Abstract
Hemophilia A in its severe form is a life-threatening hemorrhagic disease that is caused by mutations in the factor VIII (FVIII) gene (symbol F8). About 25% of patients who receive replacement therapy develop neutralizing antibodies that inhibit the function of substituted FVIII. Long-term application of high doses of FVIII has evolved as an effective therapy to eradicate the antibodies and to induce long-lasting immune tolerance. Little is known, however, about the immunologic mechanisms that cause the down-modulation of anti-FVIII antibodies by high doses of FVIII. We report that high doses of FVIII inhibit the restimulation of FVIII-specific memory B cells and their differentiation into antibody-secreting plasma cells in vitro and in vivo in a murine model of hemophilia A. The inhibition of memory B-cell responses is irreversible and not mediated by FVIII-specific T cells. Furthermore, it seems to involve the activation of caspases. We conclude that the inhibition of FVIII-specific memory B cells might be an early event in the down-modulation of anti-FVIII antibodies in patients with hemophilia A who receive high doses of FVIII.
Introduction
The development of neutralizing anti–factor VIII (FVIII) antibodies is the major complication in the treatment of patients with hemophilia A with FVIII products.1,2 Long-term application of high doses of FVIII has evolved as an effective therapy to eradicate the antibodies and to induce long-lasting immune tolerance.3-6 Despite clinical experience with the therapy, little is known about the immunologic mechanisms that cause the down-modulation of FVIII-specific immune responses and the induction of long-lasting immune tolerance against FVIII. We asked the question whether the restimulation of FVIII-specific memory B cells is affected by high concentrations of FVIII in vitro or high doses of FVIII in vivo. Memory B cells play an essential role in the maintenance of established antibody responses. On re-exposure to the same antigen, they are rapidly restimulated to proliferate and differentiate into antibody-secreting plasma cells (ASCs) that secrete high-affinity antibodies.7,8 Furthermore, memory B cells have the potential to act as very efficient antigen-presenting cells and stimulators of CD4+ T cells because of the expression of high-affinity antigen receptors, major histocompatibility complex (MHC) class II and costimulatory molecules.9 It is, therefore, reasonable to believe that memory B cells have to be eradicated or functionally inactivated during a successful immune tolerance induction therapy with FVIII inhibitors in patients with hemophilia A.
We used a murine model of hemophilia A that is characterized by complete deficiency of functionally active FVIII because of a targeted disruption of exon 17 of the F8 gene.10,11 Intravenous injection of human FVIII into these mice results in high titers of anti-FVIII antibodies that have similar characteristics to those of FVIII inhibitors in patients.12-15 Using this model, we demonstrated previously that the differentiation of FVIII-specific memory B cells into ASCs depends on the presence of activated T cells and requires CD40-CD40 ligand and CD80/CD86-CD28 costimulatory interactions.16 Here, we show that concentrations of FVIII below the physiologic plasma concentration of 0.1 μg/mL (1 U/mL) restimulate FVIII-specific memory B cells and induce their differentiation into ASCs. Concentrations above 0.1 μg/mL (1 U/mL), however, inhibit memory B-cell restimulation and prevent the formation of ASCs. This inhibition is irreversible and involves the activation of caspases.
Materials and methods
Hemophilic E-17 mice
Our colony of fully inbred hemophilic E-17 mice (characterized by a targeted disruption of exon 17 of the F8 gene) was established with a breeding pair from the original colony10,11 and crossed into the C57BL/6J background as described.17 All mice were male and aged 8 to 10 weeks at the beginning of the experiments. All studies were carried out in accordance with Austrian federal law (Act BG 501/1989) regulating animal experimentation and approved by the local authority in Vienna, Austria.
Immunization of mice with FVIII or ovalbumin
Immunization with FVIII. At weekly intervals mice received 4 intravenous doses of 0.2 μg recombinant human FVIII (approximately 80 U/kg FIII) or 8 doses of 0.4 μg recombinant B-domainless murine FVIII, both diluted in 200 μL Dulbecco phosphate-buffered saline (DPBS; Sigma-Aldrich, Irvine, United Kingdom). In preliminary experiments, the immunization schedule used for murine FVIII produced anti-FVIII antibody responses in hemophilic E-17 mice. The recombinant human FVIII used throughout the studies was albumin-free bulk material obtained from Baxter BioScience (Thousand Oaks, CA). The recombinant B-domainless murine FVIII was produced in a cell line derived from baby hamster kidney and purified as described.18
Immunization with ovalbumin. Mice were immunized with 3 intraperitoneal doses of ovalbumin (OVA; Sigma-Aldrich). The OVA contained traces of endotoxin (26 ng endotoxin per mg protein) as detected with the Limulus Amoebocyte Lysat Test (Baxter BioScience, Orth, Austria). The first dose of OVA was 20 μg and the second and third doses were both 10 μg. The interval between the first and second injection was 2 weeks; between the second and third injection, 1 week. The OVA was diluted in 100 μL DPBS. This treatment regimen was sufficiently found to develop OVA-specific immunologic memory after treatment without adjuvant.16
Spleen-cell preparation
Spleens were obtained 7 days after the last dose of FVIII or OVA. Spleen cells were prepared as described.16 Where indicated, spleen cells were depleted of T cells by incubation with Mouse pan T (anti–murine Thy 1.2) Dynabeads (Dynal ASA, Oslo, Norway) for 20 minutes at 4°C and subsequent separation with a magnetic device. T-cell depletion as determined by flow cytometry was greater than 99%.
Splenic T cells were isolated from either spleen cells or cells obtained from in vitro cultures by negative selection using a magnetic cell sorting (MACS) T-cell isolation kit (Milteny Biotec, Auburn, CA). The purity of different cell populations was analyzed by flow cytometry as described.16
Restimulation of memory B cells in vitro
The restimulation of memory B cells in vitro was achieved as described.16 Briefly, spleen cells were isolated and depleted of CD138+ ASCs. CD138 or syndecan-1 is a proteoglycan that is absent on circulating and peripheral B lymphocytes but is expressed on differentiation of B cells into plasma cells.19 CD138 serves as a marker for antibody-secreting plasma cells. CD138– spleen cells were cultured at 1.5 × 106 cells/mL in RPMI 1640 (Life Technologies, Paisley, Scotland) supplemented with 10% preselected fetal calf serum (Hyclone, Logan, Utah), 2 mM l-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin (all from Life Technologies) and 5 × 10–5 M β-mercaptoethanol (Sigma-Aldrich) at 37°C for 3 or 6 days. Different concentrations of FVIII or OVA were added to the cells on day 0 as indicated. After 3 or 6 days of culture, newly formed ASCs were detected by enzyme-linked immunospot (ELISPOT) assays as described.16,20
To test for the effect of high concentrations of FVIII on T-cell function, CD138– spleen cells were cultured for 3 days as indicated, washed twice with culture medium, and subsequently used for the isolation of T cells by negative selection as described in “Spleen-cell preparation.” T cells were then mixed in a ratio of 1:2 with freshly isolated CD138– spleen cells depleted of T cells, which were obtained from the spleens of hemophilic mice treated with 4 doses of FVIII.
To test whether inhibition of FVIII-specific memory B cells by high concentrations of FVIII is observed in the absence of FVIII-specific T cells, CD138– spleen cells, which were obtained from hemophilic mice treated with 4 doses of FVIII, were depleted of T cells and then mixed in a ratio of 1:2 with splenic T cells obtained from mice treated with 3 doses of OVA.
For the inhibition of caspases, CD138– spleen cells were preincubated with the broad-spectrum caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp-fluoromethylketone (Z-VAD-FMK; R&D Systems, Minneapolis, MN) for 4 hours before FVIII was added. A monoclonal anti–murine Fas (CD95) antibody (clone Jo2; Pharmingen, San Diego, CA) that was shown to induce apoptosis in Fas-expressing cells21 was used as a positive control for the induction of apoptosis. The anti-Fas antibody was added to the cultures together with FVIII.
For the inhibition of Fas-Fas ligand interactions, recombinant soluble Fas-Fc (extracellular domain of murine Fas fused to the crystallizable fragment [Fc] part of a human immunoglobulin G1 [IgG1]; R&D Systems) was added to the cultures at the same time as FVIII. A human monoclonal IgG1 antibody directed against an irrelevant antigen (Baxter BioScience, Orth, Austria) was used as a negative control for the Fas-Fc protein.
Restimulation of memory B cells in vivo
Spleen cells, isolated from naive hemophilic mice or hemophilic mice treated with either human or murine FVIII, were depleted of CD138+ ASCs. A total of 107 CD138– spleen cells were injected intravenously into naive hemophilic mice. One day after cell transfer, mice were injected with a single intravenous dose of either of the following: 0.2, 2, 20, or 200 μg human FVIII or 0.2, 2, 20, or 40 μg murine FVIII. Blood samples were taken from each mouse by tail snipping at 7 and 14 days after treatment with FVIII for measurement of anti-FVIII antibodies by enzyme-linked immunosorbent assay (ELISA) as described.16,20
Cytokine analysis
Murine interleukin 2 (IL-2), interleukin 4 (IL-4), interleukin 5 (IL-5), interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 12 p70 (IL-12 p70), interleukin 17 (IL-17), tumor necrosis factor α (TNF-α), and interferon γ (IFN-γ) were detected in cell culture supernatants using a Bio-Plex Mouse Cytokine 17-Plex Assay (Bio-Rad Laboratories, Hercules, CA).
Analysis of FVIII-specific T cells by intracellular cytokine staining
Analysis of FVIII-specific T cells by intracellular cytokine staining was as described.22
Proliferation assay
Proliferation assays were performed in 96-well microtiter plates (Falcon; Becton Dickinson, Franklin Lakes, NJ). A total of 1.5 × 105 CD138– spleen cells obtained from mice treated with FVIII were seeded in each well and cultured for 72 hours in the presence of different concentrations of FVIII as indicated. For the last 18 hours 1 μCi/well (0.037 MBq/well) 3H-thymidine (Amersham, Buckinghamshire, United Kingdom) was added. Cells were harvested and incorporated. 3H-thymidine was measured using a 1450 Microbeta Plus Liquid Scintillation Counter (Wallac, Turku, Finland). All proliferation assays were performed in triplicate cultures. Results are expressed as means and standard deviations of means obtained from the results of the triplicates.
Cleavage of FVIII by thrombin and purification of FVIII fragments
Human thrombin (3050 IU/mg; Baxter BioScience, Vienna, Austria) was coupled to Actigel Superflow ALD (Sterogene Bioseparations, Arcadia, CA) at a concentration of 1.5 mg thrombin/mL gel according to the manufacturers' instructions. FVIII (0.5 mL, 1700 IU/mL) was pumped at 37°C through a 4.7-mL column at 20 μL/min. Fractions containing FVIII cleavage products from 10 repetitive runs were combined and concentrated 5-fold by ultrafiltration using a YM-10 10-kDa molecular weight cut-off membrane (Amicon; Millipore, Bedford, MA). The concentrate was diafiltrated against DPBS. The final preparation had a protein concentration of 0.22 mg/mL and contained 0.17 IU FVIII/mL (0.06% residual FVIII activity) and less than 0.004% (wt/wt) thrombin antigen. Analysis by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; ExcelGel SDS gradient 8-18; Amersham Pharmacia Biotech, Piscataway, NJ) revealed that FVIII was cleaved into fragments smaller than 80 kDa.
Statistics
Arithmetic means and standard errors of means were calculated using results obtained in ELISPOT assays on different days. All results were normalized as described in the legend to Figure 1. The unpaired Student t test was used for comparison of means between groups. Differences were considered statistically significant if the P values were less than .05.
Results
High concentrations of FVIII inhibit the restimulation of FVIII-specific memory B cells in vitro and in vivo
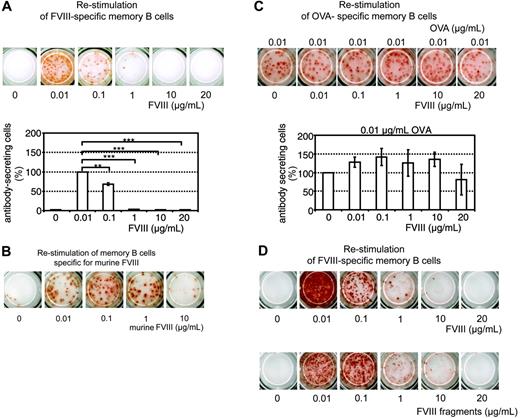
We reported previously that the differentiation of FVIII-specific memory B cells into ASCs can be stimulated in vitro.16 Here, we show that low concentrations of FVIII (0.01 μg/mL and 0.1 μg/mL), corresponding to about 10% and 100% of the normal physiologic plasma concentration of FVIII, stimulated the differentiation of FVIII-specific memory B cells into ASCs. Higher concentrations of FVIII (1, 10, and 20 μg/mL), however, induced a significant inhibition of memory B-cell differentiation and prevented the formation of ASCs (Figure 1A). We observed this inhibition both in cells obtained from mice treated with human FVIII and restimulated with human FVIII as well as in cells obtained from mice treated with murine FVIII and restimulated with murine FVIII (Figure 1B). We performed all subsequent experiments with human FVIII if not indicated otherwise. The inhibitory effect of FVIII was specific for FVIII-specific memory B cells. The restimulation of OVA-specific memory B cells was not significantly altered by high concentrations of FVIII (Figure 1C).
In normal plasma FVIII is bound noncovalently to von Willebrand factor (VWF). FVIII is proteolytically activated by thrombin and dissociates from VWF.23,24 Our in vitro culture system contained bovine VWF in fetal calf serum, which binds to human FVIII.25 Because of the multimeric structure of VWF, polyvalent FVIII-VWF complexes could form and crosslink B-cell receptors expressed by memory B cells. We investigated whether the inhibition of memory B-cell differentiation can be observed with FVIII fragments obtained after thrombin cleavage too. Our preparation of thrombin-cleaved FVIII fragments consisted of the previously characterized approximate 73-kDa cleaved light chain and the approximate 54-kDa A1 and approximate 44-kDa A2 subunits of FVIII.25 There was no evidence of the residual FVIII light chain, which in this preparation contains the VWF-binding site. Comparing the FVIII fragments with full-length FVIII we found similar inhibitory effects at concentrations greater than 0.1 μg/mL (Figure 1D). These results indicate that both full-length FVIII and thrombin-cleaved FVIII fragments inhibit the restimulation of FVIII-specific memory B cells.
Dose-dependent inhibition of memory B-cell restimulation by FVIII. CD138– spleen cells were obtained from hemophilic mice immunized with human FVIII (A,D), murine FVIII (B), or OVA (C) as described in “Materials and methods.” Newly formed anti–human FVIII (A,D), anti–murine FVIII (B), or anti-OVA (C) ASCs were detected by ELISPOT analysis after culture of CD138– spleen cells in the presence of human or murine FVIII at the concentrations indicated. (C) CD138– spleen cells were stimulated with a fixed concentration of OVA and human FVIII at the concentration indicated. (D) CD138– spleen cells were cultured with either human FVIII or thrombin-cleaved human FVIII. ELISPOTs represent the results obtained in a typical experiment. The graphs represent mean values (n = 7) and standard errors of the mean. Results were normalized for comparing experiments done on different days by setting responses obtained with 0.01 μg/mL FVIII (A) or 0.01 μg/mL OVA (C) at 100%. Spleens from 5 mice were pooled for cell preparation in each experiment. **P < .01; ***P < .001.
Dose-dependent inhibition of memory B-cell restimulation by FVIII. CD138– spleen cells were obtained from hemophilic mice immunized with human FVIII (A,D), murine FVIII (B), or OVA (C) as described in “Materials and methods.” Newly formed anti–human FVIII (A,D), anti–murine FVIII (B), or anti-OVA (C) ASCs were detected by ELISPOT analysis after culture of CD138– spleen cells in the presence of human or murine FVIII at the concentrations indicated. (C) CD138– spleen cells were stimulated with a fixed concentration of OVA and human FVIII at the concentration indicated. (D) CD138– spleen cells were cultured with either human FVIII or thrombin-cleaved human FVIII. ELISPOTs represent the results obtained in a typical experiment. The graphs represent mean values (n = 7) and standard errors of the mean. Results were normalized for comparing experiments done on different days by setting responses obtained with 0.01 μg/mL FVIII (A) or 0.01 μg/mL OVA (C) at 100%. Spleens from 5 mice were pooled for cell preparation in each experiment. **P < .01; ***P < .001.
We next investigated whether the inhibition of memory B-cell restimulation by high concentrations of FVIII could be observed in vivo. We transferred CD138– spleen cells (containing FVIII-specific memory cells), obtained from hemophilic mice treated with either human or murine FVIII, into naive mice as described.16 The resulting mice had FVIII-specific memory cells but did not have any CD138+ anti-FVIII ASCs, and, therefore, did not produce any anti-FVIII antibodies. As a control, we transferred CD138– spleen cells obtained from naive hemophilic mice into naive mice. The resulting control mice did not have any FVIII-specific memory B cells. Twenty-four hours after cell transfer, we treated mice that had memory cells specific for human FVIII with a single intravenous dose of either 0.2, 2, 20, or 200 μg human FVIII. Mice that had memory cells specific for murine FVIII were treated with a single intravenous dose of either 0.2, 2, 20, or 40 μg murine FVIII (40 μg was the highest dose that could be applied with the preparation of murine FVIII that was available). We analyzed the restimulation and differentiation of FVIII-specific memory B cells in vivo by measuring circulating levels of anti-FVIII antibodies at 7 and 14 days after treatment with FVIII. Mice that had memory cells specific for human FVIII developed detectable anti-FVIII antibodies after treatment with 0.2 or 2 μg human FVIII which indicates the induction of FVIII-specific memory responses (Figure 2A-B). When mice were treated with 20 or 200 μg FVIII, however, we did not see any significant restimulation of memory B cells (Figure 2A-B). These results indicate that high doses of FVIII inhibit the differentiation of FVIII-specific memory B cells in vivo.
Mice that had memory cells specific for murine FVIII developed detectable anti-FVIII antibody titers after treatment with 20 μg murine FVIII (Figure 2C). Higher doses of murine FVIII were required to restimulate murine FVIII-specific memory B cells in vivo than those required to restimulate human FVIII-specific memory B cells. When mice were treated with 40 μg murine FVIII, significantly lower antibody titers were developed than after treatment with 20 μg FVIII (Figure 2C). These results confirm findings obtained with human FVIII and indicate that there is a FVIII dose threshold for boosting the FVIII-specific memory B-cell response versus inhibiting this response.
Inhibition of memory B-cell restimulation is not caused by inhibition of T-cell activation and is not mediated by T cells
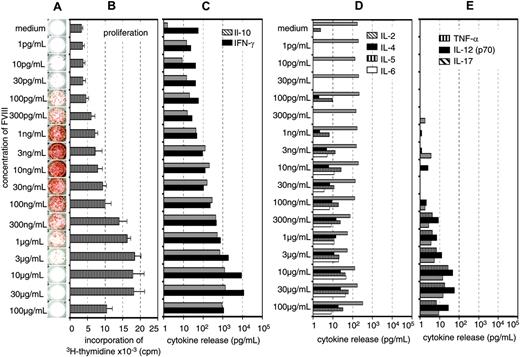
Previously, we showed that the restimulation of FVIII-specific memory B cells obtained from hemophilic mice immunized with FVIII was dependent on the presence of activated T cells.16 Therefore, we were interested to know whether the inhibition of memory B-cell restimulation by high concentrations of FVIII was caused by an inhibition of T-cell activation. We did dose-response studies using a wide range of different concentrations of FVIII and looked for the optimal concentrations required for memory B-cell restimulation and T-cell stimulation. Furthermore, we searched for the concentration of FVIII that would inhibit memory B-cell restimulation and T-cell stimulation, respectively. Restimulation of memory B cells could be detected at FVIII concentrations as small as 0.0001 μg/mL (Figure 3A). Optimal restimulation was achieved at concentrations of 0.003 to 0.01 μg/mL, which correspond to about 3% to 10% of the physiologic plasma concentration (Figure 3A). Significant inhibition of memory B-cell restimulation was observed at 0.1 to 0.3μg/mL with an almost complete inhibition at 1 μg/mL FVIII (Figure 3A). The dose-response relation for T-cell stimulation was very different from the dose-response relation for memory B-cell restimulation. Optimal stimulation of FVIII-specific T cells was observed at concentrations of 10 to 30 μg/mL FVIII (Figure 3A-C). Inhibition of T-cell stimulation was seen at concentrations of 100 μg/mL FVIII. Therefore, the concentration of FVIII required for inhibition of memory B-cell restimulation and the concentration required for inhibition of T-cell stimulation were very different (Figure 3A-B) what makes it unlikely that the inhibition of memory B-cell restimulation is due to an inhibition of T-cell stimulation.
Inhibition of memory B-cell restimulation by high-dose FVIII in vivo. Titers of anti-FVIII antibodies were measured in hemophilic mice treated with a single intravenous dose of human FVIII (A-B) or murine FVIII (C) as indicated following transfer of CD138– spleen cells obtained from naive hemophilic mice (B) or hemophilic mice immunized with 4 doses of human FVIII (A) or murine FVIII (C). The error bars represent the standard deviations (n = 7). Spleens from 5 mice were pooled for cell preparation in each experiment. **P < .01 for comparing memory responses with responses in control mice.
Inhibition of memory B-cell restimulation by high-dose FVIII in vivo. Titers of anti-FVIII antibodies were measured in hemophilic mice treated with a single intravenous dose of human FVIII (A-B) or murine FVIII (C) as indicated following transfer of CD138– spleen cells obtained from naive hemophilic mice (B) or hemophilic mice immunized with 4 doses of human FVIII (A) or murine FVIII (C). The error bars represent the standard deviations (n = 7). Spleens from 5 mice were pooled for cell preparation in each experiment. **P < .01 for comparing memory responses with responses in control mice.
The major T-cell cytokines found in culture supernatants after stimulation of spleen cells with FVIII were IL-10 and IFN-γ (Figure 3A-C), which is consistent with findings we reported previously.16,22 To further support these results, we analyzed the frequency of FVIII-specific T cells by intracellular cytokine staining 3 days after restimulation of spleen cells as described.22 We compared concentrations of 0.01 and 20 μg/mL FVIII, which stimulate and inhibit memory B-cell differentiation, respectively and observed a correlation between the frequency of FVIII-specific T cells producing IL-2, IL-10, or IFN-γ and the concentration of FVIII used for the restimulation (data not shown). We did not observe any inhibitory effects of high concentrations of FVIII on T-cell stimulation at the concentrations tested.
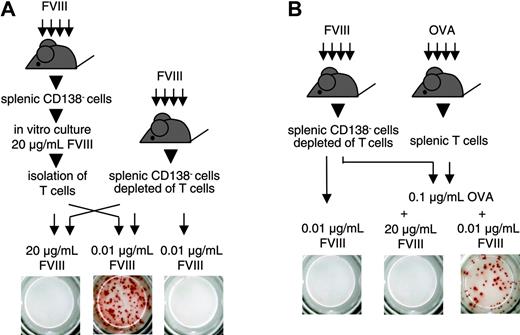
We next questioned whether T cells stimulated by high concentrations of FVIII would still be able to support the restimulation and differentiation of FVIII-specific memory B cells. For this purpose, we stimulated CD138– spleen cells with 20 μg/mL FVIII for 3 or 6 days. After this time, we isolated T cells from the cultures and mixed them with freshly isolated CD138– spleen cells that were depleted of T cells. We restimulated this cell mixture with 0.01 μg/mL FVIII, the concentration of FVIII found to be optimal for memory B-cell restimulation in vitro. We found that T cells that were initially stimulated with 20 μg/mL FVIII were still able to support the restimulation and differentiation of FVIII-specific memory B cells (Figure 4A). These results indicate that high concentrations of FVIII do not induce T cells that suppress the restimulation of memory B cells. In subsequent experiments, we investigated whether FVIII would inhibit the restimulation of FVIII-specific memory B cells in a system where FVIII-specific T cells were absent. We showed previously that activated FVIII-specific T cells that support the differentiation of FVIII-specific memory B cells could be replaced by activated bystander T cells.16 On the basis of these findings, we depleted T cells from CD138– spleen cells obtained from hemophilic mice immunized with FVIII and mixed the T-cell depleted cells with splenic T cells obtained from mice immunized with OVA. We stimulated this cell mixture in vitro with 0.1 μg/mL OVA together with either 0.01 or 20 μg/mL FVIII. Whereas OVA together with 0.01 μg/mL FVIII stimulated the differentiation of FVIII-specific memory B cells, OVA together with 20 μg/mL FVIII inhibited this process (Figure 4B). In a control, 0.01 μg/mL FVIII did not induce any differentiation in the absence of OVA-specific T cells (Figure 4B). These results indicate that the inhibitory activity of high concentrations of FVIII is caused by a direct action on FVIII-specific memory B cells and not mediated by T cells.
Concentration of FVIII determines the response of FVIII-specific memory B cells and FVIII-specific T cells. CD138– spleen cells were obtained from hemophilic mice treated with human FVIII. Cells were restimulated in vitro with human FVIII as indicated for 3 days (B) or 6 days (A,C-E). Newly formed anti-FVIII ASCs were detected by ELISPOT assay (A). ELISPOTs represent the results obtained in a typical experiment. Cell proliferation (B) and cytokine secretion into cell culture supernatants (C-E) were analyzed as described in “Materials and methods.” Presented are the means and standard deviations of triplicate cultures (B) or the medians (C-E) obtained in a typical experiment.
Concentration of FVIII determines the response of FVIII-specific memory B cells and FVIII-specific T cells. CD138– spleen cells were obtained from hemophilic mice treated with human FVIII. Cells were restimulated in vitro with human FVIII as indicated for 3 days (B) or 6 days (A,C-E). Newly formed anti-FVIII ASCs were detected by ELISPOT assay (A). ELISPOTs represent the results obtained in a typical experiment. Cell proliferation (B) and cytokine secretion into cell culture supernatants (C-E) were analyzed as described in “Materials and methods.” Presented are the means and standard deviations of triplicate cultures (B) or the medians (C-E) obtained in a typical experiment.
Inhibition of memory B-cell restimulation is irreversible and involves the activation of caspases
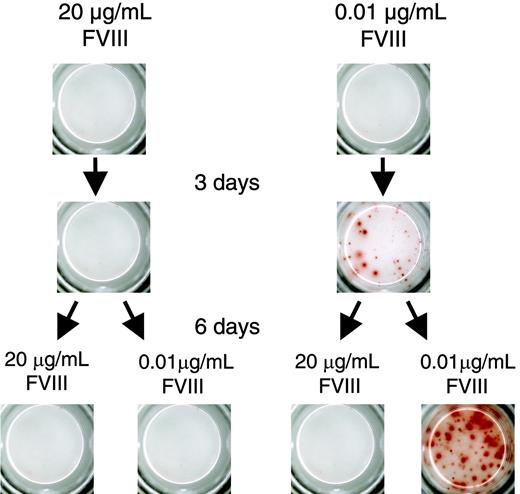
We next wanted to know whether the inhibitory effect of FVIII on the restimulation of FVIII-specific memory B cells is reversible. We restimulated FVIII-specific memory cells with either 0.01 μg/mL (stimulatory concentration) or 20 μg/mL FVIII (inhibitory concentration). After 3 days of culture, we washed cells, resuspended them in culture medium, and split them into 2 aliquots. We stimulated 1 aliquot with 0.01 μg/mL FVIII and the other aliquot with 20 μg/mL FVIII. Cells that we initially stimulated with 0.01 μg/mL FVIII could be restimulated after transfer to 0.01 μg/mL FVIII. However, cells that we initially stimulated with 20 μg/mL FVIII could not be restimulated when we transferred them to 0.01 μg/mL FVIII (Figure 5). These results indicate that the inhibition of memory B-cell restimulation by high concentrations of FVIII is irreversible. In further experiments we found that incubation for 6 hours with high concentrations of FVIII was sufficient to irreversibly inhibit the restimulation of FVIII-specific memory B cells.
Inhibition of memory B-cell restimulation is not mediated by FVIII-specific T cells. ELISPOT analysis of newly formed anti-FVIII APCs after 6 days of culture. CD138– spleen cells (obtained from hemophilic mice immunized with human FVIII) were depleted of T cells and mixed in a ratio of 1:2 with either (A) T cells obtained from a 3-day culture of CD138– spleen cells (obtained from hemophilic mice immunized with FVIII, culture in the presence of 20 μg/mL FVIII) or (B) T cells obtained from hemophilic mice immunized with 3 doses of OVA. Two aliquots of each cell mixture were obtained and cultured in the presence of FVIII or FVIII and OVA at the concentrations indicated. As a negative control, CD138– spleen cells (obtained from hemophilic mice immunized with FVIII) depleted of T cells, were cultured in the presence of 0.01 μg/mL FVIII. Presented is a typical experiment. Spleens of 5 mice were pooled for cell preparation. All experiments (n = 3) gave similar results.
Inhibition of memory B-cell restimulation is not mediated by FVIII-specific T cells. ELISPOT analysis of newly formed anti-FVIII APCs after 6 days of culture. CD138– spleen cells (obtained from hemophilic mice immunized with human FVIII) were depleted of T cells and mixed in a ratio of 1:2 with either (A) T cells obtained from a 3-day culture of CD138– spleen cells (obtained from hemophilic mice immunized with FVIII, culture in the presence of 20 μg/mL FVIII) or (B) T cells obtained from hemophilic mice immunized with 3 doses of OVA. Two aliquots of each cell mixture were obtained and cultured in the presence of FVIII or FVIII and OVA at the concentrations indicated. As a negative control, CD138– spleen cells (obtained from hemophilic mice immunized with FVIII) depleted of T cells, were cultured in the presence of 0.01 μg/mL FVIII. Presented is a typical experiment. Spleens of 5 mice were pooled for cell preparation. All experiments (n = 3) gave similar results.
Inhibition of memory B-cell restimulation by high-dose FVIII is irreversible. CD138– spleen cells were obtained from hemophilic mice immunized with human FVIII and restimulated with either 20 or 0.01 μg/mL FVIII. After 3 days of culture, cells were washed, split into 2 aliquots, and cultured for another 6 days as indicated. Newly formed ASCs were analyzed by ELISPOT. Presented is a typical experiment. Spleens of 5 mice were pooled for cell preparation. All experiments (n = 3) gave similar results.
Inhibition of memory B-cell restimulation by high-dose FVIII is irreversible. CD138– spleen cells were obtained from hemophilic mice immunized with human FVIII and restimulated with either 20 or 0.01 μg/mL FVIII. After 3 days of culture, cells were washed, split into 2 aliquots, and cultured for another 6 days as indicated. Newly formed ASCs were analyzed by ELISPOT. Presented is a typical experiment. Spleens of 5 mice were pooled for cell preparation. All experiments (n = 3) gave similar results.
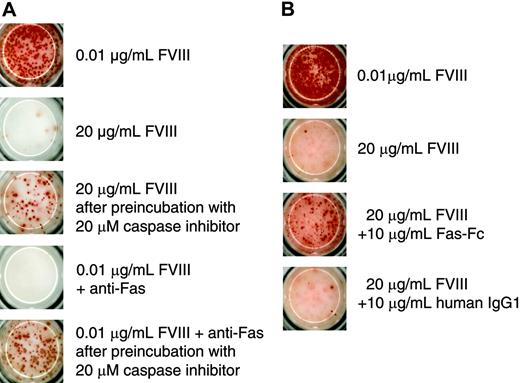
Because of the irreversible inhibition of memory B-cell restimulation, we wanted to find out whether this process involves the activation of caspases. Their involvement would indicate that the inhibition might be because of the induction of apoptosis. To address this possibility we preincubated CD138– spleen cells with a broad-spectrum caspase inhibitor before restimulation with 20 μg/mL FVIII. We used the anti–murine Fas antibody Jo2 as a positive control for the induction of apoptosis to show that the broad-spectrum caspase inhibitor Z-VAD-FMK, which we used in our system, was effective in preventing apoptosis in FVIII-specific memory B cells. We found that the inhibition of caspase activation prevented the inhibition of memory B-cell restimulation induced by either 20 μg/mL FVIII or anti-Fas antibody (Figure 6A). We next asked whether Fas-Fas ligand interactions are involved in the inhibition of memory B-cell restimulation. For this purpose, we added a soluble Fas protein to the culture of CD138– spleen cells. Soluble Fas protein was shown previously to competitively inhibit the interaction between Fas and Fas ligand.26 The addition of soluble Fas protein to CD138– spleen cells in our system prevented the inhibition of memory B-cell restimulation by 20 μg/mL FVIII (Figure 6B), partly but not completely.
Inhibition of memory B-cell restimulation by high-dose FVIII involves caspase activation and Fas-dependent mechanisms. CD138– spleen cells were obtained from hemophilic mice immunized with human FVIII and restimulated with either 0.01 or 20 μg/mL as indicated. Newly formed ASCs were analyzed after 6 days of culture by ELISPOT assays. (A) Some of the cultures were preincubated with a broad-spectrum caspase inhibitor (Z-VAD-FMK) for 4 hours before FVIII was added. As a positive control for apoptosis, part of the cultures was incubated with 0.01 μg/mL FVIII in the presence of an agonistic anti–murine Fas antibody (Jo2). (B) Some of the cultures were incubated in the presence of Fas-Fc. As a negative control for Fas-Fc, part of the cultures was incubated in the presence of a human monoclonal IgG1. Presented is a typical experiment. Spleens of 5 mice were pooled for cell preparation. All experiments (n = 3) gave similar results.
Inhibition of memory B-cell restimulation by high-dose FVIII involves caspase activation and Fas-dependent mechanisms. CD138– spleen cells were obtained from hemophilic mice immunized with human FVIII and restimulated with either 0.01 or 20 μg/mL as indicated. Newly formed ASCs were analyzed after 6 days of culture by ELISPOT assays. (A) Some of the cultures were preincubated with a broad-spectrum caspase inhibitor (Z-VAD-FMK) for 4 hours before FVIII was added. As a positive control for apoptosis, part of the cultures was incubated with 0.01 μg/mL FVIII in the presence of an agonistic anti–murine Fas antibody (Jo2). (B) Some of the cultures were incubated in the presence of Fas-Fc. As a negative control for Fas-Fc, part of the cultures was incubated in the presence of a human monoclonal IgG1. Presented is a typical experiment. Spleens of 5 mice were pooled for cell preparation. All experiments (n = 3) gave similar results.
Discussion
On the basis of the successful use of high-dose FVIII for the induction of immune tolerance in patients with hemophilia A, we questioned whether the restimulation of FVIII-specific memory B cells is affected by high concentrations of FVIII in vitro or high doses of FVIII in vivo. Our results demonstrate that concentrations of FVIII that are below the physiologic plasma concentration of 0.1 μg/mL (1 U/mL) restimulate FVIII-specific memory B cells and induce their differentiation into ASCs in vitro, whereas concentrations that are above the physiologic plasma concentration inhibit the restimulation. These results support the idea that the inactivation of FVIII-specific memory B cells might be involved in the down-regulation of established anti-FVIII antibody responses in patients. However, in vitro findings do not necessarily correlate with events that occur in vivo. Technically, it is difficult to apply daily intravenous injections to mice over several months in the same way as to patients during immune tolerance induction therapy. Therefore, we used an adoptive transfer model that we described previously16 to investigate the effects of high-dose FVIII on the differentiation of FVIII-specific memory B cells in vivo. Our results support the idea that there is a FVIII dose threshold for boosting the restimulation and differentiation of FVIII-specific memory B cells versus inhibiting it. For human FVIII, single doses of 0.2 and 2 μg FVIII restimulated memory B cells in vivo, whereas doses above 2 μg inhibited the restimulation. For murine FVIII, doses above 20 μg FVIII were required to inhibit memory B-cell responses in vivo. Whereas murine FVIII was B-domain–deleted FVIII (full-length FVIII was not available), human FVIII was recombinant full-length FVIII. It is not possible to decide whether the higher doses required with murine FVIII were due to species differences between murine and human FVIII or due to the lack of the B domain in the murine FVIII.
Patients with hemophilia A who undergo high-dose immune tolerance induction therapy (ITI) receive up to 300 U FVIII (= 30 μg)/kg per day over several months.27 From our results in mice we would like to speculate that these doses of FVIII given daily over several months might induce effects similar to those induced by the application of a single high dose that we used in mice. Clearly proof for this hypothesis can only be obtained in a clinical trial with the kinetics of FVIII-specific memory B cells monitored during the therapy. During the early phase of ITI in patients, it is to be expected that part of the FVIII is rapidly bound in immune complexes as a result of the presence of anti-FVIII antibodies in the circulation. We are currently investigating how these immune complexes might modulate the inhibition of memory B-cell restimulation by high doses of FVIII.
Previously, Gilles et al28 suggested that the induction of anti-idiotypic antibodies might be involved in the neutralization of anti-FVIII antibodies in patients during ITI. Anti-idiotypic antibodies could interact with B cells carrying the corresponding idiotypes, which might result in the functional inactivation or even deletion of these B cells.29,30 It remains to be shown whether anti-idiotypic antibodies could inhibit or delete FVIII-specific memory B cells.
Previously, we showed that the restimulation of FVIII-specific memory B cells by FVIII depends strictly on interactions with activated T cells.16 Therefore, inhibition of memory B-cell restimulation by high concentrations of FVIII could be because of either a direct action on memory B cells or an inhibition of the stimulation of FVIII-specific T cells. Our results support the idea that the inhibition is because of a direct action on memory B cells. It is likely that the inhibition is mediated by signals that are initiated after triggering the B-cell receptor (BCR) because the effect of FVIII is antigen specific. Interaction of the BCR with its specific antigen activates a series of signaling cascades that result in proliferation and differentiation, anergy or apoptosis, depending on the differentiation state of the cell, the dose, and molecular form of the antigen and additional signals that the cell receives.31 Our findings suggest that the inhibition of memory B-cell restimulation is irreversible. This could be because of either the activation of apoptosis or the induction of anergy. We found that the inhibition depends on the activation of caspases which would suggest that the irreversible inhibition of memory B-cell restimulation might be because of the induction of apoptosis. Recently, Yu et al32 showed that the caspase inhibitor that we used for our experiments is able to induce autophagy, another form of cell death. This might be the reason for our observation that inhibition of caspases could not completely rescue the restimulation of memory B cells. The partial prevention of memory B-cell inhibition by soluble Fas protein indicates that Fas-Fas ligand interactions are involved in the inhibition of memory B-cell restimulation. The question arises which cells provide the Fas ligand required for this inhibition. One possibility is the Fas ligand expressed by activated T cells that are present in the culture. Our results suggest that the inhibition of memory B-cell restimulation is mediated by a direct effect of FVIII on memory B cells. We cannot exclude, however, that activated T cells add to the direct effect of FVIII. Another possible explanation could be that activated memory B cells themselves are able to express Fas ligand if they receive a very strong stimulus via the BCR. Several previous studies have shown that activated B cells are able to express Fas ligand.33,34
Berard et al35,36 recently found that BCR ligation of human memory B cells induced apoptosis when they were previously activated through CD40 or the BCR. Neither CD40 ligand nor T-cell–derived mediators (including IL-4) were able to counteract the death-promoting effect of surrogate antigen on activated memory B cells.36 The investigators reported that transduction of the death signal via the BCR sequentially proceeded through a caspase-independent and a caspase-dependent phase.36 These results would support our hypothesis that FVIII-specific memory B cells are triggered via the BCR to undergo apoptosis and that this process depends on the activation of caspases. However, our results have to be interpreted with great caution because of the nature of the cell system that we used for our studies. CD138– spleen cells are total spleen cells that are only depleted of antibody-producing plasma cells. Any manipulation of signaling pathways or receptor-ligand interactions does not only affect memory B cells but all cell populations that are present in this cell mixture. Therefore, it is not possible to distinguish between direct actions on memory B cells and indirect actions through effects on other cell populations. Purified FVIII-specific memory B cells would be necessary to explain the exact mechanism by which high concentrations of FVIII inhibit the restimulation of these cells. However, purified memory B cells would need additional costimulatory signals for restimulation and differentiation in vitro that are normally provided by interactions with other cells such as activated T cells. Therefore, we are currently combining the restimulation of FVIII-specific memory B cells in CD138– spleen cells in vitro with single-cell analysis of individual cells in this mixture of different cell populations to obtain further information on the mechanisms that cause the inhibition of memory B-cell restimulation by high-dose FVIII.
In conclusion, the restimulation and differentiation of FVIII-specific memory B cells in hemophilic mice is sensitive to increasing doses of FVIII. There is a threshold dose of FVIII for the optimal stimulation of memory responses both in vitro and in vivo. FVIII doses above this threshold inhibit, rather than stimulate, FVIII-specific memory B-cell responses. This inhibition is irreversible. We believe that the selective inhibition of memory B-cell responses could be an important event in the down-regulation of anti-FVIII antibody responses during long-term treatment of patients with high doses of FVIII. The eradication of memory B cells would prevent their differentiation into ASCs and, moreover, may lead to a deficiency of effective antigen-presenting cells required for the restimulation of FVIII-specific T cells. The induction of regulatory T cells rather than effector T cells could be the consequence of this deficiency. Further studies are necessary to prove this hypothesis.
Prepublished online as Blood First Edition Paper, August 9, 2005; DOI 10.1182/blood-2005-03-1182.
Supported by Baxter and in part by a grant from the National Institutes of Health (R01-HL40921) and by Hemophilia of Georgia.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Elisabeth Hopfner, Gerald Brachtl, Marcus Beutel, and Monika Grewal for technical assistance. We also thank Hans-Dieter Volk for his critical review and Elise Langdon-Neuner for editing the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal