slightly modified after infection with this vector. The untreated replication-competent rV-TRICOM vector was included in the study as a positive control; as seen in Table 2, the percentage as well as the MFI value for the expression of B7-1, ICAM-1, and LFA-3 were all augmented after rV-TRICOM infection. Proliferation of allogeneic CD3+ T cells was enhanced by the use of either PUVA-rV-TRICOM–infected or rV-TRICOM–infected CLL cells, as compared with uninfected CLL cells (Figure 1A-B). Neither changes in phenotype nor proliferation of allogeneic T cells was induced when the V-WT or PUVA-V-WT control vectors were used for infection.
Flow cytometry analysis of PBMCs from patients 10 and 11 at 24 hours after infection with 10 MOI V-WT, PUVA-V-WT, rV-TRICOM, or PUVA-rV-TRICOM
Patient, vector . | B7-1, % (MFI) . | ICAM-1, % (MFI) . | LFA-3, % (MFI) . |
|---|---|---|---|
| Patient 10 | |||
| Uninfected | 12.0 (22) | 19.9 (31) | 38.9 (30) |
| V-WT | 17.1 (33) | 23.7 (28) | 37.6 (22) |
| PUVA-V-WT | 23.5 (17) | 17.4 (28) | 41.4 (29) |
| rV-TRICOM | 39.9 (130) | 54.9 (483) | 49.6 (112) |
| PUVA-rV-TRICOM | 27.5 (116) | 27.5 (125) | 39.5 (39) |
| Patient 11 | |||
| Uninfected | 18.1 (31) | 81.1 (62) | 48.4 (32) |
| V-WT | 15.4 (34) | 76.7 (37) | 36.1 (20) |
| PUVA-V-WT | 13.6 (43) | 75.7 (29) | 45.0 (19) |
| rV-TRICOM | 52.8 (237) | 96.3 (1055) | 75.1 (168) |
| PUVA-rV-TRICOM | 38.3 (129) | 89.1 (414) | 51.9 (73) |
Patient, vector . | B7-1, % (MFI) . | ICAM-1, % (MFI) . | LFA-3, % (MFI) . |
|---|---|---|---|
| Patient 10 | |||
| Uninfected | 12.0 (22) | 19.9 (31) | 38.9 (30) |
| V-WT | 17.1 (33) | 23.7 (28) | 37.6 (22) |
| PUVA-V-WT | 23.5 (17) | 17.4 (28) | 41.4 (29) |
| rV-TRICOM | 39.9 (130) | 54.9 (483) | 49.6 (112) |
| PUVA-rV-TRICOM | 27.5 (116) | 27.5 (125) | 39.5 (39) |
| Patient 11 | |||
| Uninfected | 18.1 (31) | 81.1 (62) | 48.4 (32) |
| V-WT | 15.4 (34) | 76.7 (37) | 36.1 (20) |
| PUVA-V-WT | 13.6 (43) | 75.7 (29) | 45.0 (19) |
| rV-TRICOM | 52.8 (237) | 96.3 (1055) | 75.1 (168) |
| PUVA-rV-TRICOM | 38.3 (129) | 89.1 (414) | 51.9 (73) |
Percentages are for cells that were positive for CD19 and each costimulatory molecule (B7-1, ICAM-1, and LFA-3). MFI values are for the expression of each costimulatory molecule.
We also evaluated the replication-defective MVA-TRICOM vector for infection of CLL cells. Titration experiments using 5, 10, and 20 MOI of this vector for ex vivo infection of CLL cells demonstrated 5 MOI to be optimal.
The ability of MVA-TRICOM to deliver expression of B7-1, ICAM-1, and LFA-3 on the surface of infected CLL cells was investigated in 19 patients with CLL; results are shown in Table 3. Substantial increases in both the percentage of positive cells and the MFI values for all 3 costimulatory molecules were observed after infection with 5 MOI MVA-TRICOM in all 19 patients analyzed. It should be pointed out that, although all CLL samples showed very low or undetectable levels of B7-1 surface expression, approximately 50% of uninfected CLL cells expressed considerable surface ICAM-1 or LFA-3. However, infection of these samples with MVA-TRICOM enhanced the percentage of positive cells and/or the cell surface level (MFI) for both ICAM-1 and LFA-3.
Phenotypic analysis of PBMCs from patients with CLL 24 hours after infection with MVA-TRICOM
Patient, PBMCs . | B7-1, % (MFI) . | ICAM-1, % (MFI) . | LFA-3, % (MFI) . |
|---|---|---|---|
| 1 | |||
| Uninfected | 0.9 (74) | 9.7 (25) | 61.1 (28) |
| MVA-TRICOM | 61.7 (753) | 76.9 (2310) | 88.1 (1047) |
| 2 | |||
| Uninfected | 1.0 (100) | 29.2 (71) | 21.6 (95) |
| MVA-TRICOM | 36.8 (1239) | 67.2 (3430) | 57.3 (1339) |
| 3 | |||
| Uninfected | 17.7 (7) | 83.2 (9) | 81.2 (24) |
| MVA-TRICOM | 44.5 (106) | 94.2 (134) | 85.1 (67) |
| 4 | |||
| Uninfected | 0.1 (31) | 39.0 (137) | 41.0 (118) |
| MVA-TRICOM | 10.5 (1384) | 45.5 (2318) | 44.4 (1053) |
| 5 | |||
| Uninfected | 0.7 (192) | 2.6 (118) | 13.4 (25) |
| MVA-TRICOM | 69.7 (243) | 67.2 (489) | 66.6 (216) |
| 6 | |||
| Uninfected | 10.7 (16) | 71.3 (23) | 96.3 (35) |
| MVA-TRICOM | 38.5 (383) | 79.4 (906) | 93.8 (351) |
| 7 | |||
| Uninfected | 52.1 (29) | 83.6 (35) | 97.2 (99) |
| MVA-TRICOM | 72.4 (277) | 92.2 (983) | 97.2 (406) |
| 8 | |||
| Uninfected | 0.1 (12) | 97.5 (58) | 66.0 (19) |
| MVA-TRICOM | 52.2 (348) | 98.5 (1038) | 89.2 (436) |
| 9 | |||
| Uninfected | 1.9 (53) | 18.3 (19) | 14.4 (16) |
| MVA-TRICOM | 40.3 (200) | 51.5 (506) | 52.9 (216) |
| 10 | |||
| Uninfected | 12.0 (22) | 19.9 (31) | 38.9 (30) |
| MVA-TRICOM | 64.3 (343) | 95.8 (1474) | 81.1 (539) |
| 11 | |||
| Uninfected | 18.1 (31) | 81.1 (62) | 48.4 (20) |
| MVA-TRICOM | 78.9 (261) | 97.1 (1321) | 87.3 (468) |
| 12 | |||
| Uninfected | 1.3 (141) | 75.6 (53) | 98.4 (62) |
| MVA-TRICOM | 92.6 (1052) | 98.6 (1387) | 99.9 (789) |
| 13 | |||
| Uninfected | 0.4 (134) | 78.7 (35) | 88.4 (34) |
| MVA-TRICOM | 87.5 (477) | 95.8 (952) | 96.7 (597) |
| 14 | |||
| Uninfected | 28.3 (135) | 77.8 (213) | 53.8 (96) |
| MVA-TRICOM | 84.9 (1113) | 91.9 (1974) | 95.9 (974) |
| 15 | |||
| Uninfected | 7.1 (58) | 37.2 (69) | 77.0 (69) |
| MVA-TRICOM | 57.9 (880) | 75.0 (2454) | 88.6 (1026) |
| 16 | |||
| Uninfected | 0.1 (116) | 5.1 (86) | 2.8 (98) |
| MVA-TRICOM | 39.3 (491) | 45.5 (1049) | 40.7 (500) |
| 17 | |||
| Uninfected | 0.7 (24) | 67.3 (26) | 98.7 (54) |
| MVA-TRICOM | 82.4 (1378) | 89.6 (1615) | 92.7 (865) |
| 18 | |||
| Uninfected | 0.7 (29) | 22.1 (82) | 90.5 (24) |
| MVA-TRICOM | 88.5 (601) | 86.9 (631) | 98.2 (330) |
| 19 | |||
| Uninfected | 0.3 (346) | 29.9 (47) | 31.6 (35) |
| MVA-TRICOM | 82.5 (407) | 68.4 (1062) | 92.3 (491) |
Patient, PBMCs . | B7-1, % (MFI) . | ICAM-1, % (MFI) . | LFA-3, % (MFI) . |
|---|---|---|---|
| 1 | |||
| Uninfected | 0.9 (74) | 9.7 (25) | 61.1 (28) |
| MVA-TRICOM | 61.7 (753) | 76.9 (2310) | 88.1 (1047) |
| 2 | |||
| Uninfected | 1.0 (100) | 29.2 (71) | 21.6 (95) |
| MVA-TRICOM | 36.8 (1239) | 67.2 (3430) | 57.3 (1339) |
| 3 | |||
| Uninfected | 17.7 (7) | 83.2 (9) | 81.2 (24) |
| MVA-TRICOM | 44.5 (106) | 94.2 (134) | 85.1 (67) |
| 4 | |||
| Uninfected | 0.1 (31) | 39.0 (137) | 41.0 (118) |
| MVA-TRICOM | 10.5 (1384) | 45.5 (2318) | 44.4 (1053) |
| 5 | |||
| Uninfected | 0.7 (192) | 2.6 (118) | 13.4 (25) |
| MVA-TRICOM | 69.7 (243) | 67.2 (489) | 66.6 (216) |
| 6 | |||
| Uninfected | 10.7 (16) | 71.3 (23) | 96.3 (35) |
| MVA-TRICOM | 38.5 (383) | 79.4 (906) | 93.8 (351) |
| 7 | |||
| Uninfected | 52.1 (29) | 83.6 (35) | 97.2 (99) |
| MVA-TRICOM | 72.4 (277) | 92.2 (983) | 97.2 (406) |
| 8 | |||
| Uninfected | 0.1 (12) | 97.5 (58) | 66.0 (19) |
| MVA-TRICOM | 52.2 (348) | 98.5 (1038) | 89.2 (436) |
| 9 | |||
| Uninfected | 1.9 (53) | 18.3 (19) | 14.4 (16) |
| MVA-TRICOM | 40.3 (200) | 51.5 (506) | 52.9 (216) |
| 10 | |||
| Uninfected | 12.0 (22) | 19.9 (31) | 38.9 (30) |
| MVA-TRICOM | 64.3 (343) | 95.8 (1474) | 81.1 (539) |
| 11 | |||
| Uninfected | 18.1 (31) | 81.1 (62) | 48.4 (20) |
| MVA-TRICOM | 78.9 (261) | 97.1 (1321) | 87.3 (468) |
| 12 | |||
| Uninfected | 1.3 (141) | 75.6 (53) | 98.4 (62) |
| MVA-TRICOM | 92.6 (1052) | 98.6 (1387) | 99.9 (789) |
| 13 | |||
| Uninfected | 0.4 (134) | 78.7 (35) | 88.4 (34) |
| MVA-TRICOM | 87.5 (477) | 95.8 (952) | 96.7 (597) |
| 14 | |||
| Uninfected | 28.3 (135) | 77.8 (213) | 53.8 (96) |
| MVA-TRICOM | 84.9 (1113) | 91.9 (1974) | 95.9 (974) |
| 15 | |||
| Uninfected | 7.1 (58) | 37.2 (69) | 77.0 (69) |
| MVA-TRICOM | 57.9 (880) | 75.0 (2454) | 88.6 (1026) |
| 16 | |||
| Uninfected | 0.1 (116) | 5.1 (86) | 2.8 (98) |
| MVA-TRICOM | 39.3 (491) | 45.5 (1049) | 40.7 (500) |
| 17 | |||
| Uninfected | 0.7 (24) | 67.3 (26) | 98.7 (54) |
| MVA-TRICOM | 82.4 (1378) | 89.6 (1615) | 92.7 (865) |
| 18 | |||
| Uninfected | 0.7 (29) | 22.1 (82) | 90.5 (24) |
| MVA-TRICOM | 88.5 (601) | 86.9 (631) | 98.2 (330) |
| 19 | |||
| Uninfected | 0.3 (346) | 29.9 (47) | 31.6 (35) |
| MVA-TRICOM | 82.5 (407) | 68.4 (1062) | 92.3 (491) |
Percentages indicate positive cells for CD19 and B7-1, ICAM-1, or LFA-3; MFI values are for the expression of each costimulatory molecule. There were no changes in B7-1, ICAM-1, and LFA-3 expression in CLL cells from the above 19 patients when infected with wild-type MVA-WT vector.
Functional analysis of CLL cells after infection with rV-TRICOM or PUVA-rV-TRICOM. (A-B) Mixed lymphocyte reaction using allogeneic T cells from healthy donors (1 × 105 per well) as effectors and various PBMC preparations (1 × 104 per well) as stimulators. Results are expressed as cpm ± SEM for triplicate wells.
Functional analysis of CLL cells after infection with rV-TRICOM or PUVA-rV-TRICOM. (A-B) Mixed lymphocyte reaction using allogeneic T cells from healthy donors (1 × 105 per well) as effectors and various PBMC preparations (1 × 104 per well) as stimulators. Results are expressed as cpm ± SEM for triplicate wells.
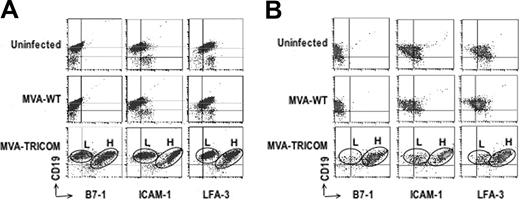
Phenotypic analysis of CLL cells after infection with either MVA-WT or MVA-TRICOM. Flow cytometry analysis of PBMCs from patients 1 (A) and 14 (B) uninfected or 24 hours after infection with 5 MOI of MVA-WT or 5 MOI of MVA-TRICOM. After infection with MVA-TRICOM, 2 populations of CLL cells that expressed either low or high levels of each costimulatory molecule were observed; these were designated here as “low MFI” (L) and “high MFI” (H) populations, respectively.
Phenotypic analysis of CLL cells after infection with either MVA-WT or MVA-TRICOM. Flow cytometry analysis of PBMCs from patients 1 (A) and 14 (B) uninfected or 24 hours after infection with 5 MOI of MVA-WT or 5 MOI of MVA-TRICOM. After infection with MVA-TRICOM, 2 populations of CLL cells that expressed either low or high levels of each costimulatory molecule were observed; these were designated here as “low MFI” (L) and “high MFI” (H) populations, respectively.
Infection of CLL cells with the control vector (MVA-WT) did not modify expression of B7-1, ICAM-1, or LFA-3 in any of the 19 patient samples analyzed in Table 3 (data not shown). Two examples are shown in Figure 2A-B. The data in Figure 2A-B and Table 4 demonstrate that there are actually 2 populations of CLL cells after infection with MVA-TRICOM, which express either low or high levels of each costimulatory molecule; these were designated as “low MFI” (L) and “high MFI” (H) populations, respectively. Data shown in Table 4 were calculated both for the combined populations (data gated on PBMCs) as well as for the low MFI and high MFI populations (data gated on each individual population of CLL cells). In the high MFI population of both patient samples shown in Figure 2, greater than 95% of the CLL cells expressed high levels of all 3 costimulatory molecules after infection with MVA-TRICOM. In the low MFI population for both patients, approximately 40% of the CLL cells were positive for B7-1, 60% to 70% were positive for ICAM-1, and almost 90% of the cells were positive for LFA-3. For succinctness, because the values for the combined populations are similar to those of the higher expressing population, the values given in Table 3 are those for the combined populations.
Flow cytometry analysis of PBMCs from patients 1 (A) and 14 (B) uninfected or 24 hours after infection with 5 MOI of MVA-WT or 5 MOI of MVA-TRICOM
Vector . | B7-1, % (MFI) . | ICAM-1, % (MFI) . | LFA-3, % (MFI) . |
|---|---|---|---|
| Patient 1 | |||
| Uninfected | 4.9 (23) | 22.2 (18) | 83.4 (25) |
| MVA-WT | 3.1 (16) | 13.6 (16) | 83.5 (25) |
| MVA-TRICOM (combined) | 68.9 (672) | 82.0 (2131) | 92.6 (987) |
| MVA-TRICOM (low MFI) | 36.5 (20) | 62.0 (32) | 88.6 (33) |
| MVA-TRICOM (high MFI) | 95.9 (920) | 96.2 (3226) | 96.1 (1949) |
| Patient 14 | |||
| Uninfected | 0.7 (2) | 26.9 (63) | 69.9 (36) |
| MVA-WT | 0.2 (2) | 18.7 (50) | 73.7 (27) |
| MVA-TRICOM (combined) | 81.8 (979) | 93.1 (2476) | 95.0 (1095) |
| MVA-TRICOM (low MFI) | 40.3 (43) | 70.5 (50) | 88.4 (45) |
| MVA-TRICOM (high MFI) | 98.8 (1534) | 99.6 (3133) | 99.2 (1766) |
Vector . | B7-1, % (MFI) . | ICAM-1, % (MFI) . | LFA-3, % (MFI) . |
|---|---|---|---|
| Patient 1 | |||
| Uninfected | 4.9 (23) | 22.2 (18) | 83.4 (25) |
| MVA-WT | 3.1 (16) | 13.6 (16) | 83.5 (25) |
| MVA-TRICOM (combined) | 68.9 (672) | 82.0 (2131) | 92.6 (987) |
| MVA-TRICOM (low MFI) | 36.5 (20) | 62.0 (32) | 88.6 (33) |
| MVA-TRICOM (high MFI) | 95.9 (920) | 96.2 (3226) | 96.1 (1949) |
| Patient 14 | |||
| Uninfected | 0.7 (2) | 26.9 (63) | 69.9 (36) |
| MVA-WT | 0.2 (2) | 18.7 (50) | 73.7 (27) |
| MVA-TRICOM (combined) | 81.8 (979) | 93.1 (2476) | 95.0 (1095) |
| MVA-TRICOM (low MFI) | 40.3 (43) | 70.5 (50) | 88.4 (45) |
| MVA-TRICOM (high MFI) | 98.8 (1534) | 99.6 (3133) | 99.2 (1766) |
The percentage of double-positive cells (for CD19 and each costimulatory molecule), as well as MFI value for the expression of each costimulatory molecule (numbers in parentheses), are shown. Data on the combined populations were calculated as the percentage of double-positive cells in the entire PBMC population; data on the low MFI and high MFI populations were calculated after gating on each individual population of CLL cells.
Samples from patients with CLL were then analyzed for proliferation of allogeneic CD3+ T cells after infection with MVA-TRICOM. Table 5 shows results from 6 patients with CLL. MVA-TRICOM–infected CLL cells strongly induced proliferation of allogeneic T cells when compared with the uninfected CLL or CLL cells infected with the MVA-WT control vector. In addition to the results of the 6 patients shown in Table 5, a marked induction of proliferation was also observed in 6 additional patients evaluated (stimulation index ranging from 86.1 to 279.7).
Allogeneic T-cell proliferation in the presence of MVA-TRICOM–infected CLL cells
Patient, stimulator cells . | Allogeneic proliferation stimulation index . |
|---|---|
| 1 | |
| Uninfected | 1.0 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 131.0 |
| 2 | |
| Uninfected | 0.6 |
| MVA-WT | 0.7 |
| MVA-TRICOM | 121.8 |
| 4 | |
| Uninfected | 0.6 |
| MVA-WT | 0.8 |
| MVA-TRICOM | 68.6 |
| 16 | |
| Uninfected | 11.7 |
| MVA-WT | 9.4 |
| MVA-TRICOM | 34.4 |
| 17 | |
| Uninfected | 2.9 |
| MVA-WT | 2.9 |
| MVA-TRICOM | 179.6 |
| 18 | |
| Uninfected | 9.0 |
| MVA-WT | 3.1 |
| MVA-TRICOM | 35.8 |
Patient, stimulator cells . | Allogeneic proliferation stimulation index . |
|---|---|
| 1 | |
| Uninfected | 1.0 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 131.0 |
| 2 | |
| Uninfected | 0.6 |
| MVA-WT | 0.7 |
| MVA-TRICOM | 121.8 |
| 4 | |
| Uninfected | 0.6 |
| MVA-WT | 0.8 |
| MVA-TRICOM | 68.6 |
| 16 | |
| Uninfected | 11.7 |
| MVA-WT | 9.4 |
| MVA-TRICOM | 34.4 |
| 17 | |
| Uninfected | 2.9 |
| MVA-WT | 2.9 |
| MVA-TRICOM | 179.6 |
| 18 | |
| Uninfected | 9.0 |
| MVA-WT | 3.1 |
| MVA-TRICOM | 35.8 |
CD3+ T cells from healthy donors were stimulated with uninfected, MVA-WT– or MVA-TRICOM–infected CLL cells, at a ratio of stimulator to effector cells equal to 1:10. Proliferation was measured as 3H-thymidine incorporation on day 5 of culture. Stimulation index was calculated as cpm (T cells + CLL cells)/cpm (T cells alone).
When compared, proliferation of allogeneic T cells was repeatedly highest in response to MVA-TRICOM, then with rV-TRICOM, and then PUVA-rV-TRICOM–infected CLL cells (data not shown). Thus, MVA-TRICOM was the most efficient of the poxviruses studied here to enhance the immunogenicity of CLL cells.
B7-1, ICAM-1, and LFA-3 all contribute to the improvement of APC function of CLL cells
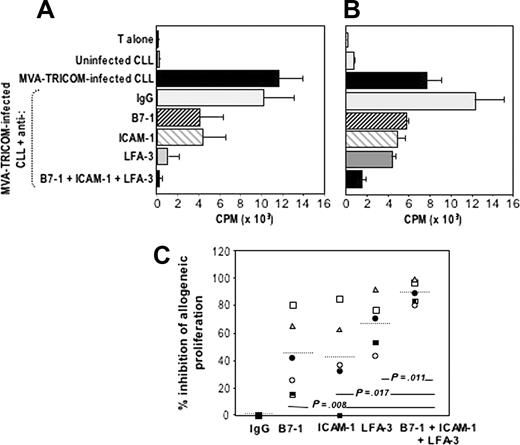
To elucidate the contribution of B7-1, ICAM-1, and LFA-3 to the enhancement of the APC potency of CLL cells after infection with MVA-TRICOM, we used specific Abs to block the function of each particular molecule. Proliferation of allogeneic T cells was conducted in the presence of MVA-TRICOM–infected CLL cells that were preincubated with various Abs or combinations of Abs specific for B7-1, ICAM-1, and LFA-3. Increased proliferation of allogeneic T cells from a normal donor was observed with MVA-TRICOM–infected CLL cells, compared with uninfected CLL cells (Figure 3A-B). Addition of specific Abs directed against B7-1, ICAM-1, or LFA-3 each resulted in an inhibition of T-cell proliferation. The most striking effect was seen in the use of Abs against all 3 costimulatory molecules, which resulted in greater than 95% reduction of T-cell proliferation (Figure 3A-B). However, evaluation of 5 patient samples has demonstrated that the effect of blocking the function of each costimulatory molecule varied among patients. Even though blocking of LFA-3 alone appears to be slightly more efficacious than blocking of either B7-1 or ICAM-1 (see Figure 3C), the highest inhibition of proliferation was consistently seen when all 3 costimulatory molecules were blocked.
Blocking of allogeneic T-cell proliferation in response to MVA-TRICOM–infected CLL cells. (A-B) Allogeneic T cells from healthy donors were stimulated in the presence of MVA-TRICOM–infected CLL cells (patients 15 and 16, respectively) that were preincubated in the presence of 5 μg/mL monoclonal Ab directed against B7-1, ICAM-1, or LFA-3, or combination of Abs (as indicated in the figure). Error bars represent the standard error of the mean (SEM) for triplicate determinations. (C) Percentage of inhibition of proliferation of allogeneic T cells in response to MVA-TRICOM–infected CLL cells in the presence of various Abs or combination of Abs, for patients 16 (○); 12 (•); 18 (□);5(▪); 15 (▵). The line represents the average for the 5 patients analyzed. Statistical comparison between groups was based on Student 2-tailed t test.
Blocking of allogeneic T-cell proliferation in response to MVA-TRICOM–infected CLL cells. (A-B) Allogeneic T cells from healthy donors were stimulated in the presence of MVA-TRICOM–infected CLL cells (patients 15 and 16, respectively) that were preincubated in the presence of 5 μg/mL monoclonal Ab directed against B7-1, ICAM-1, or LFA-3, or combination of Abs (as indicated in the figure). Error bars represent the standard error of the mean (SEM) for triplicate determinations. (C) Percentage of inhibition of proliferation of allogeneic T cells in response to MVA-TRICOM–infected CLL cells in the presence of various Abs or combination of Abs, for patients 16 (○); 12 (•); 18 (□);5(▪); 15 (▵). The line represents the average for the 5 patients analyzed. Statistical comparison between groups was based on Student 2-tailed t test.
Autologous T-cell responses to MVA-TRICOM–infected CLL cells
T cells isolated from peripheral blood of patients with CLL were stimulated in the presence of autologous CLL cells that were uninfected or infected with MVA-WT or MVA-TRICOM vector. A total of 10 CLL patient samples were analyzed (Table 6). No autologous T-cell proliferation was observed when uninfected CLL cells or CLL cells infected with the MVA-WT vector (at 1:2.5 stimulator–T cell ratio) were used as stimulators. CLL cells modified by infection with MVA-TRICOM, however, induced autologous T-cell proliferation when compared with uninfected CLL cells. The level of proliferation of autologous T cells in response to MVA-TRICOM–infected CLL cells, however, varied among patients. As shown in Table 6, strong proliferation in response to MVA-TRICOM–infected CLL cells was observed in 4 of 10 patients analyzed (patients 2, 8, 16, and 13), intermediate proliferation in 2 of 10 patients (patients 14 and 12), and low proliferation in 4 of 10 patients (patients 19, 10, 18, and 17).
Autologous T-cell proliferation in the presence of MVA-TRICOM–infected CLL cells
Patient, stimulator cells . | Autologous stimulation index . |
|---|---|
| 2 | |
| Uninfected | 1.0 |
| MVA-WT | 0.5 |
| MVA-TRICOM | 34.8 |
| 8 | |
| Uninfected | 1.3 |
| MVA-WT | 1.6 |
| MVA-TRICOM | 30.6 |
| 16 | |
| Uninfected | 1.7 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 27.2 |
| 13 | |
| Uninfected | 1.1 |
| MVA-WT | 1.0 |
| MVA-TRICOM | 13.4 |
| 14 | |
| Uninfected | 1.3 |
| MVA-WT | 0.7 |
| MVA-TRICOM | 5.3 |
| 12 | |
| Uninfected | 1.1 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 4.2 |
| 19 | |
| Uninfected | 1.7 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 2.3 |
| 10 | |
| Uninfected | 0.7 |
| MVA-WT | NA |
| MVA-TRICOM | 2.2 |
| 18 | |
| Uninfected | 1.0 |
| MVA-WT | 1.1 |
| MVA-TRICOM | 1.9 |
| 17 | |
| Uninfected | 1.1 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 1.4 |
Patient, stimulator cells . | Autologous stimulation index . |
|---|---|
| 2 | |
| Uninfected | 1.0 |
| MVA-WT | 0.5 |
| MVA-TRICOM | 34.8 |
| 8 | |
| Uninfected | 1.3 |
| MVA-WT | 1.6 |
| MVA-TRICOM | 30.6 |
| 16 | |
| Uninfected | 1.7 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 27.2 |
| 13 | |
| Uninfected | 1.1 |
| MVA-WT | 1.0 |
| MVA-TRICOM | 13.4 |
| 14 | |
| Uninfected | 1.3 |
| MVA-WT | 0.7 |
| MVA-TRICOM | 5.3 |
| 12 | |
| Uninfected | 1.1 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 4.2 |
| 19 | |
| Uninfected | 1.7 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 2.3 |
| 10 | |
| Uninfected | 0.7 |
| MVA-WT | NA |
| MVA-TRICOM | 2.2 |
| 18 | |
| Uninfected | 1.0 |
| MVA-WT | 1.1 |
| MVA-TRICOM | 1.9 |
| 17 | |
| Uninfected | 1.1 |
| MVA-WT | 1.3 |
| MVA-TRICOM | 1.4 |
CD3+ T cells from patients with CLL were stimulated with uninfected, MVA-WT-, or MVA-TRICOM–infected CLL cells, at a ratio of stimulator to effector cells equal to 1:2.5. Proliferation was measured as 3H-thymidine incorporation on day 5 of culture. Stimulation index was calculated as cpm (T cells + CLL cells)/cpm (T cells alone).
NA indicates not available.
The increase in autologous T-cell proliferative responses observed using MVA-TRICOM–infected CLL cells was also correlated with increases in the production of T-cell cytokines. Supernatants from the lymphocyte cultures were collected and assayed for various cytokines. As shown in Table 7, production of IFN-γ increased after T cells were stimulated with autologous MVA-TRICOM–infected CLL cells, as compared with T cells stimulated with uninfected CLL cells, in 4 of 7 patient samples analyzed. T cells from patients who exhibited intermediate or low proliferation in response to MVA-TRICOM–infected CLL cells, however, did not produce IFN-γ after stimulation.
IFN-γ production by T cells from patients with CLL stimulated with autologous CLL cells
. | . | IFN-γ (pg/mL) . | . | |
|---|---|---|---|---|
| Patient . | Proliferation in response to MVA-TRICOM . | Uninfected . | MVA-TRICOM . | |
| 8 | High | 0.0 | 748.2 | |
| 7 | High | 43.8 | 332.8 | |
| 11 | High | 37.4 | 220.8 | |
| 16 | High | 12.9 | 80.8 | |
| 18 | Low | 41.6 | 50.2 | |
| 14 | Intermediate | 0.0 | 0.0 | |
| 10 | Low | 0.0 | 0.0 | |
. | . | IFN-γ (pg/mL) . | . | |
|---|---|---|---|---|
| Patient . | Proliferation in response to MVA-TRICOM . | Uninfected . | MVA-TRICOM . | |
| 8 | High | 0.0 | 748.2 | |
| 7 | High | 43.8 | 332.8 | |
| 11 | High | 37.4 | 220.8 | |
| 16 | High | 12.9 | 80.8 | |
| 18 | Low | 41.6 | 50.2 | |
| 14 | Intermediate | 0.0 | 0.0 | |
| 10 | Low | 0.0 | 0.0 | |
CD3+ T cells from patients with CLL were stimulated (ratio of stimulator to effector cells, 1:2.5) with autologous CLL cells uninfected or infected with MVA-TRICOM. Supernatants were analyzed at 48 hours after stimulation.
IFN-γ indicates interferon γ.
CTL generation, proliferation, and cytotoxicity against CLL targets
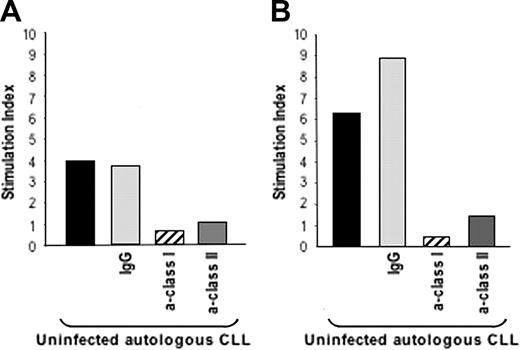
We then investigated whether T cells that have been activated to proliferate in response to autologous MVA-TRICOM–infected CLL cells could then be activated in response to uninfected/unmodified tumor cells. Repeated weekly stimulation of autologous T cells was performed as described in “Patients, materials, and methods.” T-cell lines could be established from 5 of 6 patients with CLL. After expansion for 3 IVS in the presence of irradiated autologous MVA-TRICOM–infected CLL cells, as described in “Patients, materials, and methods,” T cells were incubated with autologous uninfected CLL cells, and their ability to proliferate as well as their cytotoxic function was evaluated. Figure 4A-B shows the proliferative responses of 2 established CD3+ T-cell cultures from 2 patients with CLL in response to autologous uninfected CLL cells. Proliferation was abolished when antibodies against either class I or class II MHC molecules were added into the assay, but not when a control IgG of irrelevant specificity was used. These observations indicate that both class I–associated and class II–associated tumor antigens were being presented and recognized by the autologous T cells.
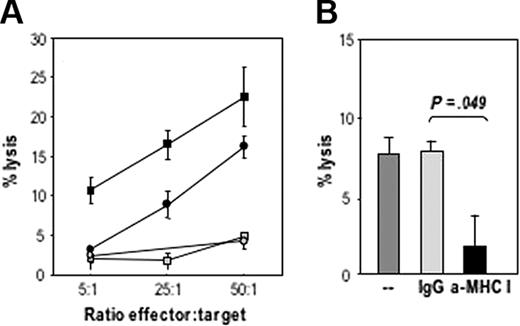
The cytotoxic activity of expanded CD8+ T cells against uninfected CLL cells was first evaluated in a 16-hour 111In release assay (data not shown). Higher specific lysis of targets was observed (60% at an E/T ratio of 50:1), although spontaneous release was also elevated. Lysis of uninfected CLL cells was then evaluated in a 5-hour 111In release assay, at various E/T ratios. Cytotoxic T-cell responses could be induced after repeated in vitro stimulation of autologous T cells in the presence of MVA-TRICOM–infected CLL cells. The intensity of the observed responses, however, varied among T cells expanded from different patients, from 4% to 25% of specific lysis of targets, at the highest ratio of effectors to target cells (Figure 5A). Ab blocking experiments demonstrated the MHC class I restriction of the cytotoxic T lymphocytes (CTL)–mediated killing (Figure 5B). Thus, these studies support the MHC restriction results in Figure 4A-B. It should be pointed out that by analyzing CTL-mediated lysis in short-term 111In release assays (5 hours), one could be prevented from observing apoptosis of CLL cells after incubation with T cells.
Cytotoxic activity of expanded CD4+ T cells from patient 7 was also evaluated. No lysis of uninfected autologous CLL cells was observed in a 5-hour or a 16-hour 111In release assay. However, the CD4+-expanded T cells were able to proliferate in response to uninfected CLL cells, and the proliferation was blocked with anti–class II Ab, with results similar to those shown in Figure 4A-B.
In vitro–expanded T cells were able to proliferate in response to uninfected autologous CLL cells. T cells expanded in vitro after 3 rounds (21 days) of stimulation with autologous MVA-TRICOM–infected CLL cells and exogenous IL-2 were incubated in the presence of autologous, uninfected CLL cells. Panels A and B correspond to the results with 2 different T-cell cultures, established from 2 different patients with CLL (patients 8 and 11, respectively). Abs (20 μg/mL) directed against MHC class I, class II; or an IgG control were added to the uninfected CLL cells for 1 hour before the addition of the T cells. Results are expressed as Stimulation index, calculated as cpm (T cells + CLL cells) / cpm (T cells alone).
In vitro–expanded T cells were able to proliferate in response to uninfected autologous CLL cells. T cells expanded in vitro after 3 rounds (21 days) of stimulation with autologous MVA-TRICOM–infected CLL cells and exogenous IL-2 were incubated in the presence of autologous, uninfected CLL cells. Panels A and B correspond to the results with 2 different T-cell cultures, established from 2 different patients with CLL (patients 8 and 11, respectively). Abs (20 μg/mL) directed against MHC class I, class II; or an IgG control were added to the uninfected CLL cells for 1 hour before the addition of the T cells. Results are expressed as Stimulation index, calculated as cpm (T cells + CLL cells) / cpm (T cells alone).
Specific CTL-mediated lysis of autologous CLL cells. CD8+ isolated T cells that were in vitro expanded by 3 rounds of stimulation with autologous MVA-TRICOM–infected CLL cells and exogenous IL-2 were assessed for their ability to lyse autologous, uninfected 111In-labeled CLL cells. (A) Four different T-cell cultures established from 4 CLL patients (patients 6, 7, 8, and 9) were assayed. (B) Abs (20 μg/mL) directed against MHC class I or an IgG control were added to 111In-labeled target cells for 1 hour before the addition of the T cells (patient 8). Statistical comparison between groups was based on Student 2-tailed t test. Error bars represent the SEM for triplicate determinations.
Specific CTL-mediated lysis of autologous CLL cells. CD8+ isolated T cells that were in vitro expanded by 3 rounds of stimulation with autologous MVA-TRICOM–infected CLL cells and exogenous IL-2 were assessed for their ability to lyse autologous, uninfected 111In-labeled CLL cells. (A) Four different T-cell cultures established from 4 CLL patients (patients 6, 7, 8, and 9) were assayed. (B) Abs (20 μg/mL) directed against MHC class I or an IgG control were added to 111In-labeled target cells for 1 hour before the addition of the T cells (patient 8). Statistical comparison between groups was based on Student 2-tailed t test. Error bars represent the SEM for triplicate determinations.
The results demonstrated that MVA-TRICOM–infected CLL cells could be used for the generation of autologous T-cell immune responses, and that these T cells could mediate killing of unmodified autologous CLL cells.
Discussion
In previous studies, CD40L activation has been used to up-regulate expression of various costimulatory molecules on the surface of CLL cells and, as a consequence, to enhance their ability to present antigens to T cells.37 Coculture of CLL cells in the presence of CD40L+ feeder cells12,13,15,17 as well as direct gene transfer of CD40L into CLL cells via adenovirus vectors9,14 have been used. A phase 1 clinical trial studying administration of a single intravenous infusion of adenovirus-CD40L–transduced autologous leukemia cells to patients with CLL has shown increases in absolute blood T-cell counts as well as in the number of leukemia-specific T cells after treatment.9 These results support the concept that increasing immunogenicity of CLL cells could be a path to overcome the host immune tolerance against the tumor cells.
Gene transfer into CLL cells is a difficult procedure with the currently available gene transfer techniques, such as electroporation, lipofection, and viral vector systems.38-40 For instance, very high multiplicities of infection (MOI from 500 to 1000) of adenovirus vectors are needed to achieve sufficient gene transfer into CLL cells9,14,16,41 ; some investigators have postulated that low levels of adenovirus receptor and coreceptors are responsible for the refractoriness of CLL cells to adenovirus infection.16 In the search for alternate vector systems that could efficiently be used for ex vivo modification of leukemia cells, we have evaluated the efficiency of poxvirus vectors for infection and transgene expression into CLL cells. We have compared various poxvirus vectors encoding for the human TRICOM molecules (B7-1, ICAM-1, and LFA-3) for direct modification of CLL cells in vitro and subsequently studied the potential of TRICOM-modified leukemia cells for antigen presentation to T cells.
In a previous report,24 we have shown that freshly isolated human B cells from healthy individuals can be turned into more efficient APCs to activate antigen-specific human T cells via infection with the nonreplicative rF-TRICOM vector. This vector markedly increases the surface expression of B7-1 and moderately increases the expression of ICAM-1 and LFA-3 on normal B cells. We have also reported32 that murine splenocytes (greater than 80% B cells) could be efficiently infected with the rF-TRICOM vector (encoding for the murine TRICOM molecules) to enhance their APC potency up to the level of DCs. The same rF-TRICOM (murine) vector was used for modification of murine A20 lymphoma cells, which were successfully used as a tumor vaccine to promote immunity in a murine B-cell lymphoma model.33 In the studies reported here, however, the recombinant fowlpox rF-TRICOM vector was ineffective in enhancing the immunogenicity of CLL cells. One possible explanation for this is that CLL cells infected with rF-TRICOM expressed B7-1 but little, if any, ICAM-1 and LFA-3 above the levels of the uninfected CLL cells.
Because patients with CLL are commonly immunocompromised individuals, and adverse reactions could potentially arise from the use of a replicating virus vector, such as vaccinia, we used an inactivated vector, that is, rV-TRICOM treated with psoralen and UV light irradiation.30,31 The procedure, which does not affect transcription of early genes, has been previously used for viral inactivation of vaccinia recombinants administered to patients with metastatic melanoma in a phase 1-2 clinical trial.35 The nonreplicative vector PUVA-rV-TRICOM was capable of inducing expression of B7-1, ICAM-1, and LFA-3 in the infected CLL cells. It was also capable of enhancing the immunogenicity of infected CLL cells, although results varied among different patients.
MVA is a highly attenuated strain of vaccinia virus that does not replicate productively in human cells and has been used in the final stages of the smallpox eradication campaign in Germany and Turkey, showing efficacy and no undesired effects in a large number of vaccinated individuals, even in those who were immunocompromised.26,42,43 The safety of MVA has been confirmed in animal studies showing no toxicities in immunosuppressed non–human primates that have received high doses of MVA.44 Results from a clinical trial have ascertained the safety of MVA for vaccination of individuals infected with HIV-1 with possible immune deficiencies.43,45 Thus, the safety profile of MVA makes it an ideal vector to use in immunotherapeutic approaches in patients with CLL. The recombinant MVA-TRICOM has proven to be the most efficient of the vectors compared in the studies reported here for in vitro infection of CLL cells. A multiplicity of infection as low as 5 MOI of the vector was able to markedly increase the expression of B7-1, ICAM-1, and LFA-3 on the surface of CLL cells. The data in Figure 2A-B demonstrated that there are actually 2 populations of CLL cells after infection with MVA-TRICOM, which express either low or high levels of each costimulatory molecule, respectively. It should be pointed out that the low MFI population does not reflect uninfected cells, because both the percentage of positive cells and the MFI value for one or more costimulatory molecules encoded by the TRICOM vector are greater in this population than those observed for uninfected or control vector-infected cells. The reason for the appearance of 2 populations after infection with MVA-TRICOM is unknown at the present time but may well reflect the heterogeneity in the CLL population for any given patient.
In a previous report from our laboratory we have used MVA vectors encoding for the murine TRICOM molecules for induction of potent and effective antigen-specific T-cell responses in mouse preclinical models.46 Here, we have shown that MVA-TRICOM–infected CLL cells were highly efficient in stimulating proliferation of allogeneic and autologous T cells. Results from the Ab-blocking experiments clearly indicated the participation of B7-1, ICAM-1, and LFA-3 in boosting the APC function of the CLL cells, as blocking of any individual molecule resulted in significant loss of T-cell proliferation.
The rationale for a therapeutic use of MVA-TRICOM–infected CLL cells as a whole tumor-cell vaccine is that they could potentially induce a host immune response against the tumor. Induction of autologous antileukemia-specific T cells was observed when MVA-TRICOM–infected CLL cells were used in vitro to repeatedly stimulate autologous T cells. Furthermore, these leukemia-specific T cells proliferated in response to uninfected autologous CLL cells and mediated lysis of unmodified/uninfected autologous CLL cells. The fact that T cells, expanded in the presence of MVA-TRICOM–infected CLL cells, were able to proliferate and lyse uninfected leukemia cells clearly indicates that leukemia-specific T cells have been expanded.
We have analyzed patient characteristics (Table 1) for correlations with the various biologic parameters described in this study. First, MVA-TRICOM was able to increase expression of the encoded B7-1, ICAM-1, and LFA-3 molecules in 19 of 19 CLL samples analyzed, independent of the patients' characteristics. Second, proliferation of allogeneic T cells was highly enhanced in response to MVA-TRICOM–infected CLL cells for all 12 patients analyzed here. Finally, even though results on autologous T-cell proliferation varied among patients, there was no obvious correlation between the level of autologous T-cell proliferation and any of treatment status, disease status, stage, CD38 expression, or chromosomal aberrations. Thus, unfortunately, at this time there is no obvious clinical correlate with the ability of MVA-TRICOM to enhance the proliferation of allogeneic or autologous T cells in response to CLL cells.
Numerous preclinical studies have shown that introduction of costimulatory molecules into tumor cells enhances their immunogenicity for use as antitumor vaccines.47,48 We have previously demonstrated that poxvirus vectors could be used for expression of murine B7-1 in both live and X-irradiated whole tumor-cell vaccines, and effective antitumor responses were shown when the poxvirus-modified tumor cells were used as vaccines in mouse preclinical studies.49 One of the concerns in the use of a vaccine containing a poxvirus vector is that previous immunity via the smallpox vaccine would decrease the effectiveness of the vaccine after subsequent vaccinations. In the case of a whole tumor-cell vaccine infected prior to administration with a virus such as MVA, immune responses to MVA should not play a negative role in the efficacy of the vaccine because MVA is nonreplicating. Moreover, other investigators have reported that MVA is less immunogenic than other virulent poxvirus, and much lower cellular responses are elicited against MVA antigens and significantly higher responses against a foreign MVA-encoded antigen.50
These findings thus suggest that MVA-TRICOM can potentially be used for the therapy of CLL by either ex vivo infection of CLL cells as a vaccine or via direct intranodal or intravenous injection of MVA-TRICOM in patients with CLL.
Prepublished online as Blood First Edition Paper, August 4, 2005; DOI 10.1182/blood-2005-03-1214.
Supported by a grant from the Mario Lemieux Foundation (K.A.F.) and by the Intramural Research Program of the National Cancer Institute (NCI), National Institutes of Health. The Laboratory of Tumor Immunology and Biology, NCI, has a Collaborative Research and Development Agreement (CRADA) with Therion Biologics Corporation.
Several of the authors (D.P., A.G.Y., and J.C.) are employed by a company (Therion Biologics Corp) whose potential product was studied in the present work. Some of the authors (J.S., D.P., and J.W.H.) hold patents relating to a potential product.
C.P. and K.Y.T. designed and performed the research, analyzed the data, and wrote the paper; K.A.F. designed the research, contributed vital new reagents/analytic tools, and analyzed the data; D.P., A.G.Y., and J.C. contributed vital new reagents/analytic tools; J.W.H. designed and performed the research; and J.S. designed the research, analyzed the data, and wrote the paper.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Michelle Mail and James Monroe for their effort in constructing the viruses used in this study, and Debra Weingarten for her editorial assistance in the preparation of the manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal