Abstract
High-dose melphalan followed by stem cell transplantation (HDM-SCT) has become the treatment of choice for patients with immunoglobulin light chain amyloidosis (AL). Unfortunately, treatment mortality can be excessive in certain subpopulations. We have noted that patients who gained more than 2% body weight during mobilization had a poorer outcome following HDM-SCT. Excluding 2 patients for lack of weight record and denial of consent, 126 patients between July 1997 and June 2003 were retrospectively studied. Weight increased more than 2.0% during mobilization in 51.6% of the patients. Patients who accumulated more than 2.0% tended to have more proteinuria, more organs involved, lower serum albumin, more diuretic use, and dosage adjustment during mobilization. First-year mortality was significantly higher in those with more than 2% weight gain (33.9% versus 9.8%, P = .002). Multivariate analysis showed weight gain, glomerular filtration rate, and septal thickness to be independent predictors of first-year mortality. The increase in mortality was noted even after the excess weight was diuresed prior to conditioning. The impact on mortality dissipated after the first year. Weight gain during mobilization appears to be a new marker of adverse outcome following HDM-SCT. Better prognostication may improve the treatment mortality rate of these patients.
Introduction
Immunoglobulin light chain amyloidosis (AL) is a plasma cell dyscrasia characterized by systemic deposition of fibrillar monoclonal light chain fragments. Systemic involvement carries a poor prognosis, as the relentless deposition of amyloid eventually leads to organ failure and death. Until recently, effective therapeutic options for AL were limited. In several clinical trials, median survival was marginally increased to approximately 18 months with conventional chemotherapy.1-6 Patients who were able to receive longer duration of therapy did better, but many patients died before response could be achieved.2,3 This is especially true for patients with symptomatic cardiac involvement whose life expectancy was less than 6 months.2,3
The outlook for these patients markedly improved with the advent of high-dose melphalan followed by stem cell transplantation (HDM-SCT). The more rapid reduction of plasma cell clones significantly enhanced survival. In the largest study to date, Boston University reported its results on 312 patients treated with HDM-SCT.7 Their overall median survival was 4.6 years. Outcome of patients with symptomatic cardiac involvement remained poor (median survival of 1.6 years) but appeared better than the results with conventional chemotherapy.1 Patients without cardiac involvement had not reached their median survival. The intensive treatment resulted in complete hematologic response in 40% at 1 year, which correlated with better organ response and overall survival. Similar results had also been reported by the Mayo Clinic.8 In a case-controlled analysis, patients who underwent HDM-SCT were shown to have a survival advantage over those who received conventional chemotherapy.9
Despite its superiority over conventional chemotherapy, HDM-SCT is not suitable for all cases of AL. The treatment mortality rate can be prohibitively high in certain subgroups of patients. The overall treatment mortality rate reported by medical centers specializing in AL is approximately 13%.7,8 This rate, however, is often much higher at centers with less experience.10,11 A recent study of a small cohort of patients suggests patient selection has an enormous impact on the overall outcome.10 This one factor was felt to be responsible for reducing the treatment mortality rate from 50% to 20% at one medical center. Others have identified cardiac involvement, reduced glomerular filtration rate (GFR), high proteinuria, advanced age, poor Eastern Cooperative Oncology Group (ECOG) performance status, multiorgan involvement, and elevated liver function tests as additional risk factors for poor outcome in this population.7,10,12 The significance of these risk factors varies between centers, probably as a reflection of their experience in performing transplants in AL patients. In our program, we have observed that excessive fluid accumulation during stem cell mobilization carries a poor prognosis for AL patients undergoing HDM-SCT. This study was conducted to examine the impact of fluid accumulation during mobilization on the outcome of patients after HDM-SCT.
Patients, materials, and methods
Patients
The amyloid database was queried for AL patients who underwent HDM-SCT between July 1997 and June 2003. Diagnosis of amyloidosis was made by demonstration of Congo Red birefringence on tissue biopsies. AL was confirmed by immunohistologic evidence of light chains in the amyloid deposits and the presence of a plasma cell dyscrasia (circulating monoclonal paraprotein or light chain restriction of a plasma cell population in bone marrow). Details of eligibility for HDM-SCT were described elsewhere.13 Patients must have evidence of visceral involvement to qualify for the high-dose therapy. Those with amyloidosis other than AL or localized disease were excluded. A minimum of 1 year of follow-up is required for inclusion into this study. Written informed consent for the data collection was obtained. This study was approved by the institutional review board at the Mayo Foundation in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act (HIPPA) guidelines.
Mobilization and conditioning
Prior to July of 2001, stem cells were mobilized by either granulocyte colony-stimulating factor (G-CSF, 5 μg/kg per day) alone or granulocyte macrophage colony-stimulating factor (GM-CSF, 250 μg/m2) with cyclophosphamide (1.5 g/m2). After July 2001, the practice of cyclophosphamide pulsing was discontinued. Collection continued until a minimum of 2 × 106 CD34+ cells/kg was collected. Conditioning was performed with melphalan (140 mg/m2) with total body irradiation (12 Gy) or melphalan (200 mg/m2) until January of 1999. Afterward, melphalan alone was used in dosages of 200 mg/m2, 140 mg/m2, or 100 mg/m2.
Data collection and definitions
Baseline (prior to mobilization) demographics and clinical data were collected retrospectively from the electronic medical records. Septal thickness and ejection fraction were measured by echocardiography. Renal function in this study was represented by glomerular filtration rate (GFR) corrected for body surface area. GFR was determined by nonradiolabeled iothalamate clearance.14 Proteinuria was measured by 24-hour urine collection. The criteria for organ involvement were previously described.12 All tissue biopsies were reviewed for vascular involvement by AL. This was defined by demonstration of either Congo Red birefringence or amyloid fibril within the vascular wall of the tissue examined. Hematologic response was defined by a 50% reduction of circulating monoclonal protein or complete eradication if the paraprotein was too small to be quantified.15
Cardiac biomarkers were available and collected on a number of patients in this study. These were measured prior to HDM-SCT. Based on earlier work, cardiac troponin T (cTnT) and N-terminal pro–brain natriuretic peptide (NT-proBNP, amino acids 1-76) were chosen for their prognosticating ability in this population.16,17 Details of the analytic techniques were previously described.16
Fluid accumulation was characterized by a rapid increase in body weight. Each patient is weighed the same way (with or without shoes and jacket) by a nurse during each visit to the Blood and Marrow Transplant Clinic. Baseline weight was measured prior to mobilization. Weight was measured daily during mobilization and collection. The highest weight during mobilization and collection was used to calculate (peak) weight increase. Weight was also recorded prior to conditioning. Patients were divided into 2 groups based on whether the peak weight gain exceeded 2%. Diuretic use was recorded at baseline and during mobilization and collection, along with any changes. Diuretics were administered at the discretion of the primary provider.
Statistical analysis
Statistical calculations were performed using JMP software package version 5.1.2 from SAS Institute (Cary, NC). Pearson chi-square tests were performed on categoric data. 2-Sample t tests and Wilcoxon rank sum tests were used to compare continuous variables between the 2 weight groups. Variables with P values below .05 were considered significant. A multivariate logistic regression was later adopted with respect to the weight gain using all statistically significant pretransplant variables and separately with posttransplant variables. In the multivariate analysis, cardiac biomarkers were treated as categoric variables based on a cutoff of 0.035 ng/mL for cTnT and 332 pg/mL for NT-proBNP, while the rest of the variables were evaluated as continuous. Overall survival was calculated from the time of HDM-SCT to the last day of follow-up for each patient, and the survival curve was constructed using the Kaplan-Meier method. The log-rank tests were used to detect overall survival differences of each categoric factor, and the multivariate Cox proportional hazard model with hazard ratio and its 95% confidence intervals (CIs) were used to utilize backward elimination of the least significant factors. The nominal logistic model was used to identify variables related to first-year mortality.
Results
Patient characteristics
One-hundred and twenty-eight patients underwent HDM-SCT during the study period. One patient declined consent for the study and another did not have a complete weight record. Analysis was performed on the remaining 126 patients. No one was lost to follow-up. Age and sex were well matched between the 2 groups (Table 1). Patients who gained more than 2% body weight during mobilization, however, were more likely to have greater proteinuria, more organs involved, lower serum albumin, and more diuretic use. No differences were noted in the mobilization and conditioning regimens.
Baseline characteristics and clinical data of the 126 patients
. | Weight gain of 2% or less . | Weight gain of more than 2% . | P . |
|---|---|---|---|
| No. patients | 61 | 65 | NA |
| Age, y, median (range) | 53.7 (39.4-68.2) | 54.5 (30.6-70) | .21 |
| Sex, % male | 59.0 | 52.3 | .45 |
| Ejection fraction, %, median (range) | 65 (28-84) | 65 (38-83) | .71 |
| Septal thickness, mm, mean (range) | 12 (7-24) | 12 (8-25) | .52 |
| Elevated troponin T, % | 7.7 | 23.1 | .05 |
| Elevated NT-proBNP, % | 46.2 | 46.7 | .97 |
| GFR, mL/min/1.73m2, median (range) | 76.5 (9-155) | 65 (10-133) | .18 |
| Proteinuria, g/d, median (range) | 3.5 (0.03-18.5) | 5.4 (0.02-26.2) | .01* |
| Albumin, g/dL, median (range) | 3.0 (1.1-4.3) | 2.3 (1.0-4.4) | .03* |
| Alkaline phosphatase, U/L, median (range) | 171 (85-1350) | 186 (67-936) | .3 |
| Organ involvement, kg, median (range) | 1 (1-3) | 2 (1-4) | .002* |
| Baseline weight, median (range) | 79.0 (49-141.2) | 75.0 (43.3-113.1) | .38 |
| Weight change, %, median (range) | 0.16 (-8.4-2.0) | 4.6 (2.1-23.0) | < .001* |
| Weight at conditioning, kg, median (range) | 78.3 (47.6-145) | 78.0 (45.3-113.0) | .86 |
| Diuretic use, % | |||
| Baseline | 37.7 | 60.0 | .012* |
| End of collection | 42.6 | 78.5 | < .001* |
| Change in diuretic dose, % | 9.8 | 43.1 | < .001* |
| Mobilization, % | .60 | ||
| G-CSF | 80.3 | 70.8 | |
| G-CSF + GM-CSF | 1.6 | 4.6 | |
| GM-CSF | 3.3 | 3.1 | |
| Cytoxan + G-CSF | 4.9 | 10.8 | |
| Cytoxan + GM-CSFF | 9.8 | 10.8 | |
| Conditioning, % | .57 | ||
| Melphalan, 200 mg/m2 | 62.3 | 50.8 | |
| Melphalan, 140 mg/m2 | 19.7 | 29.2 | |
| Melphalan, 100 mg/m2 | 8.2 | 9.2 | |
| 140 mg/m2 + TBI | 9.8 | 10.8 |
. | Weight gain of 2% or less . | Weight gain of more than 2% . | P . |
|---|---|---|---|
| No. patients | 61 | 65 | NA |
| Age, y, median (range) | 53.7 (39.4-68.2) | 54.5 (30.6-70) | .21 |
| Sex, % male | 59.0 | 52.3 | .45 |
| Ejection fraction, %, median (range) | 65 (28-84) | 65 (38-83) | .71 |
| Septal thickness, mm, mean (range) | 12 (7-24) | 12 (8-25) | .52 |
| Elevated troponin T, % | 7.7 | 23.1 | .05 |
| Elevated NT-proBNP, % | 46.2 | 46.7 | .97 |
| GFR, mL/min/1.73m2, median (range) | 76.5 (9-155) | 65 (10-133) | .18 |
| Proteinuria, g/d, median (range) | 3.5 (0.03-18.5) | 5.4 (0.02-26.2) | .01* |
| Albumin, g/dL, median (range) | 3.0 (1.1-4.3) | 2.3 (1.0-4.4) | .03* |
| Alkaline phosphatase, U/L, median (range) | 171 (85-1350) | 186 (67-936) | .3 |
| Organ involvement, kg, median (range) | 1 (1-3) | 2 (1-4) | .002* |
| Baseline weight, median (range) | 79.0 (49-141.2) | 75.0 (43.3-113.1) | .38 |
| Weight change, %, median (range) | 0.16 (-8.4-2.0) | 4.6 (2.1-23.0) | < .001* |
| Weight at conditioning, kg, median (range) | 78.3 (47.6-145) | 78.0 (45.3-113.0) | .86 |
| Diuretic use, % | |||
| Baseline | 37.7 | 60.0 | .012* |
| End of collection | 42.6 | 78.5 | < .001* |
| Change in diuretic dose, % | 9.8 | 43.1 | < .001* |
| Mobilization, % | .60 | ||
| G-CSF | 80.3 | 70.8 | |
| G-CSF + GM-CSF | 1.6 | 4.6 | |
| GM-CSF | 3.3 | 3.1 | |
| Cytoxan + G-CSF | 4.9 | 10.8 | |
| Cytoxan + GM-CSFF | 9.8 | 10.8 | |
| Conditioning, % | .57 | ||
| Melphalan, 200 mg/m2 | 62.3 | 50.8 | |
| Melphalan, 140 mg/m2 | 19.7 | 29.2 | |
| Melphalan, 100 mg/m2 | 8.2 | 9.2 | |
| 140 mg/m2 + TBI | 9.8 | 10.8 |
Values in parentheses indicate the range.
TBI indicates total body irradiation; NA, not applicable.
Significant P values.
Sixty-five (51.6%) patients experienced weight gain of more than 2% during mobilization. The median weight increase during mobilization by all patients was 2.25%. This ranged from –8.9% to +23%. Both diuretic use at baseline and dosage adjustment during mobilization were more common in the more-than-2% weight gainers (Table 1). At the end of collection, 78.5% of the more-than-2% weight gainers were on diuretics versus 42.6% of the 2%-or-less weight gainers. Despite the differences in weight accumulation, the preconditioning weight was similar between the 2 groups.
Cardiac biomarkers were not available on every patient in this study. Thirty-nine patients from each group had cTnT measured. NT-proBNP was measured in 26 patients with 2% or less weight gain and 30 of those with more. Overall levels of cTnT or NT-proBNP were similar between the 2 groups. However, patients with more than 2% weight gain were more likely to have cTnT levels higher than 0.035 ng/mL (23.1% vs 7.7%, Table 1). No differences were found with NT-proBNP.
Bone marrow biopsy was available on all but one patient. Vascular involvement was demonstrated in 76% of the biopsies. Thirty-five percent of the patients had at least one renal biopsy. Sixty-five percent of the renal biopsies showed vascular involvement. Biopsies of the heart and gastrointestinal tract, including liver, were performed on 12% and 25% of the patients, respectively. All had evidence of vascular involvement by AL. Evidence of vascular involvement on tissue biopsies was demonstrated in 55 (90.2%) patients with 2% or less weight gain and 57 (87.7%) patients who gained more than 2% body weight. No significant difference was noted between the 2 groups.
Impact on survival
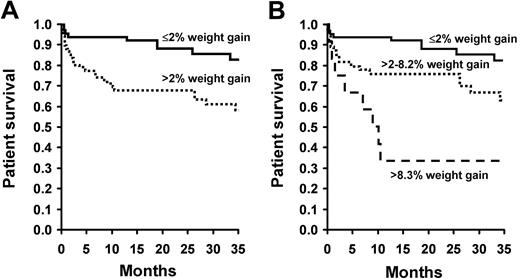
A gain of 2% or more body weight during mobilization had an unfavorable and significant impact on survival after HDM-SCT (Figure 1A). The impact was most evident during the first year. The difference in survival became significant at 100 days and continued up to 1 year (Table 2). The 1-year mortality rate was 33.9% for those who gained more than 2% during mobilization versus 9.8% for those with 2% or less (odds ratio [OR] = 4.69, CI = 1.84 to 13.66, P = .002). The risk was even more pronounced for the top 10% weight gainers (> 8.3% body weight). Their 1-year mortality rate was 66.7% (OR = 18.77, CI = 10.26-99.23, P = .001, Figure 1B). The leading cause of death for the entire cohort was cardiac-related complications. Overwhelming infections and hepatic failure were responsible for a number of deaths in the more-than-2% weight gainers (Table 3). The association of weight gain and mortality was most important in the first year. No relationship was noted between the weight at baseline or conditioning with the outcome after HDM-SCT. We also examined whether successful diuresis prior to conditioning would improve the outcome of the more-than-2% weight gainers. The results suggested that once these patients gained more than 2% of their body weight during mobilization, diuresis back to baseline did not improve their 1-year mortality.
Survival comparison after HDM-SCT, by weight gain
. | No. who gained 2% or less . | No. who gained more than 2% . | P . |
|---|---|---|---|
| 30-day survival | 95.1 | 87.7 | .13 |
| 100-day survival | 93.4 | 80.0 | .02 |
| 1-year survival | 90.2 | 66.2 | .001 |
. | No. who gained 2% or less . | No. who gained more than 2% . | P . |
|---|---|---|---|
| 30-day survival | 95.1 | 87.7 | .13 |
| 100-day survival | 93.4 | 80.0 | .02 |
| 1-year survival | 90.2 | 66.2 | .001 |
Causes of death in the first year after HDM-SCT, by weight gain
Cause of death . | No. who gained 2% or less . | No. who gained more than 2% . |
|---|---|---|
| Cardiac | 4 | 8 |
| Hepatic failure | 0 | 3 |
| Sepsis | 1 | 4 |
| ARDS/multiorgan failure | 1 | 2 |
| Pneumonia | 0 | 2 |
| Brain hemorrhage | 0 | 1 |
| Disseminated fungal infection | 0 | 1 |
| Pulmonary embolism | 0 | 1 |
| Total | 6 | 22 |
Cause of death . | No. who gained 2% or less . | No. who gained more than 2% . |
|---|---|---|
| Cardiac | 4 | 8 |
| Hepatic failure | 0 | 3 |
| Sepsis | 1 | 4 |
| ARDS/multiorgan failure | 1 | 2 |
| Pneumonia | 0 | 2 |
| Brain hemorrhage | 0 | 1 |
| Disseminated fungal infection | 0 | 1 |
| Pulmonary embolism | 0 | 1 |
| Total | 6 | 22 |
ARDS indicates acute respiratory distress syndrome.
Patient survival by weighted gain. (A) Overall survival of all 126 patients following HDM-SCT by Kaplan-Meier method. Differences in survival were calculated by log-rank test. Patients with 2% or less weight gain during mobilization are represented by the solid line, while the dotted line represents those with more than 2%. Survival was significantly worse for patients who gained more than 2% body weight (P = .004). (B) Survival analyzed by the percentage of body weight gained (≤ 2%, 2%-8.29%, > 8.3%). Mortality was highest in the top 10% (> 8.3%) weight gainers (P = .001).
Patient survival by weighted gain. (A) Overall survival of all 126 patients following HDM-SCT by Kaplan-Meier method. Differences in survival were calculated by log-rank test. Patients with 2% or less weight gain during mobilization are represented by the solid line, while the dotted line represents those with more than 2%. Survival was significantly worse for patients who gained more than 2% body weight (P = .004). (B) Survival analyzed by the percentage of body weight gained (≤ 2%, 2%-8.29%, > 8.3%). Mortality was highest in the top 10% (> 8.3%) weight gainers (P = .001).
Univariate and multivariate analyses
In addition to weight gain, univariate analysis identified lower GFR, increased septal thickness, more organs involved, higher alkaline phosphatase, diuretic use, and elevated cTnT (> 0.035 ng/mL) and NT-proBNP (> 332 pg/mL) as predictors of first-year mortality following HDM-SCT (Table 4). Only weight gain, GFR, and septal thickness remained independent predictors in the multivariate analysis (Table 5). The mortality risk was highest with patients who gained more than 8.3% body weight. Normal cTnT and NT-proBNP levels and melphalan dose of 200 mg/m2 were associated with a better long-term survival, but were not independently predictors of first-year outcome. Hematologic response strongly correlated with a favorable first-year and long-term outcome (P < .001). Hematologic response was more common in the 2%-or-less weight gainers than the more-than-2% weight gainers (73.8% vs 56.9%, P = .04). Even when hematologic response was considered, GFR (P = .01) and weight gain (P = .01) remained independent predictors of first-year mortality.
Univariate analysis of baseline variables related to first-year mortality following HDM-SCT
Baseline characteristic . | OR . | P . |
|---|---|---|
| Diuretic use | 0.3 | .001 |
| Weight gained, % | 292.8 | .002 |
| GFR | 0.01 | .002 |
| Troponin T (> 0.035 ng/mL) | 5.60 | .01 |
| NT-proBNP (> 332 pg/mL) | 6.22 | .03 |
| Organs involved | .03 | |
| Septal thickness | 9.94 | .05 |
| Alkaline phosphatase | 8.86 | .05 |
| Change in diuretic dose | 0.47 | .10 |
| Conditioning | .12 | |
| Albumin | 0.42 | .29 |
| Proteinuria | 2.49 | .36 |
| Age | 1.44 | .72 |
| Mobilization | .77 | |
| Sex | 1.13 | .78 |
| Ejection fraction | 1.42 | .80 |
| Pretransplant weight | 1.29 | .84 |
| Vascular involvement | 0.95 | .94 |
Baseline characteristic . | OR . | P . |
|---|---|---|
| Diuretic use | 0.3 | .001 |
| Weight gained, % | 292.8 | .002 |
| GFR | 0.01 | .002 |
| Troponin T (> 0.035 ng/mL) | 5.60 | .01 |
| NT-proBNP (> 332 pg/mL) | 6.22 | .03 |
| Organs involved | .03 | |
| Septal thickness | 9.94 | .05 |
| Alkaline phosphatase | 8.86 | .05 |
| Change in diuretic dose | 0.47 | .10 |
| Conditioning | .12 | |
| Albumin | 0.42 | .29 |
| Proteinuria | 2.49 | .36 |
| Age | 1.44 | .72 |
| Mobilization | .77 | |
| Sex | 1.13 | .78 |
| Ejection fraction | 1.42 | .80 |
| Pretransplant weight | 1.29 | .84 |
| Vascular involvement | 0.95 | .94 |
Independent variables predictive of first-year mortality after HDM-SCT by nominal logistic model
. | OR . | CI . | P . |
|---|---|---|---|
| GFR | 0.014 | 0.0006-0.268 | .007 |
| Septal thickness | 16.23 | 1.21-257.65 | .038 |
| Weight gained | |||
| More than 2.0% and less than 8.29% | 3.18 | 1.10-10.27 | .039 |
| More than 8.3% | 18.77 | 4.26-99.23 | .001 |
. | OR . | CI . | P . |
|---|---|---|---|
| GFR | 0.014 | 0.0006-0.268 | .007 |
| Septal thickness | 16.23 | 1.21-257.65 | .038 |
| Weight gained | |||
| More than 2.0% and less than 8.29% | 3.18 | 1.10-10.27 | .039 |
| More than 8.3% | 18.77 | 4.26-99.23 | .001 |
Discussion
In this study, patients who gained an excessive amount of fluid during mobilization had a significantly higher mortality rate after HDM-SCT than those who did not. A 3-fold increase in mortality was noted in the first year after HDM-SCT if the baseline weight was increased 2% or more during mobilization. Patients who gained more than 8.3% body weight carried the highest risk. The impact on survival was most influential during the first 12 months after transplantation. This effect was independent of cardiac, renal, or hepatic dysfunction. Subgroup analysis showed that diuresis was not beneficial once the 2% threshold was breached, indicating that the excess fluid was not directly responsible for the higher mortality. Instead, fluid accumulation appears to be a surrogate marker rather than a direct cause.
Weight gain was the strongest predictor of first-year mortality among all of the other variables tested. While analysis showed weight gain, GFR and septal thickness were the best independent predictors of first-year survival; weight gain and elevated cTnT were also found to be independent predictors. However, when either cTnT or NT-proBNP was added to the model with septal thickness and GFR, all 3 variables became insignificant, leaving weight gain as the only independent predictor. This suggested that cTnT and NT-proBNP were linked to GFR and septal thickness. Since cardiac biomarkers were not available for every patient, we chose to use the model with weight gain, GFR, and septal thickness, as it was more representative of this patient population. It is important to remember that weight gain remained a significant and independent predictor in both models.
The cause of the excess fluid accumulation remains undetermined. While it is commonly seen in cardiac, renal, or hepatic dysfunction, all 3 had been ruled out. One condition that can mimic these organ dysfunctions is capillary leak syndrome (CLS). CLS can be seen after hematopoietic cell transplantation, especially if growth factors are used to accelerate engraftment.18,19 Although cytotoxics are often thought to be important, they are by no means a prerequisite.20 Most agree that CLS is the result of activation of proinflammatory cytokines.21 The incident may be higher with GM-SCF than G-CSF.19 In severe cases, systemic inflammatory response syndrome (SIRS) can also develop.18
There is some evidence to suggest that AL patients may be more prone to CLS/SIRS following growth factors. Gertz et al reported a case of fatal noncardiogenic pulmonary edema in an AL patient undergoing stem cell mobilization.22 During autopsy, advance vascular and interstitial amyloid that was not seen on premortem radiologic examination was found in the lung. This has prompted us to stop using growth factor after HDM-SCT in AL patients in order to avoid this complication. Vascular infiltration by amyloid appears to play a key role in the development of CLS/SIRS. Our data showed vascular involvement was common if not ubiquitous in this population. Simply having vascular amyloid deposition does not appear to be enough since nearly every patient in both groups had evidence of it. The development of CLS might indicate actual vascular dysfunction. This is equivalent to cardiac amyloid, which can be found in every AL patient, but only a portion develops cardiac dysfunction.23 If proven, vascular dysfunction should be viewed as a separate organ dysfunction. Unfortunately, measuring the vascular dysfunction may be difficult. In addition, it is unclear whether vascular dysfunction or the development of CLS is responsible for the increase in mortality. Comparison with nonamyloid patients should help shed light on the picture.
We acknowledge there were several limitations in this study. The retrospective nature of this study subjected it to all of the restrictions associated with this design. The most significant drawback was the diuretic use, especially after mobilization began. The lack of a protocol for dosage adjustment made it difficult to determine whether intervention could have made a difference. In addition, significant differences existed between the 2 groups at baseline. Fortunately, these differences did not influence the first-year mortality in the multivariate analysis. Finally, cardiac biomarkers were not available on every patient. As mentioned earlier in this section, this could potentially alter the multivariate analysis.
Despite the limitations, the current study suggests weight gain during mobilization is a useful risk assessment tool for AL patients. Proper patient selection has been shown to be important in reducing the treatment-related mortalities in this patient population.10 Excessive weight gain during mobilization can serve as a final screening test for those undergoing intensive therapy. For those who experience enormous weight gain (especially > 8.3%), alternative therapy could be considered to avoid excessive mortality risk. Oral melphalan and dexamethasone was recently shown to be a well-tolerated and, more importantly, effective therapy in patients who were not candidates for HDM-SCT.24 As this and other effective alternatives to HDM-SCT become available, pretransplant risk assessment will become increasingly more important to ensure that patients receive the most appropriate treatment.
Prepublished online as Blood First Edition Paper, July 21, 2005; DOI 10.1182/blood-2005-03-1206.
Supported in part by the Hematologic Malignancies fund of the Mayo Foundation.
N.L. performed the study design, manuscript preparation, and data accumulation; T.R.L. collected data accumulation; S.S.C. performed statistical analysis of data. A.D. contributed to writing and the database. M.Q.L. contributed to the database. M.A.G. cowrote the paper and maintained the database.
Presented in abstract form at the 46th American Society of Hematology Annual Meeting, San Diego, CA, December 4, 2004.25
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal