Abstract
It has been proposed that direct and indirect mechanisms contribute to the unresolved issue of CD4+ T-cell depletion that results from HIV-1 infection. We recently reported that plasma levels of tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) are elevated in HIV-1–infected patients and that they correlate with viral load. The present study investigates the expression of TRAIL death receptor 5 (DR5) in the peripheral-blood mononuclear cells (PBMCs) of HIV-1–infected patients and its role in CD4+ T-cell death. DR5 expression was elevated and associated with the apoptotic marker annexin V. Apoptosis was reduced in CD4+ T cells when cultured with anti-DR5 antibody. CD4+, but not CD8+, T cells from uninfected donors expressed TRAIL, DR5, and activated caspase-3 when cultured with infectious or noninfectious HIV-1, resulting in preferential apoptosis of CD4+ T cells. TRAIL, caspase-3 expression, and apoptosis were type 1 interferon (IFN) dependent. Induction of apoptosis and DR5 expression required glycoprotein 120 (gp120)–CD4 interaction. Finally, we analyzed DR5 expression by CD4+ T cells in highly active antiretroviral therapy (HAART)–treated patients. The decreased viral loads and increased CD4 counts of HAART-responsive patients were associated with a decrease in DR5 mRNA expression by CD4+ T lymphocytes. We propose a novel model in which a type 1 IFN–regulated TRAIL /DR5 mechanism induces apoptosis of HIV-1–exposed CD4+ T cells.
Introduction
The pathogenic mechanisms responsible for CD4+ T-cell depletion in AIDS are not completely understood because evidence supports direct and indirect mechanisms for loss of this key lymphocyte population. During primary infection, a high frequency of CD4+ T cells is infected by HIV-1, and lysis or immunologic clearance of these infected cells accounts for the substantial early depletion of CD4+ T cells, particularly when mucosal tissues are sampled.1,2 Thus, direct killing of infected cells appears to contribute to the loss of CD4+ T cells in primary HIV-1 infection. However, other observations suggest that immune mechanisms contribute to HIV-1–induced death of CD4+ T cells.3,4 Apoptosis of uninfected CD4+ T cells was suggested as a mechanism,5 particularly during the chronic stage of infection and during progression to AIDS. Because the loss of circulating CD4+ T cells during HIV-1 disease progression is greater than that of CD8+ T cells, we were interested in mechanisms of T-cell death that might preferentially affect CD4+ T cells.
The Fas/Fas ligand (FasL) apoptotic pathway has been studied extensively and was suggested as a mechanism that contributes to apoptosis of CD4+ T cells in AIDS.6 Several models showed that CD4 cross-linking and Fas ligation resulted in apoptosis of CD4+ and CD8+ T cells.7-9 However, death mechanisms other than Fas/FasL may contribute to apoptosis of CD4+ T cells during AIDS.10,11 Because the primary immunologic consequence of HIV-1 infection is CD4+ T-cell depletion, our objective was to develop a model that selectively affects CD4+ T cells on exposure to HIV-1.
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a TNF superfamily member,12 induces the apoptosis of virus-infected13 and tumor cells.14 TRAIL has 2 death receptors capable of inducing apoptosis15 (DR4 and DR5), and 3 other receptors that engage ligand without initiating apoptosis.16 TRAIL protein is expressed on cell membrane or secreted, and both the soluble and membrane-bound forms induce the apoptosis of cells expressing death receptors.17
Several studies suggested a role for TRAIL in the apoptosis of CD4+ T cells in HIV infection. For example, CD4+ and CD8+ T cells from HIV-1–infected patients were more susceptible to TRAIL-induced apoptosis in vitro than T cells from healthy donors.18-20 TRAIL induced selective apoptosis of uninfected CD4+ T cells in HIV-1–infected human peripheral-blood leukocyte–nonobese diabetic–severe combined immunodeficient (hu-PBL-NOD-SCID) mice.21 TRAIL produced by monocytes exposed to the HIV-1 transactivating (Tat) protein resulted in the apoptosis of uninfected CD4+ T cells.22 HIV-1–positive encephalitic brain tissue contained TRAIL-expressing macrophages and neurons that expressed TRAIL death receptors.23 Moreover, we recently reported that plasma TRAIL levels in HIV-1–infected patients directly correlate with viral load, suggesting that this pathway contributes to CD4+ T-cell depletion in AIDS.24 However, the expression and regulation of TRAIL death receptors on primary T lymphocytes in HIV-1–infected patients remain to be established.
The TRAIL gene is regulated by type 1 interferon (IFN)–α/β.25 IFN-α/β is produced mainly by plasmacytoid dendritic cells (pDCs)26 and has broad antiviral activity, including activity against HIV-1.27 Therefore, IFN-α/β may contribute to TRAIL-mediated apoptosis of virus-exposed cells. Less than 1% of HIV-1 virions in plasma is typically associated with culturable infectivity,28,29 and exposure of peripheral-blood mononuclear cells (PBMCs) to chemically inactivated virions induces T-cell death.30 We recently reported that HIV-1 virions chemically inactivated by treatment with aldrithiol-2 (AT-2 HIV-1), a process that maintains the structural and functional integrity of the viral envelope glycoproteins,31 induce TRAIL production by monocytes.24 Furthermore, studies of lymphoid tissues suggest that apoptosis of CD4+ T cells in HIV-1–infected persons and simian immunodeficiency virus (SIV)–infected macaques occurs mainly in uninfected T-helper cells.5 We hypothesize that exposure to noninfectious HIV-1 particles induces type 1 IFN–dependent, TRAIL/DR5-mediated selective apoptosis of uninfected CD4+ T cells.
We report here that the percentage of apoptotic CD4+ T cells expressing DR5 was higher in HIV-1–infected patients than in uninfected donors and that blocking anti-DR5 antibodies decreased the apoptosis of CD4+ T cells isolated from patients. In vitro experiments demonstrated that infectious and noninfectious HIV-1 virions induced expression of TRAIL and activated caspase-3 and DR5 by CD4+ but not CD8+ T cells. This selective expression of TRAIL/DR5 resulted in preferential apoptosis of CD4+ T cells, an effect that was blocked by anti-TRAIL or anti-DR5 antibodies. Expression of TRAIL, activated caspase-3, and apoptosis was inhibited by anti–IFN-α/β antibodies. pDCs were responsible for most of the IFN-α/β production in our model. HIV-1–induced apoptosis and DR5 expression in CD4+ T cells required glycoprotein 120 (gp120)–CD4 interaction but not coreceptor binding. To evaluate the clinical relevance of TRAIL-mediated CD4+ T-cell death in AIDS, we analyzed blood samples of patients receiving highly active antiretroviral therapy (HAART). The observed treatment-associated increase in CD4+ T cells paralleled a decrease in the DR5/CD4 mRNA ratio in T cells from 6/7 HAART-responsive patients.
Patients, materials, and methods
HIV-1–infected patients
Blood was collected from 22 HIV-1–infected patients and 16 uninfected donors. In addition, 8 patients from a different cohort involved in a longitudinal study of HAART were followed up for 40 weeks. These 8 patients received the combination of tenofivir (Viread; Gilead Sciences, Foster, City, CA), stavudine (Zerit; Bristol-Myers Squibb, New York, NY), and lopinavir/ritonavir (Kaletra; Abbott Laboratories, North Chicago, IL) or the combination of zidovudine/lamivudine/abacavir (Trizivir; GlaxoSmithKline), zidovudine/lamivudine (Combivir; GlaxoSmithKline, Research Triangle Park, NC), and efavirenz (Sustiva; DuPont Pharmaceuticals, Wilmington, DE). Plasma HIV-1 RNA was measured by a quantitative reverse transcription–polymerase chain reaction (RT-PCR) assay; all data are expressed as copies per milliliter (Amplicor Monitor; Roche Diagnostic Systems, Branchburg, NY; detection limit, 50 copies/mL). All patients were studied under institutional review board (IRB)–approved protocols and gave written, informed consent on forms from the National Cancer Institute and Wilford Medical Center.
Isolation and culture of T cells and pDCs
All in vitro experiments were performed using PBMCs isolated by Ficoll density centrifugation from blood obtained from HIV-1–seronegative blood bank donors. Cells were cultured in RPMI 1640 medium (Invitrogen, Gaithersburg, MD) containing 10% fetal bovine serum (Sigma, St Louis, MO) and 1% Pen-Strep-Glut (Invitrogen). CD4+ and CD8+ cells were purified from PBMCs by using positive or negative selection kits (Miltenyi Biotech, Auburn, CA). The purity of CD3+ CD4+ and CD3+ CD8+ cells after enrichment was greater than 98%. Similar results were obtained using positive or negative selection. T cells from the HAART patients were obtained by negative selection, using adherence, followed by treatment with a cocktail of lytic antibodies and complement (T-Qwick; One-Lambda, Los Angeles, CA). Enriched T-cell subpopulations were greater than 90% positive for the selected marker, as assessed by flow cytometry. Plasmocytoid dendritic cells (CD4+ CD123+ BDCA-2+) were isolated from PBMCs using the BDCA-2 isolation kit (Miltenyi Biotech).
Preparation of noninfectious AT-2 HIV
HIV-1MN (X4-tropic) and HIV-1Ada (R5-tropic) were produced and inactivated with 1 mM AT-2 for 18 hours at 4°C, as described.31 Elimination of detectable infectivity of HIV-1 virions by such treatment has been documented32 and was verified for the AT-2 HIV-1 preparations used. Noninfectious AT-2 HIV-1 retains functionally intact envelope glycoproteins and interacts with cell receptors.31 Microvesicles, isolated from uninfected cell cultures matched to the cultures to produce the virus, were used as negative controls.33
Cultures of leukocytes with HIV-1
Isolated CD4+ and CD8+ cells were cultured with noninfectious AT-2 HIV-1MN or AT-2 HIV-1Ada at 500 ng/mL p24CA equivalent. Infectious HIV-1MN and HIV-1Ada were used at the same concentration. We verified infection in the CD4+ cells cultured with infectious HIV-1 and absence of infection in the cells cultured to AT-2 HIV-1 by p24 enzyme-linked immunosorbent assay (ELISA) of supernatants (Beckman-Coulter, Miami, FL) after 3 days of culture.
Type 1 IFN and soluble TRAIL detection
Type 1 IFN levels were detected by ELISA (R&D Systems, Minneapolis, MN). Soluble TRAIL was detected using Function ELISA TRAIL Kit (Active Motif, Carlsbad, CA).
Apoptosis
Cells were washed in annexin buffer and incubated for 20 minutes with medium only (negative control) or with fluorescein isothiocyanate (FITC)–conjugated annexin V (Caltag, Burlingame, CA) at room temperature. After 2 washes, propidium iodide (100 μg/mL) (Caltag) was added, and analysis was performed by flow cytometry using FACSCalibur (Becton Dickinson, San Jose, CA).
Activated caspase-3 levels in CD4+ T cells cultured 6 hours with AT-2 HIV-1MN were determined using the anti–active caspase 3 kit (PharMingen, San Jose, CA).34 The antibody used is specific for the activated cleaved form of caspase-3.
Membrane TRAIL and DR5 expression by T cells
Isolated CD4+ and CD8+ cells were cultured for 24 hours in the absence or presence of AT-2 HIV-1MN or AT-2 HIV-1Ada. Membrane TRAIL and DR5 expression was determined by incubating cells for 20 minutes at room temperature with phycoerythrin (PE)–conjugated mouse immunoglobulin G1 (IgG1) anti–human TRAIL monoclonal antibody (RIK-2), anti-DR5 monoclonal antibody (eBioscience, San Diego, CA), or control isotype-matched antibodies (at 5 μg/mL each) in phosphate-buffered saline (PBS) containing 2% mouse serum (Sigma). Cells were also incubated with anti–CD3-allophycocyanin (APC) (Becton Dickinson). Samples were run on FACSCalibur (Becton Dickinson), and 50 000 CD3+ events were acquired using CellQuest software (Becton Dickinson) and analyzed using FlowJo software (Ashland, OR). Results are given for standard error difference (SED) positive cells. Confidence in difference was greater than 99.9% for each experiment.
Real-time PCR
Total RNA was extracted from CD4+ cells after 12 hours of culture with HIV-1MN using the acid guanidinium thiocyanate-phenol-chloroform method,35 modified for TRIzol (Invitrogen). For the HAART longitudinal study, RNA was extracted from T cells from the 9 patients. The use of CD4 mRNA in place of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA for the HAART study was necessary to avoid variations of the internal standard because of changes in the CD8+ T-cell number. Real-time PCR was conducted with the ABI Prism 7900HT (Applied Biosystems, Foster City, CA) using SYBR green PCR mix (QuantiTect SYBR Green PCR; Qiagen, Valencia, CA), with the following thermal profile: denaturation at 95°C for 15 seconds, annealing at 60°C (61°C for TRAIL) for 15 seconds, extension at 72°C for 15 seconds. Primer sequences were designed using Primer Express Software version 2.0 (Applied Biosystems, Foster City, CA) (TRAIL forward, 5-GCTCTGGGCCGCAAAAT-3; TRAIL reverse, 5-TGCAAGTTGCTCAGGAATGAA-3; DR-5 forward, 5-GGGCCACAGGGACACCTT-3; DR-5 reverse, 5-GCATCTCGCCCGGTTTT-3; GAPDH forward, 5-CCACCCATGGCAAATTCC-3; GAPDH reverse, 5-TGGGATTTCCATTGATGACAAG-3; CD4 forward, 5-AAGCATGGAGCATGGGACTG-3; CD4 reverse, 5-TCCATCCTTGACTGGCTTGG-3). Data analysis was performed using SDS2.1 software (Applied Biosystems, Foster City, CA). Standards were obtained by amplification of a control sample in a PCR reaction, using the same primers, reagents, and conditions optimized for the real-time analysis. Results are presented as ratios between the target gene and the GAPDH or CD4 mRNA.
Blocking assays
PBMCs isolated from 5 HIV-1–infected patients were cultured in media supplemented with 50% autologous plasma for 24 and 48 hours in the absence or presence of anti-DR5 antibodies (Ebioscience) at 5 μg/mL. Apoptosis of CD3+CD4+ T cells was analyzed by flow cytometry using annexin V.
CD4+ T cells isolated from PBMCs of healthy donors were cultured for 24 hours with AT-2 HIV-1 and anti-TRAIL (RIK-2)36 monoclonal antibodies at 1, 5, and 10 μg/mL. For analysis of inhibitory effects associated with blockade of DR5, cells were cultured with microvesicles plus anti-DR5 (control), AT-2 HIV-1MN plus anti-DR5 antibody (eBioscience), and AT-2 HIV-1MN plus isotype (eBioscience). After 24 hours, control and HIV-1MN plus anti–DR5-treated cells were stained using an anti–mouse PE antibody (BD Bioscience), and cells cultured with HIV-1MN plus isotype were stained using the anti-DR5 antibody, followed by anti–mouse PE antibody. This technique allowed us to block and then detect cells that were not undergoing apoptosis (annexin V negative) but were expressing DR5 (DR5+). Polyclonal anti–IFN-α/β antibodies were used at 2000 and 500 neutralizing U/mL, respectively (BioSource International, Camarillo, CA). Mouse IgG isotype control antibody (BD Bioscience) was used at 5 μg/mL. Apoptosis was assessed by the annexin V method.
We also tested whether HIV-1–induced apoptosis and DR5 mRNA expression would be inhibited by soluble CD4-IgG (sCD4-IgG) and by AMD-3100 and regulated on activated/normal T-cell expressed and secreted (RANTES). CD4+ cells were cultured for 24 hours with AT-2 HIV-1MN/Ada in the presence of sCD4 (2 μg/mL), AMD-3100 (2 μg/mL) (AIDS Research and Reagents Program, National Institute of Allergy and Infectious Diseases, Bethesda, MD), or RANTES (2 μg/mL). We also performed titration for sCD4 ranging from 0.001 to 10 μg/mL.
Statistical analysis
Experiments were repeated at least 4 times, and probability (P) was calculated using a 2-tailed Student t test. P values of less than .05 were considered statistically significant. The relationship between DR5-mediated apoptosis (DR5+ annexin V+) and all apoptotic cells (annexin V+) in HIV-1 patients was analyzed by linear regression using Excel software version 2003 (Microsoft, Seattle, WA). Univariate distributions of flow cytometric data were performed by probability binning, in 300 bins using FlowJo software.37
Results
Detection of annexin V and DR5 in HIV-1–infected patients and controls
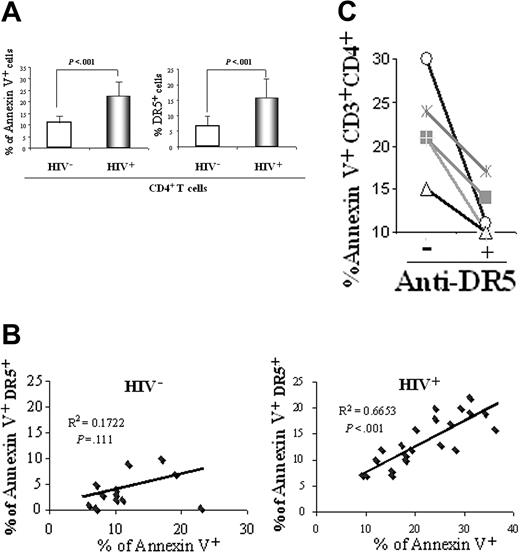
We tested 22 HIV-1–infected patients and 16 healthy, uninfected controls for plasma TRAIL levels. HIV-1–infected patients exhibited increased levels of plasma TRAIL compared with uninfected controls (2617 ± 729 pg/mL vs 730 ± 320 pg/mL; [mean ± SEM]; P = .0001), confirming our previous finding in a separate cohort of HIV-1–infected patients.24 Apoptosis and DR5 expression were measured in CD4+ T cells from patients and controls directly after Ficoll separation and without culture. We also observed an increased percentage of CD4+ T cells that expressed annexin V (23% ± 8%) and DR5 (16% ± 6%) compared with levels of annexin V (11% ± 3%; P = 3 × 10-5) and DR5 expression (7% ± 3%; P = 2 × 10-6) in healthy controls (Figure 1A). To estimate the proportion of CD4+ T cells entering apoptosis because of DR5 expression, we plotted annexin V+ cells versus annexin V+ DR5+ cells. We found a statistically significant correlation between the expression of DR5 and annexin V by CD4+ T cells from HIV-1–infected patients (R2 = 0.67; P = 3 × 10-6; n = 22). In contrast, there was no correlation between these 2 parameters in healthy, HIV-1–seronegative persons (R2 = 0.17; P = .11; n = 16) (Figure 1B). Thus, most apoptotic (annexin V+) CD4+ T cells in these patients expressed the death receptor, DR5, in contrast to the few dying DR5+CD4+ T cells from healthy controls. Furthermore, to determine whether TRAIL/DR5-mediated apoptosis contributes to CD4+ T-cell death in patients, we cultured PBMCs from HIV-1–infected patients for 48 hours with or without a DR5-specific blocking antibody. We found that the percentage of annexin V+ CD4+ T cells was reduced in all 5 patients tested (Figure 1C). Our findings that DR5 expression increased on annexin V+ CD4+ T cells from HIV-1–infected patients and that DR5 antibody blocked CD4+ T-cell apoptosis of HIV-1–infected patients suggested that TRAIL-mediated apoptosis contributes to CD4+ T-cell depletion. Therefore, we performed in vitro experiments and developed a model for HIV-1–induced TRAIL/DR5-mediated apoptosis.
Analysis of apoptosis and DR5 in HIV-1–infected patients and uninfected controls. (A) Flow cytometric analysis of annexin V+ and DR5+ CD4+ T cells from 16 uninfected control donors (HIV-) and 22 HIV-1–infected patients (HIV+). P values were calculated using 2-tailed Student t test. (B) Linear regression analysis of DR5-mediated CD4+ T-cell apoptosis (DR5+annexin V+) compared with all apoptotic CD4+ T cells (annexin V+) performed in the same cohort. (C) PBMCs from 5 HIV-1–infected patients were cultured for 48 hours with (+) or without (-) anti-DR5 blocking antibody. CD3+ CD4+ T cells were analyzed for annexin V by FACS.
Analysis of apoptosis and DR5 in HIV-1–infected patients and uninfected controls. (A) Flow cytometric analysis of annexin V+ and DR5+ CD4+ T cells from 16 uninfected control donors (HIV-) and 22 HIV-1–infected patients (HIV+). P values were calculated using 2-tailed Student t test. (B) Linear regression analysis of DR5-mediated CD4+ T-cell apoptosis (DR5+annexin V+) compared with all apoptotic CD4+ T cells (annexin V+) performed in the same cohort. (C) PBMCs from 5 HIV-1–infected patients were cultured for 48 hours with (+) or without (-) anti-DR5 blocking antibody. CD3+ CD4+ T cells were analyzed for annexin V by FACS.
Annexin V, TRAIL, and DR5 expression by T cells after HIV-1 exposure
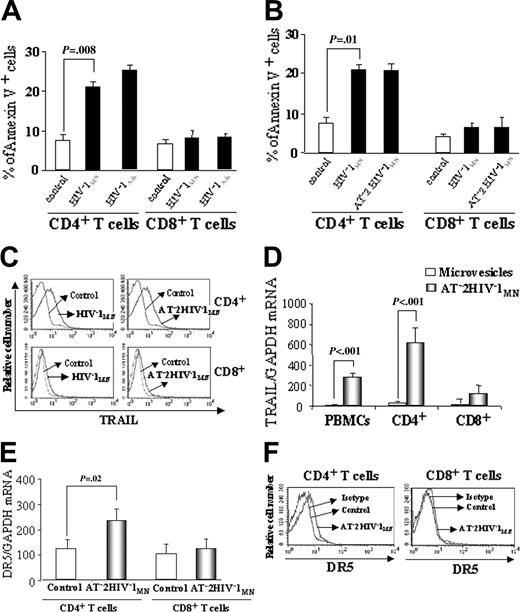
We investigated whether culture of unfractionated PBMCs from HIV-1–seronegative donors with infectious HIV-1 would result in the apoptosis of T cells. We found that culture of PBMCs with infectious HIV-1MN (CXCR4-tropic) or HIV-1Ada (CCR5-tropic) for 24 hours induced annexin V expression by 20% to 26% of CD4+ T cells (P = .008 and P = .006, respectively, compared with control) but by only 9% of CD8+ T cells (P = .09 and .08, respectively, compared with control) (Figure 2A).
We then tested whether isolated CD4+ and CD8+ cells from HIV-1–seronegative donors would also express annexin V when cultured with infectious HIV-1 for 24 hours. This was insufficient time for productive viral replication in our culture system, suggesting a mechanism that involved virus exposure but not viral replication. Furthermore, we compared the effects of culturing CD4+ and CD8+ cells with infectious and noninfectious AT-2 HIV-1. Our experiments indicated that infectious HIV-1MN and AT-2 HIV-1MN were equally effective at inducing annexin V expression in isolated CD4+ T cells, but neither induced significant apoptosis of CD8+ T cells (Figure 2B). These results were similar to those seen for the CD4+ and CD8+ T-cell subsets when unfractionated PBMCs were cultured with infectious HIV-1 (compare Figure 2B and 2A). Thus, CD4+ but not CD8+ T cells expressed annexin V, irrespective of exposure to HIV-1 as unfractionated PBMCs or purified cells or to infectious or noninfectious HIV-1. Preferential apoptosis of CD4+ T cells compared with CD8+ T cells (34% ± 5% vs 12% ± 3%; P = .002) was still seen, even after 3 days of culture with AT-2 HIV-1MN and HIV-1Ada. We also verified that annexin V+ cells underwent the final stages of apoptosis by propidium iodide (PI) staining. CD4+ T cells (24% ± 5%) were annexin V+ and PI+ when cultured for 3 days with infectious or noninfectious HIV-1 (P < .001, compared with control).
We recently reported that HIV-1–infected patients have elevated levels of plasma TRAIL and that culture of monocytes from HIV-1–seronegative donors with infectious or noninfectious HIV-1 induced TRAIL production.24 Therefore, we tested isolated CD4+ and CD8+ cells cultured with HIV-1MN for TRAIL expression. Infectious HIV-1MN and AT-2 HIV-1MN increased TRAIL expression on CD4+ T cells (SED % positive, 69% compared with control; P < .001), but only marginally on CD8+ T cells (SED % positive, 10% compared with control; P = .01) (Figure 2C). AT-2 HIV-1MN induced TRAIL mRNA expression in CD4+, but only marginally in CD8+, cells (Figure 2D).
TRAIL-mediated apoptosis requires the expression of TRAIL death receptor DR4 or DR5.16 We tested whether culture of CD4+ and CD8+ cells with AT-2 HIV-1MN increased DR5 mRNA expression. Figure 2E indicates that DR5 mRNA was increased in CD4+ but not in CD8+ T cells cultured with AT-2 HIV-1MN for 24 hours. We obtained similar data when CD4+ T cells were cultured with infectious HIV-1 (data not shown). AT-2 HIV-1MN did not induce DR4 mRNA expression on either CD4+ or CD8+ cells (data not shown). Importantly, we showed that the culture of CD4+ cells with AT-2 HIV-1MN induced 25% more DR5+ CD4+ T cells than did incubation with control microvesicle preparations (P = .0001) (Figure 2F). In contrast, culture of CD8+ T cells with AT-2 HIV-1MN induced less than a 1% increase of DR5 expression compared with controls (Figure 2F). Thus, AT-2 HIV-1MN induced expression of the DR5 TRAIL death receptor on CD4+ but not on CD8+ T cells.
Role of type 1 IFN and DR5 in T-cell apoptosis
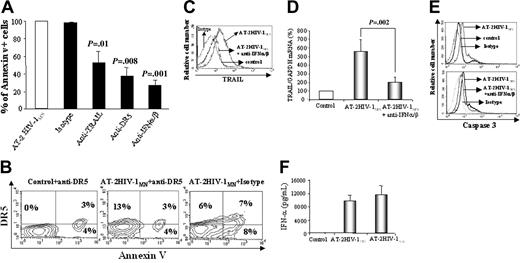
These in vitro results suggest that TRAIL/DR5 contributes to apoptosis induced by culturing CD4+ cells with infectious or noninfectious HIV-1. We then tested whether CD4+ T-cell apoptosis could be blocked by monoclonal antibodies to TRAIL or DR5. TRAIL antibody reduced annexin V+ cells by 50% (compared with isotype control; P = .01), whereas anti-DR5 monoclonal antibody inhibited CD4+ T-cell annexin V positivity by 62% (compared with isotype control; P = .008) (Figure 3A). In contrast, a Fas-specific antibody did not block apoptosis, suggesting that Fas/FasL-mediated death was not involved in our in vitro model of HIV-1–induced CD4+ T-cell apoptosis (data not shown). To show that annexin V expression is a direct consequence of DR5 engagement, we added a DR5 blocking/staining antibody to CD4+ T-cell cultures before or after 24-hour incubation with HIV-1. This technique allowed us to inhibit DR5-positive cells from undergoing apoptosis and to detect them as DR5-positive but annexin V-negative cells. CD4+ T cells were cultured for 24 hours with microvesicles plus anti-DR5 (control), microvesicles plus AT-2 HIV-1 plus anti-DR5 antibody, or AT-2 HIV-1MN plus isotype control (Figure 3B). After 24 hours, control and AT-2 HIV-1 plus anti–DR5-treated cells were stained using an anti–mouse PE-labeled antibody, and cells cultured with AT-2 HIV-1 plus isotype were stained using the anti-DR5 antibody, followed by anti–mouse PE-labeled antibody. Cells were then acquired and analyzed by flow cytometry. Anti-DR5 blocking antibody increased by 2-fold the percentage of CD4+ T cells that were not apoptotic but that expressed DR5+ (Figure 3B, upper left) compared with isotype cultured with HIV-1 (P = .001). Thus, expression of DR5 precedes and is required for HIV-1–mediated apoptosis of CD4+ T cells.
Annexin V, TRAIL, and DR5 expression in T cells. Percentages of annexin V+ cells from HIV-1–uninfected donors determined by flow cytometry in (A) unfractionated PBMCs cultured for 24 hours with microvesicles (control), infectious HIV-1MN, or HIV-1Ada; or (B) isolated CD4+ and CD8+ T cells cultured with microvesicles, or with infectious or AT-2 HIV-1MN. (C) FACS analysis of TRAIL expression on CD4+ and CD8+ T cells cultured for 24 hours with infectious or noninfectious HIV-1MN. (D) TRAIL mRNA expression after 24-hour culture of PBMCs, isolated CD4+ and CD8+ T cells with AT-2 HIV-1MN, or microvesicles. (E) DR5 mRNA expression in CD4+ and CD8+ T cells cultured for 24 hours with AT-2 HIV-1MN or microvesicles. (F) FACS analysis of DR5 expression on CD4+ and CD8+ T cells after 24 hours of culture with AT-2 HIV-1MN (identical results obtained using infectious HIV-1MN; data not shown). Eight percent to 13% of CD4+ T cells expressed DR5, whereas less than 1% of CD8+ T cells did. (A-B,D-E) Mean values with standard errors of 6 independent experiments for each condition tested. (C,F) Representative results of 4 independent experiments. (A-B,E) P values determined by 2-tailed Student t test.
Annexin V, TRAIL, and DR5 expression in T cells. Percentages of annexin V+ cells from HIV-1–uninfected donors determined by flow cytometry in (A) unfractionated PBMCs cultured for 24 hours with microvesicles (control), infectious HIV-1MN, or HIV-1Ada; or (B) isolated CD4+ and CD8+ T cells cultured with microvesicles, or with infectious or AT-2 HIV-1MN. (C) FACS analysis of TRAIL expression on CD4+ and CD8+ T cells cultured for 24 hours with infectious or noninfectious HIV-1MN. (D) TRAIL mRNA expression after 24-hour culture of PBMCs, isolated CD4+ and CD8+ T cells with AT-2 HIV-1MN, or microvesicles. (E) DR5 mRNA expression in CD4+ and CD8+ T cells cultured for 24 hours with AT-2 HIV-1MN or microvesicles. (F) FACS analysis of DR5 expression on CD4+ and CD8+ T cells after 24 hours of culture with AT-2 HIV-1MN (identical results obtained using infectious HIV-1MN; data not shown). Eight percent to 13% of CD4+ T cells expressed DR5, whereas less than 1% of CD8+ T cells did. (A-B,D-E) Mean values with standard errors of 6 independent experiments for each condition tested. (C,F) Representative results of 4 independent experiments. (A-B,E) P values determined by 2-tailed Student t test.
Role of type 1 IFN and DR5 in T-cell apoptosis. (A) Effect of monoclonal RIK-2 anti-TRAIL antibody, anti-DR5 antibody, and anti–IFN-α/β antibodies on the percentages of annexin V+ cells resulting from 24-hour cultures of CD4+ T cells with AT-2 HIV-1MN. (B) Two-color flow cytometric analysis of annexin V/DR5 double-positive CD4+ T cells cultured for 24 hours with microvesicles plus anti-DR5 (control), AT-2 HIV-1MN plus isotype, or AT-2 HIV-1 plus anti-DR5 antibody. Control and AT-2 HIV-1 plus anti-DR5–treated cells were then stained using an anti–mouse PE antibody, and cells cultured with AT-2 HIV-1 plus isotype were stained using an anti-DR5 PE antibody. (Top left) Cells are nonapoptotic but DR5+. (Top right) Cells are apoptotic (annexin V+) and DR5+. (C) Effect of IFN-α/β–specific antibodies on TRAIL expression by CD4+ T cells cultured with AT-2 HIV-1MN. (D) Effect of anti–IFN-α/β antibodies on TRAIL mRNA expression induced by culture with AT-2 HIV-1MN for 24 hours. (E) Activated caspase-3 levels in CD4+ T cells cultured for 6 hours with or without AT-2 HIV-1MN (top panel) and in the presence or absence of anti–IFN-α/β antibodies (bottom panel). (F) IFN-α levels, measured by ELISA, in supernatants of isolated pDCs, cultured for 24 hours with microvesicles, AT-2 HIV-1MN, or AT-2HIV-1Ada. (A,D,F) Mean values (± SD) of 6 experiments. (B-C,E) Representative results of 4 independent experiments. (A,D) P values were determined by 2-tailed Student t test.
Role of type 1 IFN and DR5 in T-cell apoptosis. (A) Effect of monoclonal RIK-2 anti-TRAIL antibody, anti-DR5 antibody, and anti–IFN-α/β antibodies on the percentages of annexin V+ cells resulting from 24-hour cultures of CD4+ T cells with AT-2 HIV-1MN. (B) Two-color flow cytometric analysis of annexin V/DR5 double-positive CD4+ T cells cultured for 24 hours with microvesicles plus anti-DR5 (control), AT-2 HIV-1MN plus isotype, or AT-2 HIV-1 plus anti-DR5 antibody. Control and AT-2 HIV-1 plus anti-DR5–treated cells were then stained using an anti–mouse PE antibody, and cells cultured with AT-2 HIV-1 plus isotype were stained using an anti-DR5 PE antibody. (Top left) Cells are nonapoptotic but DR5+. (Top right) Cells are apoptotic (annexin V+) and DR5+. (C) Effect of IFN-α/β–specific antibodies on TRAIL expression by CD4+ T cells cultured with AT-2 HIV-1MN. (D) Effect of anti–IFN-α/β antibodies on TRAIL mRNA expression induced by culture with AT-2 HIV-1MN for 24 hours. (E) Activated caspase-3 levels in CD4+ T cells cultured for 6 hours with or without AT-2 HIV-1MN (top panel) and in the presence or absence of anti–IFN-α/β antibodies (bottom panel). (F) IFN-α levels, measured by ELISA, in supernatants of isolated pDCs, cultured for 24 hours with microvesicles, AT-2 HIV-1MN, or AT-2HIV-1Ada. (A,D,F) Mean values (± SD) of 6 experiments. (B-C,E) Representative results of 4 independent experiments. (A,D) P values were determined by 2-tailed Student t test.
Type 1 IFN is important for inducing apoptosis of virus-infected cells, including HIV-1–infected cells.27 Therefore, we investigated whether antibodies against IFN-α/β would inhibit AT-2 HIV-1–induced apoptosis and TRAIL expression. These antibodies reduced the percentage of annexin V+ cells by 70% (P = .001 compared with isotype) (Figure 3A). Furthermore, anti–IFN-α/β completely blocked AT-2 HIV-1MN–induced TRAIL protein (Figure 3C) and mRNA expression (Figure 3D). Thus, TRAIL expression and apoptosis of CD4+ T cells induced by HIV-1 in our in vitro experiments are type 1 IFN dependent. We also verified that these CD4+ T cells were activated caspase-3+, a late marker of apoptosis. We showed that caspase-3 activation in AT-2 HIV-1–exposed CD4+ T cells was blocked by anti–IFN-α/β antibodies (Figure 3E).
pDCs were recently shown to be potent producers of type 1 IFN after HIV-1 exposure.38 Therefore, we tested whether isolated pDCs cells would produce IFN-α after exposure to AT-2 HIV-1. We found that the pDCs produced 10 to 11 ng/mL IFN-α per 105 cells after 24-hour exposure to AT-2 HIV-1MN and AT-2 HIV-1Ada (Figure 3F). Similar results were obtained using infectious HIV-1 (data not shown). Up to 1% of our CD4+ positively selected cells were pDCs (CD4+ CD123+ BDCA2+), which could account for the type 1 IFN produced.
Effect of HIV-1 entry inhibitors on apoptosis and DR5 expression in CD4+ T cells
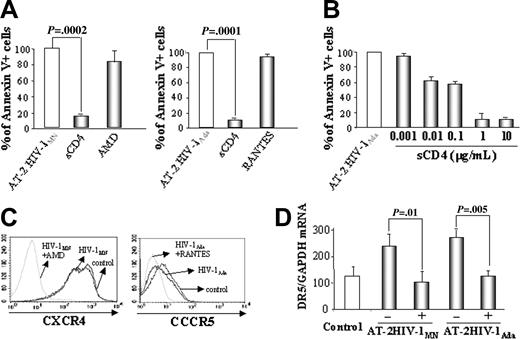
We tested whether HIV-1–induced apoptosis and DR5 mRNA expression would be inhibited by sCD4-IgG, which blocked the interaction between viral gp120 and CD4, and by AMD-3100 and RANTES, which blocked CXCR4 and CCR5 coreceptor binding, respectively. Results (Figure 4A) demonstrate that sCD4-IgG, but not AMD-3100 or RANTES, inhibited AT-2 HIV-1MN and AT-2 HIV-1Ada from inducing apoptosis of CD4+ T cells. Results (Figure 4B) also indicate that 1 μg/mL to 10 μg/mL sCD4 maximally blocked AT-2 HIV-1MN–induced CD4+ T-cell apoptosis. We verified that AMD-3100 and RANTES bound CXCR4 and CCR5— and were, therefore, functional in our experiment—by staining the CD4+ cells with anti-CXCR4 or anti-CCR5 antibodies. AMD-3100 and RANTES bound CXCR4 and CCR5, preventing the binding of anti–coreceptor antibodies (Figure 4C).
Effect of entry inhibitors on apoptosis and DR5 mRNA expression in T cells. (A) CD4+ cell were cultured for 24 hours with AT-2 HIV-1MN/Ada in the presence of sCD4-IgG (2 μg/mL), AMD-3100 (2 μg/mL), or RANTES (2 μg/mL). Apoptosis was analyzed by flow cytometry using the annexin V method. (B) Titration of sCD4-IgG in blocking AT-2 HIV-1MN–induced apoptosis of CD4+ T cells (μg/mL) (C) Flow cytometric analysis of CXCR4 or CCR5 expression by CD4+ T cells cultured for 24 hours in the presence of AT-2 HIV-1MN with or without AMD-3100 (2 μg/mL) or with or without RANTES (2 μg/mL). (D) DR5 mRNA expression by CD4+ T cells cultured in the presence of HIV-1MN/Ada and with (+) or without (-) sCD4-IgG.
Effect of entry inhibitors on apoptosis and DR5 mRNA expression in T cells. (A) CD4+ cell were cultured for 24 hours with AT-2 HIV-1MN/Ada in the presence of sCD4-IgG (2 μg/mL), AMD-3100 (2 μg/mL), or RANTES (2 μg/mL). Apoptosis was analyzed by flow cytometry using the annexin V method. (B) Titration of sCD4-IgG in blocking AT-2 HIV-1MN–induced apoptosis of CD4+ T cells (μg/mL) (C) Flow cytometric analysis of CXCR4 or CCR5 expression by CD4+ T cells cultured for 24 hours in the presence of AT-2 HIV-1MN with or without AMD-3100 (2 μg/mL) or with or without RANTES (2 μg/mL). (D) DR5 mRNA expression by CD4+ T cells cultured in the presence of HIV-1MN/Ada and with (+) or without (-) sCD4-IgG.
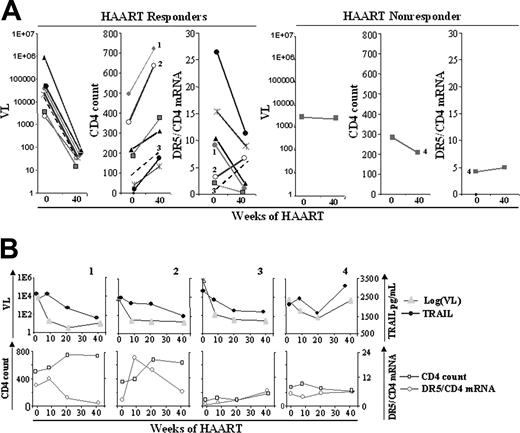
Effect of HAART on DR5 mRNA expression in T cells. Eight HIV-1–infected patients started HAART therapy and were followed up for 40 weeks. Samples were collected at weeks 0, 8, 20, and 40. (A) Viral load, CD4 count, and DR5/CD4 mRNA data for weeks 0 and 40 of therapy for 7 HAART responder patients (3 left panels) and 1 HAART nonresponder (3 right panels) patients. (B) Data for weeks 0, 8, 20, and 40 are shown for viral load and plasma TRAIL (top panels) and for CD4 count and DR5/CD4 mRNA ratio (bottom panels) for a representative example (patient 1) of 5 HAART responder patients, the 2 exceptions shown in Figure 4A (second panel) (patients 2 and 3), and the HAART nonresponder (patient 4). Plasma TRAIL levels of patients 1 and 4 were shown in our earlier study.24 Plasma TRAIL levels of patients 2 and 3 are new data.
Effect of HAART on DR5 mRNA expression in T cells. Eight HIV-1–infected patients started HAART therapy and were followed up for 40 weeks. Samples were collected at weeks 0, 8, 20, and 40. (A) Viral load, CD4 count, and DR5/CD4 mRNA data for weeks 0 and 40 of therapy for 7 HAART responder patients (3 left panels) and 1 HAART nonresponder (3 right panels) patients. (B) Data for weeks 0, 8, 20, and 40 are shown for viral load and plasma TRAIL (top panels) and for CD4 count and DR5/CD4 mRNA ratio (bottom panels) for a representative example (patient 1) of 5 HAART responder patients, the 2 exceptions shown in Figure 4A (second panel) (patients 2 and 3), and the HAART nonresponder (patient 4). Plasma TRAIL levels of patients 1 and 4 were shown in our earlier study.24 Plasma TRAIL levels of patients 2 and 3 are new data.
Finally, we demonstrated that sCD4-IgG also inhibited DR5 mRNA expression by CD4+ T cells cultured with AT-2 HIV-1MN or AT-2 HIV-1Ada (Figure 4C). These results are consistent with a triggering mechanism that is dependent on very early CD4/gp120 binding events.
DR5 expression, CD4 count, and viral load in a longitudinal study of HAART patients
To assess the potential clinical relevance of our in vitro findings, we compared the changes in DR5 mRNA expression in CD4+ T lymphocytes with CD4 counts and viral loads in 8 drug-naive, HIV-1–infected patients who received HAART during a 40-week longitudinal study. Because only CD4+ T cells, and not CD8+ T cells, expressed DR5, we used CD4 mRNA as an internal standard to normalize DR5 mRNA expression. Comparisons of the viral loads, CD4 counts, and DR5/CD4 mRNA expression are shown for weeks 0 and 40 in Figure 5A. HAART reduced viral loads and increased CD4 counts in 7 of 8 patients, indicated as HAART responders. The DR5/CD4 mRNA ratio decreased in 5 of 7 of these responders, along with HAART-induced viral load reductions, and increased CD4 counts (Figure 5A). Data from the patient who appeared to be a HAART nonresponder (patient 4) showed no appreciable decrease in viral load, a decrease in CD4 count, and a slight increase in DR5/CD4 mRNA at 40 weeks. Figure 5B presents details of 4 of the 8 patients at 0, 8, 20, and 40 weeks of HAART. Patient 1 provides an example of 5 HAART responders in whom a direct correlation was observed between viral load and plasma TRAIL levels, and an inverse correlation was observed between CD4 count and DR5/CD4 mRNA throughout 40 weeks. In patient 2, a direct correlation was observed between viral load and plasma TRAIL, but no apparent correlation was observed between CD4 count and DR5/CD4 mRNA (Figure 4A). However, between week 8 and week 40, a dramatic decrease in DR5/CD4 mRNA was observed in patient 2, concomitant with an increase in CD4 count (Figure 5B). In patient 3, a parallel decrease was observed in viral load and plasma TRAIL, but this was exceptional in that both the CD4 count and the DR5/CD4 mRNA remained low, despite a slight increase, throughout the 40 weeks. In patient 4, who appeared to be a HAART nonresponder in Figure 5A, a decrease in viral load and plasma TRAIL was observed at weeks 0 to 20, and parallel rebounds of viremia and TRAIL were observed at week 40. Similarly, the initial increase (weeks 0-8) and subsequent decrease (weeks 8-40) of CD4 count inversely correlated with DR5/CD4 mRNA. Thus, the data of patient 4 showed a dynamic change in which viral load and plasma TRAIL exhibited parallel declines and rebounds and inverse changes in CD4 count and DR5/CD4/mRNA level. These results show an inverse pattern between CD4 count and DR5/CD4 mRNA level in HAART patients.
Discussion
The percentage of HIV-1–infected CD4+ T cells is too low to account for the extensive depletion of T-helper cells in patients who are chronically infected with HIV-1 and progressing to AIDS.39,40 Thus, it was suggested that uninfected CD4+ T cells die by apoptosis during HIV-1 disease progression.5 Here we propose a model in which either infectious or noninfectious HIV-1 can induce apoptosis of uninfected CD4+ T cells by a type 1 IFN-induced TRAIL/DR5-mediated mechanism. We tested this model by performing in vitro experiments and by analyzing CD4+ T cells from HIV-1–infected patients.
Our in vitro results indicate that HIV-1 induced TRAIL and DR5 expression and preferential apoptosis of CD4+ T cells irrespective of HIV-1 coreceptor usage. We demonstrated that infectious and noninfectious HIV-1 induced selective apoptosis of CD4+ T cells, suggesting that noninfectious HIV-1 particles, which constitute a large portion of plasma virus,28,29 contribute to the decline in CD4 counts in patients. Other studies report that Tat-exposed monocytes22 and HIV-1–exposed dendritic cells41 produce TRAIL, which induces the death of uninfected CD4+ T cells. Our data show that uninfected CD4+ T cells express TRAIL, DR5, and activated caspase-3 when exposed to HIV-1, resulting in their apoptosis. These observations suggest that DR5+ CD4+ T cells can be killed by CD4+ T cells, TRAIL-expressing monocytes,22 dendritic cells,41 soluble TRAIL,24 or combinations of these sources of TRAIL.
We demonstrated that anti-TRAIL antibodies partially inhibited apoptosis of CD4+ T cells cultured with HIV-1. Furthermore, DR5-specific antibodies partly blocked most HIV-1–induced apoptosis, underscoring the essential role of DR5. This incomplete inhibition suggests that other death mechanisms are also involved. Such mechanisms, however, may not involve Fas/FasL because anti-Fas antibodies did not block apoptosis in our experiments.
Inhibition of TRAIL expression and CD4+ T-cell death by IFN-α/β–specific antibodies is consistent with the known regulation of TRAIL expression by type 1 IFN.25 However, our findings that TRAIL expression, caspase-3 activation, and preferential apoptosis of CD4+ T cells were induced by noninfectious HIV-1 are novel. Our results that primary CD4+ T-cell apoptosis induced by noninfectious HIV-1 was regulated by type 1 IFN raises the possibility that IFN-α/β induces not only the death of HIV-1–infected cells but the apoptosis of uninfected CD4+ T cells that have interacted with noninfectious HIV-1.
We showed that pDCs cultured with infectious or noninfectious HIV-1 produced IFN-α, which is in agreement with findings of a recent report.38 Therefore, pDCs present in the isolated CD4+ cells are probably a major source of IFN-α/β that induced TRAIL expression and apoptosis of CD4+ T cells. Our results suggest that IFN-producing cells play an important role in this model. We are investigating the role of pDCs in HIV-1–mediated TRAIL expression on CD4+ T cells.
It has been suggested that type 1 IFN produced by pDCs protects against AIDS progression42,43 and has been used for therapy in AIDS patients.44 However, type 1 IFN induced in lymph nodes of macaques infected with SIV-1 did not control viral replication.45 Our findings raise the possibility that type 1 IFN could be harmful by setting the stage for the death of uninfected CD4+ T cells activated to express TRAIL and DR5 without becoming productively infected. Thus, apoptosis of CD4+ T cells induced by interaction with noninfectious HIV-1 may use the same type 1 IFN mechanism proposed for the elimination of virus-infected cells.46,47 Based on our observations that antibodies against IFN-α/β reduced AT-2 HIV-1–induced CD4+ T-cell death and blocked TRAIL expression, we raise the possibility of considering anti–type 1 IFN antibodies in the HIV-1 therapeutic arsenal. This strategy is consistent with reports that HIV-1–mediated immune suppression is induced by IFN-α and can be blocked by anti–IFN-α antibodies48 and that immunization against IFN-α reduced the rate of HIV-1 disease progression.49
Our finding that sCD4-IgG effectively inhibited AT-2 HIV-1–induced DR5 expression and apoptosis of CD4+ T cells demonstrate that the binding of gp120 to CD4 is essential for inducing TRAIL/DR5 apoptosis. This result also demonstrates that the apoptosis observed was caused by HIV-1 particles. In contrast, coreceptor inhibitors did not have any effect, suggesting that the binding coreceptor step is not essential for inducing apoptosis. This is in agreement with another report demonstrating that CXCR4- and CCR5-tropic HIV isolates are equally cytopathic to CD4+ T cells in human lymphoid tissue.50 However, we cannot exclude the possibility that these 2 HIV-1 isolates might have used other coreceptors for inducing these effects.
Our analysis of clinical samples showed that the percentage of CD4+ T cells entering apoptosis correlates with expression of the TRAIL DR5 in HIV-1–infected patients. In contrast, no correlation was observed in healthy controls. To explore the link between DR5 and CD4 count, we studied samples from patients receiving HAART. We quantified DR5 and CD4 mRNA expression in T lymphocytes before and after HAART. We showed that 6 of 7 patients who were responsive to HAART, as evidenced by decreased viral loads and increased CD4 counts, exhibited decreases in DR5/CD4 mRNA ratios. Furthermore, the CD4 count decrease seen in the patient with a viral rebound was associated with an increase in the DR5/CD4 mRNA ratio, which was predicted by our model. The inverse association between CD4 count and DR5/CD4 mRNA level could be the reflection of 2 nonmutually exclusive possibilities: general decrease of DR5 mRNA expression in the existing CD4+ T cells and reduction of DR5/CD4 mRNA ratio resulting from increased numbers of CD4+ T cells that do not express elevated DR5. In either case, the overall result was a reduction of DR5 mRNA expression by the CD4+ T-cell population as the CD4 count increased. This result was consistent with the findings in our cross-sectional study of HIV-1–infected patients (Figure 1). Our finding that DR5-specific blocking antibody inhibited apoptosis in the CD4+ T cells of HIV-1–infected patients suggests a cause-and-effect relationship between TRAIL/DR5-mediated apoptosis and CD4+ T-cell loss. These ex vivo data are consistent with our in vitro demonstration that DR5 expression precedes HIV-1–induced apoptosis of CD4+ T cells.
We propose a model in which uninfected CD4+ T cells undergo apoptosis by a type 1 IFN and TRAIL/DR5 mechanism after HIV-1 exposure. Therefore, TRAIL is likely to be a major death molecule in HIV-1 immunopathogenesis. Why CD4+ T cells become sensitive to death in HIV-1 infection was a question recently raised.51 The present study indicates the answer is that DR5 is expressed on CD4+ T cells. Our results provide a novel, clinically supported model for the depletion of uninfected CD4+ T cells in HIV-1 disease involving the TRAIL/DR5 pathway and noninfectious HIV-1.
Prepublished online as Blood First Edition Paper, July 26, 2005; DOI 10.1182/blood-2005-03-1243.
Supported by the Intramural Program of the Center for Cancer Research, NCI, NIH; by the NIH Intramural AIDS Targeted Antiviral Program; and in part by NCI NIH contract N01-CO-12400.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human services, nor does mention of trade names, commercial products, or organization imply endorsement by the US Government.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal