Epidermal growth factor receptor-1 (EGFR-1/HER-1/ErbB-1) regulates proliferation and cell fate during epidermal development. HER-1 is activated by several EGF-family ligands including heparin-binding epidermal growth factor–like growth factor (HB-EGF), a mitogenic and chemotactic molecule that participates in tissue repair, tumor growth, and other tissue-modeling phenomena, such as angiogenesis and fibrogenesis. We found that mesenchymal stem cells (MSCs), the precursors of different mesenchymal tissues with a role in processes in which HB-EGF is often involved, normally express HER-1, but not HB-EGF itself. Under the effect of HB-EGF, MSCs proliferate more rapidly and persistently, without undergoing spontaneous differentiation. This effect occurs in a dose-dependent fashion, and is specific, direct, and HER-1 mediated, as it is inhibited by anti–HER-1 and anti–HB-EGF blocking antibodies. Moreover, HB-EGF reversibly prevents adipogenic, osteogenic, and chondrogenic differentiation induced with specific media. These data show that HB-EGF/HER-1 signaling is relevant to MSC biology, by regulating both proliferation and differentiation.
Introduction
HER-1 (EGFR-1, ErbB), the receptor for epidermal growth factor (EGF), is involved in regulating proliferation and cell fate during epidermal development.1 Other receptors (HER-2, HER-3, HER-4) and other factors belonging to the EGF family, including betacellulin, heparin-binding epidermal growth factor–like growth factor (HB-EGF), epiregulin, transforming growth factor-α (TGF-α), and amphiregulin have been discovered.1-2 Among them, HB-EGF binds to HER-1 and HER-4 with high affinity; is expressed in a wide range of cell types of both epithelial and mesenchymal origin3-8 ; and promotes adhesion, proliferation, and chemotaxis on fibroblasts and smooth muscle cells (SMCs), chemotaxis on endothelial cells and astrocytes, and proliferation and survival on some epithelial cells.3-11 HB-EGF participates in tissue-modeling events and plays a role in complex proliferation and differentiation-related phenomena, such as blastocystis implantation,12 cutaneous wound healing,13 branching morphogenesis of the submandibular gland, angiogenesis, and normal heart development and function.14 HB-EGF is also involved in pathologic processes such as atheromatous lesions,7,15 cardiac hypertrophy,16 cystic fibrosis,17 and epithelial neoplastic growth.7 In all these phenomena, a stem cell pool of mesenchymal origin is necessary to build up the stromal and vascular architecture, to renew the damaged tissues, and to support normal or neoplastic proliferation.
Mesenchymal stem cells (MSCs) have been initially identified in the bone marrow (BM) as multipotent nonhematopoietic progenitor cells that differentiate into osteoblasts and adipocytes, chondrocytes, tenocytes, skeletal myocytes, and cells of visceral mesoderm.18-22 Recently, an MSC pool has been shown in tissues other than BM,23 and circulating MSCs can be normally detected in peripheral blood,24 thus suggesting that the mesenchymal tissue compartment may be supported by circulating BM MSCs, similarly to what happens for hematopoietic stem cells. In addition, exogenously administered MSCs spread into all tissues, but preferentially survive and proliferate in the presence of regenerating tissues and proliferating malignant cells, thus being incorporated into the tissue architecture as stromal fibroblasts, and preferentially interacting with neoplastic cells. For this reason, engineered MSCs may be used as an intratumor source of therapeutics.25 Although MSCs are rare in adult human BM (1/105), MSCs constitute approximately one third of the initial adherent BM-derived stromal colonies in vitro. They do not express hematopoietic markers, but a specific pattern of molecules, such as CD105, CD73, CD106 (vascular cell adhesion molecule-1 [VCAM-1]), CD54 (intercellular adhesion molecule-1 [ICAM-1]), CD44, CD90, CD29, and stromal derived factor-1 (STRO-1).18-22 MSCs interact with hematopoietic stem cells, influencing their homing and differentiation through cell-cell contact and the production of factors and chemokines. MSCs can be cotransplanted with hematopoietic stem cells to improve their engraftment in BM,26 and they may be used to repair or regenerate damaged or mutated bone, cartilage, and myocardial or hepatic tissues.19-22,27-28 Finally, MSCs have a significant immunoregulatory function, both toward developing thymocytes29 and peptide-specific mature T cells,30 which might play a role in tumor–T cell interaction during the neoplastic growth.
We show here that MSCs express HER-1 and that HB-EGF/HER-1 signaling profoundly affects MSC biology.
Materials and methods
Generation of MSCs
MSCs were generated from BM aspirates of healthy donors, collected after informed consent. BM mononuclear cells were obtained with density gradient centrifugation (Lymphoprep; Nycomed Pharma, Oslo, Norway) and cultured in 25-cm2 flasks (BD Falcon; Becton Dickinson, Milan, Italy) at a concentration of 30 × 106 cells in 5 mL Dulbecco modified Eagle medium (DMEM), with high glucose concentration, GLUTAMAX I, 15% heat-inactivated fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin (all from GibcoBRL/Life Technologies, Milan, Italy). Cultures were incubated at 37°C in a 5% CO2 atmosphere. After 72 hours, nonadherent cells were removed. When 70% to 80% confluent, adherent cells were trypsinized (0.05% trypsin at 37°C for 5 minutes; GibcoBRL/Life Technologies), harvested, and washed with medium to remove trypsin, and expanded in larger flasks. A homogenous cell population was obtained after 2 to 3 weeks of culture.
Flow cytometric analysis
MSCs were recognized by immunophenotype using monoclonal antibodies (MoAbs) specific for CD105 (endoglin), CD73, CD106 (VCAM-1), CD29, CD44, and CD90. In addition, we assessed the lack of endothelial cell (with anti-CD31 antibodies) and hematopoietic (with anti-CD45, -CD14, -CD11c, -CD123, and -CD34 antibodies) marker expression. All antibodies were purchased from Pharmingen/Becton Dickinson (Milan, Italy). MSCs were also stained with anti-CD10 and -CD56 (to assess adipocyte and osteoblast differentiation, respectively) and anti–HER-1 and –HER-4 (EGF/HB-EGF receptors) antibodies (all were purchased from Pharmingen/Becton Dickinson except anti–HER-4, which was from Neomarkers, Westinghouse, CA), and with polyclonal anti–HB-EGF Ab (Oncogene Research Products, Boston, MA). For immunophenotypic analysis, MSCs were detached using trypsin/EDTA (ethylenediaminetetraacetic acid) for 5 minutes, immediately washed with phosphate-buffered saline (PBS) to remove trypsin, and resuspended at 106/mL. No difference in marker expression (including MSC-related and hematopoietic molecules) was observed by gently detaching the cells using a cell scraper and then mixing the cell suspension by a syringe to disaggregate cell clusters. Cell suspension (100 μL) was incubated at +4°C for 10 minutes with 15% FCS, followed by incubation with the specific antibody at +4°C for 30 minutes. Cells were washed with PBS. At least 10 000 events were analyzed by flow cytometry (FACScalibur; Becton Dickinson, Milan, Italy) using Cell Quest software.
HB-EGF, HER-1, and HER-4 RNA expression
HB-EGF, HER-1, and HER-4 RNA expression by MSCs was evaluated with reverse-transcriptase–polymerase chain reaction (RT-PCR). Total RNA was isolated from human MSCs cultured without and with HB-EGF; total RNA was isolated from the A431 human breast cancer cell line as a positive control for HER-1 expression31 ; and the U937 cell line for HB-EGF and HER-4,4 respectively. An aliquot of 4 μg RNA was reverse-transcribed, as previously reported,4,31,32 and c-DNA was analyzed using the following primers: (1) HB-EGF sense 5′-TGGTGCTGAAGCTCTTTCTGG-3′ and antisense 5′-GTGGGAATTAGTCATGCCCAA-3′, to span exons 1 to 5 of the gene giving a fragment of 605 bp (complete form of HB-EGF c-DNA); (2) HER-1 sense 5′-GATACCCAGGACCCAG-3′ and antisense 5′-GCGACAATGAAAAACT-3′ (290-bp fragment); and (3) HER-4 sense 5′-AGATGGAGGTTTTGCTGCTGAACA-3′ and antisense 5′-TTACACCACAGTATTCCGGTGTCT-3′ (726-bp fragment). ACTB (beta-actin) served as quantitative control, detected by the following primers: sense 5′-GGCACCCAGCACAATGAAG-3′ and antisense 5′-GCTGATCCACATCTGCTGG-3′ (200-bp fragment). HB-EGF and HER-4 c-DNA was amplified as previously reported.4 The cycling parameters for HER-1 were as follows: 30 runs in a thermal cycler (GeneAmp PCR System 2400; Perkin Elmer, Norwalk, CT) using 1.25 U Taq polymerase (Perkin Elmer) in 50 μL (94°C for 30 seconds, 49°C for 60 seconds, 72°C for 90 seconds, followed by 7 minutes at 72°C). PCR products were separated by electrophoresis on 1.5% agarose gel.
MSC proliferation assays
To assess the enhancement of MSC number under the effect of HB-EGF, short-term (72 hours) 3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay was carried out as previously described.4,32 Briefly, 10 μL per well of 5 mg/mL MTT solution was added and the plates were incubated at 37°C for 4 hours. After adding 100 μL/well of 0.04 N HCl in isopropanol and thoroughly mixing, the plates were read (wave-lengths: test, 570; reference, 630 nm) on an AutoReader III (Ortho Diagnostic Systems, Raritan, NJ). Results were expressed as “additional proliferation,” that is, the percentage of increase of MSC proliferation following HB-EGF addition (P1) compared with the spontaneous MSC proliferation that normally occurred without HB-EGF (P2). In other words, (P1–P2)/P2 × 100. Blocking anti–HB-EGF and anti–HER-1 goat antibodies (from R&D Systems Europe, Abingdon, United Kingdom) were used at saturating concentration (50 μg/mL) to assess the HB-EGF/HER-1 signaling contribution to MSC proliferation. Indifferent goat immunoglobulins (Igs) were used as negative control for the blocking antibodies.
To assess the proliferating cell rate, 3H-thymidine incorporation assay was run in parallel for 72 hours and 7 days, and the effect of HB-EGF was compared with that of 1 ng/mL basic fibroblast growth factor (bFGF), which is currently used for MSC expansion.33 We also assessed whether FCS, a normal constituent of the complete culture medium, might simulate the effect of HB-EGF. Proliferation assay was performed in round-bottom 96-well plates (Costar, Cambridge, MA) in a total volume of 0.2 mL complete DMEM. A total of 1 μCi (0.037 MBq) [3H]-thymidine (ICN, Costa Mesa, CA) was added into each well 2 or 6 days after the addition of HB-EGF or bFGF, and cells were harvested on day 3 or day 7, respectively, onto glass fiber filters using an LKB 96-well harvester (Wallac Oy, Turku, Finland) after an additional 24 hours. [3H] thymidine uptake was measured on an LKB Betaplate counter (Wallac Oy). The results were expressed as mean counts per minute (cpm) for triplicate cultures (standard errors were routinely < 10%).
Long-term MSC proliferation was assessed without and with different HB-EGF concentrations, starting from 5 × 103 and 104 MSCs seeded into 24- or 6-well plates or 25-, 75-, or 175-cm2 flasks (BD Falcon; Becton Dickinson), by counting with flow cytometry all the cells harvested at 1, 2, and 3 weeks of culture before reaching cell confluence. Cell acquisition continued up to the end of the samples and was stopped as soon as no other cells were detected, thus preventing aspirated air bubbles, which could be incorrectly counted as cells. In addition, the flow cytometer was thoroughly washed between each sample to avoid any cross-contamination.
MSC differentiation assay
MSCs were tested for their ability to differentiate into adipocytes, osteoblasts, and chondrocytes, as previously described,18-22,26,30,34 in the presence or absence of human recombinant HB-EGF (R&D Systems Europe). Adipocyte differentiation was achieved after 2-week culture of MSCs with adipogenic medium (AM), containing 10–6 M dexamethasone, 10 μg/mL insulin, and 100 μg/mL 3-isobutyl-l-methylxantine (all from Sigma Immunochemicals, Milan, Italy). Osteoblast differentiation was achieved after 2-week culture with osteogenic medium (OM) containing 10–7 M dexamethasone, 50 μg/mL ascorbic acid, and 10 mM β-glycerophosphate (Sigma Immunochemicals). Chondrocyte differentiation was achieved after 2-week culture with chondrogenic medium (CM), containing 10–7 M dexamethasone and 10 ng/mL TGF-β (Sigma Immunochemicals). However, one-week culture with differentiation medium is sufficient to irreversibly prime MSCs toward a specific differentiation, with a good cell morphology preservation. Oil-red-O, von Kossa, and toluidine blue dyes were used to identify adipocytes, osteoblasts, and chondrocytes, respectively. More than 90% of the cells differentiated depending on the time left in culture with the differentiating agent.
Differentiation gene expression
We analyzed the expression of differentiation genes by MSCs under the effect of 2 conditioning media (AM and OM) with or without HB-EGF (50 ng/mL) or bFGF (1 ng/mL), by using quantitative real-time RT-PCR. Total RNA was isolated from human MSCs, and 1 μg RNA was converted to c-DNA with random primers by the First Strand c-DNA Synthesis Kit (AMV; Roche Diagnostic, Mannheim, Germany). Real-time quantitative PCR analysis was performed on an ABI Prism 7000 SDS (Applied Biosystems, Foster City, CA) using the TaqMan PCR Master Mix (Applied Biosystems). The TaqMan assays were chosen from the list of the Assays-on-Demand (Applied Biosystems). They included Hs00234592_m1 for PPAR-γ (peroxisome proliferative activated receptor, gamma), Hs00173452_m1 for LPL (lipoprotein lipase), and Hs00609791_m1 for FABP4 (fatty acid–binding protein 4, adipocyte) to test adipogenic differentiation35 ; Hs00173720_m1 for IBSP (integrin-binding sialoprotein bone sialoprotein, bone sialoprotein II) and Hs00231692_m1 for RUNX2 (runt-related transcription factor 2) to test osteogenic differentiation.36-37 All PCR reactions contained primers and probe diluted 1:20 and 5 ng c-DNA (total RNA equivalent) in 25 μL total volume, and samples were analyzed in triplicate. Thermal cycling included an initial incubation at 50°C for 10 minutes followed by 95°C for 10 minutes then 50 cycles of 15 seconds at 95°C for denaturation and 1 minute at 60°C for extension. Fluorescence emission of 6-carboxyfluorescein (FAM) was automatically measured during PCR run. A cycle threshold value of 45 was considered the end of the PCR run. The expression of each mRNA was calculated by relative quantification using the average of GAPD (glyceraldehyde-3-phosphate dehydrogenase) (Hs99999905_m1) and ACTB (beta-actin) (Hs99999903_m1) transcript level as reference. Data were analyzed as indicated in User Bulletin no. 2 (Applied Biosystems).38
HER-1 phosphorylation assay
The effect of HB-EGF by HB-EGF–producing cells (LP1 cell line; F.V., personal data, February 2000) was evaluated through its effect on HER-1 pY1068 phosphorylation in MSCs in a coculture system (cell-to-cell contact). MSCs were cultured in DMEM plus 15% FCS in 6-well plates (Costar) and then incubated in the absence or presence of 100 μg/mL anti–HB-EGF neutralizing moAbs as a control (R&D, Minneapolis, MN). LP1 cells (3 × 106) were directly added to the monolayer of MSCs and cocultured for 20 minutes. Subsequently, LP1 cells were removed and MSCs washed with cold PBS, collected, and stored at –80 °C until use to analyze the phosphorylation status of HER-1 1068 tyrosine (pY1068). MSCs were also stimulated for 20 minutes with 50 ng/mL recombinant human HB-EGF (R&D) to test their reactivity and as a control of the culture experiments.
Full-length HER-1 (EGFR) and HER-1(EGFR)-P (pY1068) ELISA
Cell lysate preparation. Cell pellets were washed twice with cold PBS and lysed for 30 minutes in 150 μL ice-cold cell extraction buffer (Biosource, Camarillo, CA) additioned with protease inhibitor cocktail (Sigma, St Louis, MO) and 1 mM phenylmethylsulfonyl fluoride (PMSF). The lysates were harvested, incubated for 30 minutes on ice, and vortexed at 10-minute intervals. After centrifugation at 16 060g (13 000 rpm) for 10 minutes at 4°C, aliquots of clear lysate were transferred to microfuge tubes and used for enzyme-linked immunosorbent assay (ELISA).
ELISA. Full-length EGFR and EGFR phosphorylated at tyrosine 1068 were measured using commercially available ELISA kits (Biosource). Briefly, 100 μL of samples (diluted 1:10) prepared as described above was incubated in duplicate overnight at 4°C. After washing, detection antibodies specific for human C-terminus of EGFR or EGFR phosphorylated at tyrosine 1068 was added to the wells. After removal of excess detection antibody, samples were treated with a horseradish peroxidase–labeled anti–rabbit IgG. After a third incubation and washing, the substrate solution was added. The enzymatic reaction was stopped and read within 30 minutes at 450 nm on a AutoReader III (Ortho Diagnostic Systems). Values of EGFR (pY1068) were normalized for full-length EGFR content. Results were expressed as percentage (x ± SD) of phosphorylation compared with the basal control (3 experiments). Each sample had comparable cell numbers and amount of HER-1 molecules.
Statistics
Statistical comparison of the proliferation assay arms (achieved on the basis of the variable cell number, HB-EGF culture concentration, and presence/absence of anti–HB-EGF or anti–HER-1 blocking antibodies, but homogeneous as far as their composition) was carried out according to the Student t test. Differences were considered statistically significant when P < .05.
Results
MSCs express and modulate surface HER-1 but not HB-EGF
MSC immunophenotype is shown in Table 1. Proliferating MSCs normally expressed surface CD105, CD73, CD29, CD44, and CD90 at high levels, and CD106 less intensely (Figure 1A), but they were negative for all the hematopoietic (CD45, CD14, CD11c, CD123, CD34) and endothelial (CD31) markers. Along with the differentiation into preadipocytes, preosteocytes, and prechondrocytes, cells progressively reduced the expression of CD105, CD73, and VCAM-1. At the same time, preadipocytes and preosteocytes became positive for CD10 and CD56, respectively (Table 1).
Immunophenotype of proliferating and differentiating MSCs
. | Proliferating MSCs . | HB-EGF-treated MSCs . | Preadipocytes . | Preosteocytes . | Prechondrocytes . |
|---|---|---|---|---|---|
| CD105 | ++ | ++ | ± | ± | ± |
| CD73 | ++ | ++ | ± | ± | ± |
| CD106 | ± | ± | ± | ± | ± |
| CD29 | ++ | ++ | ± | ± | ± |
| CD44 | +++ | +++ | + | + | + |
| CD90 | +++ | +++ | + | + | + |
| CD45 | - | - | - | - | - |
| CD14 | - | - | - | - | - |
| CD11c | - | - | - | - | - |
| CD123 | - | - | - | - | - |
| CD34 | - | - | - | - | - |
| CD31 | - | - | - | - | - |
| HB-EGF | - | - | - | - | - |
| HER-1 | + | - | - | - | - |
| HER-4 | - | - | - | - | - |
| CD10 | - | - | + | - | - |
| CD56 | - | - | - | + | - |
. | Proliferating MSCs . | HB-EGF-treated MSCs . | Preadipocytes . | Preosteocytes . | Prechondrocytes . |
|---|---|---|---|---|---|
| CD105 | ++ | ++ | ± | ± | ± |
| CD73 | ++ | ++ | ± | ± | ± |
| CD106 | ± | ± | ± | ± | ± |
| CD29 | ++ | ++ | ± | ± | ± |
| CD44 | +++ | +++ | + | + | + |
| CD90 | +++ | +++ | + | + | + |
| CD45 | - | - | - | - | - |
| CD14 | - | - | - | - | - |
| CD11c | - | - | - | - | - |
| CD123 | - | - | - | - | - |
| CD34 | - | - | - | - | - |
| CD31 | - | - | - | - | - |
| HB-EGF | - | - | - | - | - |
| HER-1 | + | - | - | - | - |
| HER-4 | - | - | - | - | - |
| CD10 | - | - | + | - | - |
| CD56 | - | - | - | + | - |
MSC immunophenotype have been analyzed by flow cytometry in standard conditions (proliferating cells), after 2-hour incubation with 25 ng/mL HB-EGF, and after culture with differentiation (adipogenic, osteogenic, chondrogenic) media. HB-EGF-treated MSCs lose HER-1 surface expression, which is restored a few hours after removing HB-EGF from the medium (data not shown). Results are expressed as intensity of expression (-, negative; ± weak expression; +, 1 log shift from negative control; ++, 2 log shifts from negative control; +++, 3 log shifts from negative control) and derive from 6 different experiments.
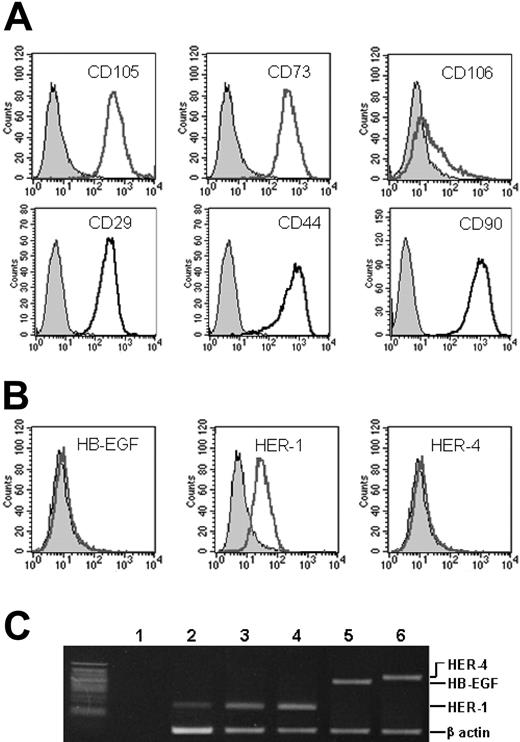
MSC marker expression. Proliferating MSCs were analyzed for the surface expression of (A) CD105 (endoglin), CD73, CD106 (VCAM-1), CD29, CD44, and CD90, which are all constitutively expressed, and (B) HB-EGF, HER-1, and HER-4 molecules. In panels A and B, the x axis measures positive marker, open curves, cells positive for the marker; and shaded curves, negative controls. Only HER-1 is expressed in standard conditions. The presence of the mRNA for HB-EGF, HER-1, and HER-4 was evaluated by RT-PCR on MSCs (C). Lane 1 indicates negative control (water); lane 2, MSCs; lane 3, MSCs after the addition of 25 ng/mL HB-EGF to culture; lane 4, A431 cell line (HER-1 positive control); and lanes 5-6, U937 cell line (HB-EGF and HER-4 positive controls). Beta-actin is the quantitative control.
MSC marker expression. Proliferating MSCs were analyzed for the surface expression of (A) CD105 (endoglin), CD73, CD106 (VCAM-1), CD29, CD44, and CD90, which are all constitutively expressed, and (B) HB-EGF, HER-1, and HER-4 molecules. In panels A and B, the x axis measures positive marker, open curves, cells positive for the marker; and shaded curves, negative controls. Only HER-1 is expressed in standard conditions. The presence of the mRNA for HB-EGF, HER-1, and HER-4 was evaluated by RT-PCR on MSCs (C). Lane 1 indicates negative control (water); lane 2, MSCs; lane 3, MSCs after the addition of 25 ng/mL HB-EGF to culture; lane 4, A431 cell line (HER-1 positive control); and lanes 5-6, U937 cell line (HB-EGF and HER-4 positive controls). Beta-actin is the quantitative control.
All proliferating MSCs showed surface membrane expression of HER-1 (Table 1; Figure 1B), except those treated with HB-EGF (Table 1). MSCs were always negative for HB-EGF and HER-4 (Figure 1B). These features were confirmed by RT-PCR (Figure 1C). MSCs were negative for HB-EGF and HER-4 (Figure 1C lanes 2-3) RNA, if compared with the U937 cell line (Figure 1C lanes 5-6). HER-1 RNA was present in proliferating MSCs (Figure 1C lane 2), like in the A431 cell line (Figure 1C lane 4), and still detectable after the addition of HB-EGF to the culture (Figure 1C lane 3), although MSCs down-regulated completely the surface HER-1 expression by one hour after HB-EGF addition, and expressed it again a few hours after HB-EGF removal from culture (data not shown). By contrast, differentiating MSCs (Table 1), and MSCs that were no longer proliferating, permanently lost the expression of surface HER-1 (data not shown).
Effect of HB-EGF on MSC expansion. Enhancement of MSC proliferation was induced by HB-EGF in a dose-dependent fashion with concentrations between 5 to 50 ng/mL, starting from both 5 × 103 (A) and 104 (B) cells. MSC expansion with 5 ng/mL (▦), 25 ng/mL (▪), and 50 ng/mL (□) HB-EGF was assessed by MTT method at 72 hours and expressed as additional proliferation (the percentage of increase of MSC proliferation following HB-EGF addition [P1] compared with the spontaneous MSC proliferation that normally occurs without HB-EGF [P2]). In other words, (P1–P2)/P2 × 100. P < .01 refers to the difference between the spontaneous expansion without HB-EGF and the additional proliferation with 5, 25, and 50 ng/mL HB-EGF. The figure shows means ± SD of 5 different experiments.
Effect of HB-EGF on MSC expansion. Enhancement of MSC proliferation was induced by HB-EGF in a dose-dependent fashion with concentrations between 5 to 50 ng/mL, starting from both 5 × 103 (A) and 104 (B) cells. MSC expansion with 5 ng/mL (▦), 25 ng/mL (▪), and 50 ng/mL (□) HB-EGF was assessed by MTT method at 72 hours and expressed as additional proliferation (the percentage of increase of MSC proliferation following HB-EGF addition [P1] compared with the spontaneous MSC proliferation that normally occurs without HB-EGF [P2]). In other words, (P1–P2)/P2 × 100. P < .01 refers to the difference between the spontaneous expansion without HB-EGF and the additional proliferation with 5, 25, and 50 ng/mL HB-EGF. The figure shows means ± SD of 5 different experiments.
HB-EGF is a proliferation factor for MSCs without inducing differentiation
The addition of HB-EGF to MSC cultures at a concentration range of 5 to 50 ng/mL enhanced MSC expansion in a dose-dependent fashion, starting from both 5 × 103 and 104 cells. Higher concentrations (500 ng/mL) led to an inhibitory effect on MSC proliferation, as described for other cell types.4 After 72-hour culture, the mean increase of MSC number, compared with the 5 × 103 basal cell concentration, was 2.6 ± 1.4 times with no HB-EGF. This spontaneous MSC expansion was increased by 5, 25, and 50 ng/mL HB-EGF of an additional 11.9 ± 7.4%, 20.2 ± 6.9%, and 29.4 ± 14.4%, respectively. The difference between the expansion without HB-EGF and with 5, 25, and 50 ng/mL HB-EGF was statistically significant (P < .01) (Figure 2A).
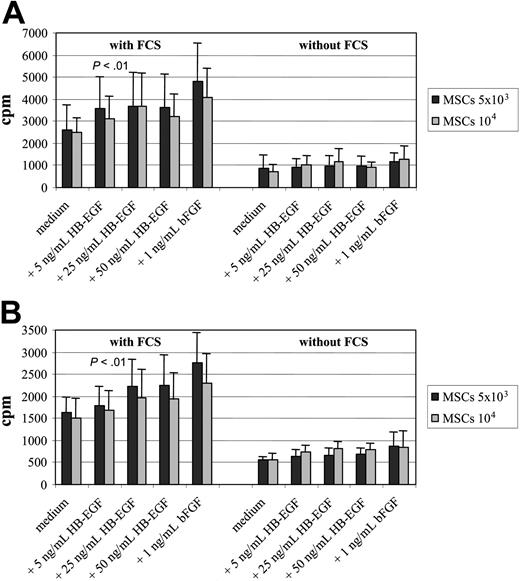
Comparison of HB-EGF with bFGF and contribution of FCS to proliferation enhancement. To assess the proliferating cell rate, 3H-thymidine incorporation assay was run for 72 hours (A) and 7 days (B), and the effect of HB-EGF was compared with that of 1 ng/mL basic fibroblast growth factor (bFGF), with or without FCS, a normal constituent of the complete culture medium that might contain growth factors. The enhancement of MSC proliferation rate by HB-EGF is dose dependent, with little difference between 25 and 50 ng/mL, and the effect with 50 ng/mL concentration is lower but not far from that achieved by adding 1 ng/mL bFGF to the culture. With both growth factors, as well as with normal medium, MSC proliferation depends on the presence of FCS, thus indicating that the increase of proliferation rate is actually due to the specific effect of those growth factors and not to FCS components. In the presence of FCS, the difference between the arm without HB-EGF and the others is statistically significant (P < .01). The figure shows means ± SD of 5 different experiments.
Comparison of HB-EGF with bFGF and contribution of FCS to proliferation enhancement. To assess the proliferating cell rate, 3H-thymidine incorporation assay was run for 72 hours (A) and 7 days (B), and the effect of HB-EGF was compared with that of 1 ng/mL basic fibroblast growth factor (bFGF), with or without FCS, a normal constituent of the complete culture medium that might contain growth factors. The enhancement of MSC proliferation rate by HB-EGF is dose dependent, with little difference between 25 and 50 ng/mL, and the effect with 50 ng/mL concentration is lower but not far from that achieved by adding 1 ng/mL bFGF to the culture. With both growth factors, as well as with normal medium, MSC proliferation depends on the presence of FCS, thus indicating that the increase of proliferation rate is actually due to the specific effect of those growth factors and not to FCS components. In the presence of FCS, the difference between the arm without HB-EGF and the others is statistically significant (P < .01). The figure shows means ± SD of 5 different experiments.
We observed similar findings when we used a starting MSC number of 104, by which cells are nearly confluent in a 96-well plate; with no HB-EGF, the mean increase of MSC number was slightly lower (2.0 ± 1.0 times), probably because of cell-cell contact. Nevertheless, MSC expansion was enhanced by 5, 25, and 50 ng/mL HB-EGF of an additional 13.6 ± 8.8%, 21.0 ± 7.8%, and 30.9 ± 15.0%, respectively. Again, the difference between the expansion without HB-EGF and with 5, 25, and 50 ng/mL HB-EGF was statistically significant (P < .01) (Figure 2B).
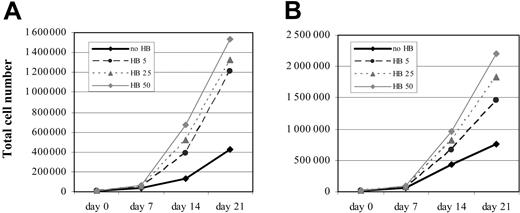
Long-term effect of HB-EGF on MSC proliferation. Dose-dependent enhancement of MSC proliferation induced by HB-EGF persists for several weeks, starting from both 5 × 103 (A) and 104 (B) cells. No HB indicates MSC proliferation without HB-EGF; HB 5, HB 25, and HB 50: MSC proliferation with 5, 25, and 50 ng/mL HB-EGF, respectively. The figure shows a representative case from those analyzed in Figure 2.
Long-term effect of HB-EGF on MSC proliferation. Dose-dependent enhancement of MSC proliferation induced by HB-EGF persists for several weeks, starting from both 5 × 103 (A) and 104 (B) cells. No HB indicates MSC proliferation without HB-EGF; HB 5, HB 25, and HB 50: MSC proliferation with 5, 25, and 50 ng/mL HB-EGF, respectively. The figure shows a representative case from those analyzed in Figure 2.
Effect of blocking antibodies against HER-1 and HB-EGF on MSC proliferation. Starting from both 5 × 103 (A) and 104 (B) cells, the enhancement of MSC proliferation induced by 5 ng/mL (▦), 25 ng/mL (▪), or 50 ng/mL (□) HB-EGF (1) is completely abolished by the addition of blocking antibodies against HER-1 (2) and HB-EGF (3). No inhibitory effect is obtained by adding to HB-EGF the same concentration of indifferent goat immunoglobulins (4), the blocking antibody isotype. Proliferation rate is assessed by MTT method at 72 hours and expressed as additional proliferation (the percentage of increase of MSC proliferation following HB-EGF addition [P1] compared with the spontaneous MSC proliferation that normally occurs without HB-EGF [P2]). In other words, (P1–P2)/P2 × 100. P < .01 refers to the difference between the arms without blocking antibodies and the others. The figure shows means ± SD of 5 different experiments.
Effect of blocking antibodies against HER-1 and HB-EGF on MSC proliferation. Starting from both 5 × 103 (A) and 104 (B) cells, the enhancement of MSC proliferation induced by 5 ng/mL (▦), 25 ng/mL (▪), or 50 ng/mL (□) HB-EGF (1) is completely abolished by the addition of blocking antibodies against HER-1 (2) and HB-EGF (3). No inhibitory effect is obtained by adding to HB-EGF the same concentration of indifferent goat immunoglobulins (4), the blocking antibody isotype. Proliferation rate is assessed by MTT method at 72 hours and expressed as additional proliferation (the percentage of increase of MSC proliferation following HB-EGF addition [P1] compared with the spontaneous MSC proliferation that normally occurs without HB-EGF [P2]). In other words, (P1–P2)/P2 × 100. P < .01 refers to the difference between the arms without blocking antibodies and the others. The figure shows means ± SD of 5 different experiments.
The enhancement of MSC proliferation rate by HB-EGF, measured by 3H-thymidine assay, was dose dependent with little difference between 25 and 50 ng/mL, and the effect with 50 ng/mL concentration was lower but not far from that achieved by adding 1 ng/mL bFGF to the culture. For both growth factors, the effect depended on the presence of FCS in the culture medium. However, FCS removal hampered not only the advantage in proliferation conferred to MSCs by HB-EGF and bFGF, but also MSC normal expansion, thus indicating that the increase of proliferation rate is actually due to the specific effect of those growth factors and not to FCS components (Figure 3), and that serum-free medium, even with HB-EGF addition, does not allow normal MSC proliferation.
Dose-dependent enhancement of MSC proliferation induced by HB-EGF persisted for several weeks (Figure 4), starting from both 5 × 103 (Figure 4A) and 104 (Figure 4B) cells, sustained by a clear-cut increase of proliferation rate of HB-EGF–treated MSCs compared with the initial cell concentrations.
At any time of the culture of MSCs with HB-EGF, no spontaneous differentiation into any of the mesenchymal lineages was observed, as assessed by specific staining. HB-EGF–cultured MSCs continued to proliferate more quickly than untreated MSCs regardless of the HER-1 down-regulation, as previously mentioned. In addition, MSCs expanded with HB-EGF were still able to differentiate into adipocytes, chondrocytes, and osteoblasts using the appropriate differentiation media without HB-EGF.
HB-EGF–induced proliferation is mediated by HER-1
To show that the proliferative effect of HB-EGF on MSCs was exerted through the interaction with its receptor HER-1, blocking MoAbs against HER-1 or HB-EGF were added to MSC cultures in conjunction with HB-EGF and the resulting proliferation was measured. Starting from both 5 × 103 (Figure 5A) and 104 cells (Figure 5B), the enhancement of MSC expansion beyond the spontaneous expansion (which was increased of an additional 9.8 ± 2.2%, 18.9 ± 6.6%, and 25.8 ± 15.4% starting from 5 × 103 MSCs with 5, 25, and 50 ng/mL HB-EGF, respectively; and 12.4 ± 8.0%, 19.5 ± 9.2%, and 30.8 ± 10.4% starting from 104 MSCs, with 5, 25, and 50 ng/mL HB-EGF, respectively; lane 1) was completely abolished by the addition of anti–HER-1 (lane 2) and anti–HB-EGF (lane 3) blocking MoAbs (P < .01). In the presence of HB-EGF and indifferent goat Ig, the blocking antibody isotype, no difference was observed in MSC proliferation compared with HB-EGF only (starting from 5 × 103 cells: additional 10.4 ± 1.6%, 18.3 ± 5.9%, and 25.0 ± 13.0% proliferation by 5, 25, and 50 ng/mL HB-EGF, respectively; starting from 104: additional 12.4 ± 7.6%, 21.1 ± 9.4%, and 31.4 ± 11.2%, respectively; Figure 5A-B lane 4).
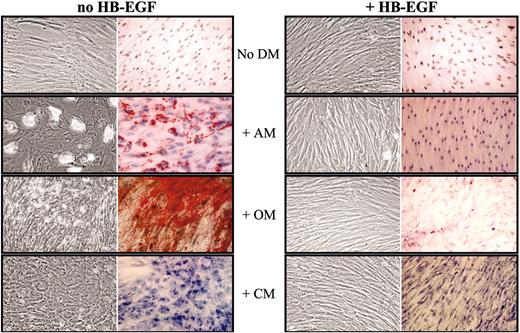
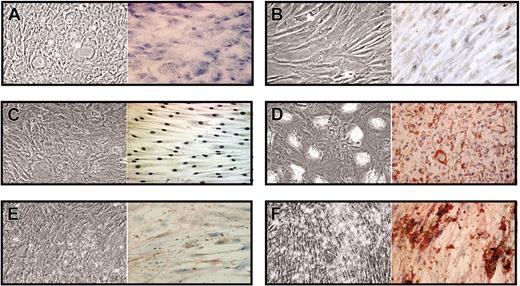
HB-EGF prevents MSC differentiation induced by specific media. Confluent MSCs were cultured for 14 days in flasks and culture slides with no differentiation medium (No DM) or in the presence of adipogenic (AM), osteogenic (OM), or chondrogenic (CM) medium, without and with 50 ng/mL HB-EGF; then cells were stained with Oil-red-O, von Kossa, and toluidine blue methods, respectively, to assess MSC differentiation. MSCs cultured in the presence of HB-EGF did not show any specific differentiation. The figure shows a representative case of 3 different experiments. In each box, left images show unstained cells and right images show cells stained with specific dyes. Images were visualized through an Olympus BX50 microscopic equipped with UPlan FL 100×/1.30 oil objective lens (Olympus, Tokyo, Japan). Acquisition was performed with Nikon ACT-1 2.20 software (Nikon, Tokyo, Japan); processing, with Adobe Photoshop 7.0 (Adobe, San Jose, CA).
HB-EGF prevents MSC differentiation induced by specific media. Confluent MSCs were cultured for 14 days in flasks and culture slides with no differentiation medium (No DM) or in the presence of adipogenic (AM), osteogenic (OM), or chondrogenic (CM) medium, without and with 50 ng/mL HB-EGF; then cells were stained with Oil-red-O, von Kossa, and toluidine blue methods, respectively, to assess MSC differentiation. MSCs cultured in the presence of HB-EGF did not show any specific differentiation. The figure shows a representative case of 3 different experiments. In each box, left images show unstained cells and right images show cells stained with specific dyes. Images were visualized through an Olympus BX50 microscopic equipped with UPlan FL 100×/1.30 oil objective lens (Olympus, Tokyo, Japan). Acquisition was performed with Nikon ACT-1 2.20 software (Nikon, Tokyo, Japan); processing, with Adobe Photoshop 7.0 (Adobe, San Jose, CA).
Simple addition of antibodies (anti–HER-1, anti–HB-EGF, or indifferent goat Ig) without HB-EGF did not modify MSC spontaneous proliferation (data not shown); moreover, we had previously assessed that anti–HB-EGF blocking antibodies have no effect on proliferation of HB-EGF– and HER-1–negative cell lines.4
HB-EGF inhibits MSC differentiation
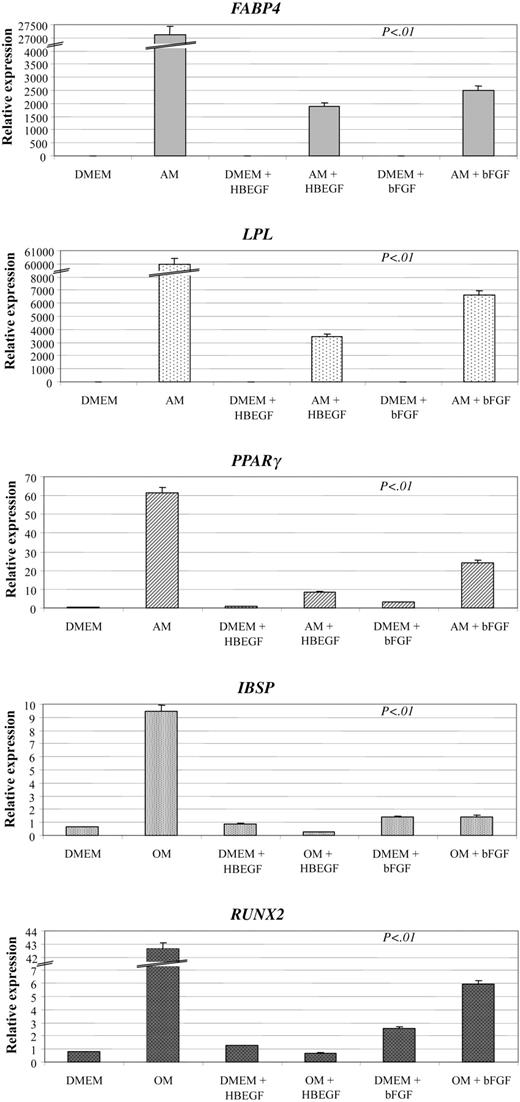
Confluent MSCs were cultured for 14 days in flasks and culture slides in the presence of AM, CM, or OM, in the presence or absence of 50 ng/mL HB-EGF. Then cells were stained with Oil-red-O, von Kossa, and toluidine blue, respectively, to assess MSC differentiation. MSCs cultured in the presence of HB-EGF did not show any evidence of differentiation (Figure 6). To achieve this result, HB-EGF had to be added to the culture immediately before or together with the differentiation medium and left for the entire culture time. Analysis of differentiation gene expression by quantitative RT-PCR showed that the presence of HB-EGF together with differentiation media is associated with the lack of specific differentiation gene up-regulation, more evidently than using bFGF (Figure 7), which had been suggested to not interfere with MSC multilineage differentiation potential.33
HB-EGF preserves multilineage stem-cell potential
As the treatment with HB-EGF had prevented MSC differentiation, we assessed whether HB-EGF–treated MSCs retained their multilineage stem cell potential in the presence of differentiation medium (Figure 8). MSCs were cultured for one week in the presence of CM without and with 50 ng/mL HB-EGF, which was added 2 hours before CM and left for the entire culture. At the end of the culture, HB-EGF and CM were removed and MSCs cultivated for 2 weeks with AM or OM. MSCs treated with CM without HB-EGF underwent differentiation into chondrocytes, as suggested by morphology and Toluidine blue (Figure 8A), while MSCs cultured with HB-EGF and CM did not (Figure 8B). Consequently, adipogenic and osteogenic differentiation were achieved only with HB-EGF–pretreated MSCs (Figure 8D and F, respectively), but not in the absence of HB-EGF preincubation (Figure 8C and E, respectively). This evidence shows that the presence of HB-EGF in the preliminary culture with CM had not only prevented MSC differentiation into chondrocytes, but also let them maintain their multilineage stem cell potential. Similar results were achieved by culturing MSCs for one week in the presence of AM or OM without and with 50 ng/mL HB-EGF, and then for 2 weeks with CM or OM (those previously cultured with AM ± HB-EGF), and with CM or AM (those previously cultured with OM ± HB-EGF). In all cases, differentiation was achieved only with HB-EGF–pretreated MSCs (data not shown).
HB-EGF–producing cells activate HER-1 signaling in MSCs
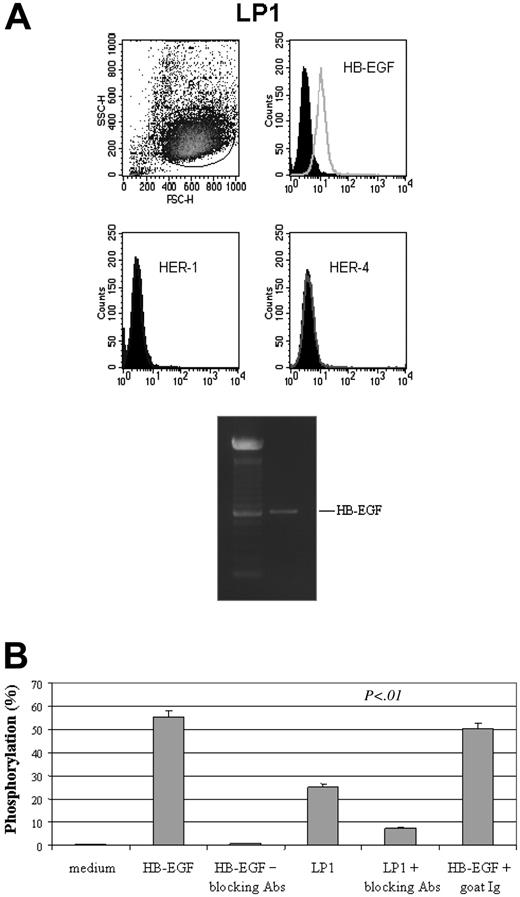
Coculture of MSCs with cells that normally produce HB-EGF, such as LP1 myeloma cell line (membrane HB-EGF positive, soluble HB-EGF producing, and membrane HER-1 and HER-4 negative; M.K., personal data, October 2003) led to a significant HER-1 phosphorylation (25.1 ± 5.2%) in MSCs, an event that triggers all HER-1–dependent intracellular activities,9 compared with that observed using human recombinant HB-EGF (55.2 ± 6.6%). In both cases, phosphorylation was significantly prevented (7.3 ± 3.4% and 0.9 ± 0.3%, respectively) by the use of blocking antibodies against HB-EGF, while indifferent goat Igs were ineffective (Figure 9).
Analysis of differentiation gene expression by quantitative RT-PCR. The presence of HB-EGF (50 ng/mL) together with differentiation media (AM, adipogenic medium; OM, osteogenic medium) in a 2-week culture is associated with the lack of specific differentiation gene up-regulation. This result is less evident with bFGF (1 ng/mL). FABP4 indicates fatty acid-binding protein 4, adipocyte differentiation; LPL, lipoprotein lipase, adipocyte differentiation; PPARγ, peroxisome proliferative active receptor-γ, adipocyte differentiation; IBSP, integrin-binding sialoprotein II, osteoblast differentiation; and RUNX2, runt-related transcription factor 2, osteoblast differentiation. The expression of each mRNA was calculated by relative quantification using the average of GAPD (glyceraldehyde-3-phosphate dehydrogenase) and ACTB (beta-actin) transcript level as reference (4 duplicates). P < .01 refers to the difference between the arms with differentiation medium (AM or OM) without growth factors and those with either HB-EGF or bFGF, and to the reciprocal comparison of the 2 growth factors. DMEM indicates culture medium without growth factors or differentiation agents. Error bars indicate means ± SD of 4 different experiments.
Analysis of differentiation gene expression by quantitative RT-PCR. The presence of HB-EGF (50 ng/mL) together with differentiation media (AM, adipogenic medium; OM, osteogenic medium) in a 2-week culture is associated with the lack of specific differentiation gene up-regulation. This result is less evident with bFGF (1 ng/mL). FABP4 indicates fatty acid-binding protein 4, adipocyte differentiation; LPL, lipoprotein lipase, adipocyte differentiation; PPARγ, peroxisome proliferative active receptor-γ, adipocyte differentiation; IBSP, integrin-binding sialoprotein II, osteoblast differentiation; and RUNX2, runt-related transcription factor 2, osteoblast differentiation. The expression of each mRNA was calculated by relative quantification using the average of GAPD (glyceraldehyde-3-phosphate dehydrogenase) and ACTB (beta-actin) transcript level as reference (4 duplicates). P < .01 refers to the difference between the arms with differentiation medium (AM or OM) without growth factors and those with either HB-EGF or bFGF, and to the reciprocal comparison of the 2 growth factors. DMEM indicates culture medium without growth factors or differentiation agents. Error bars indicate means ± SD of 4 different experiments.
Discussion
This study provides the first evidence that HB-EGF/HER-1 signaling is mitogenic for MSCs and may reversibly prevent their differentiation, leading to self-renewal rather than differentiative cell divisions. MSCs are the precursors of different mesenchymal tissues that play a crucial role in the construction of normal and pathologic microenvironments, mainly through the interaction with other cell types, such as endothelial cells, vascular SMCs, and leukocytes. HB-EGF is involved in these processes, in which it exerts a complex role entailing chemotaxis, proliferation, differentiation, and apoptosis, depending on both HER-1 or HER-4 stimulation and reverse signaling through the cytoplasmic tail of HB-EGF.4-10,12-15,39
HB-EGF preserves multilineage stem cell potential. MSCs were cultured for one week in the presence of CM without and with 50 ng/mL HB-EGF, which was added 2 hours before CM and left for the entire culture. Then both groups were cultured for 2 weeks with AM or OM, without HB-EGF and CM. MSCs treated with CM without HB-EGF underwent differentiation into chondrocytes, as suggested by morphology and Toluidine blue (A), while MSCs cultured with HB-EGF and CM did not (B). Adipogenic and osteogenic differentiation were not achieved with untreated MSCs (C,E), but only with HB-EGF–pretreated MSCs (D,F). The presence of HB-EGF in the preliminary culture with CM not only prevented MSC differentiation into chondrocytes, but also preserved their multilineage stem cell potential. The figure shows a representative case of 3 different experiments. Image visualization, acquisition, and processing were performed as for Figure 6.
HB-EGF preserves multilineage stem cell potential. MSCs were cultured for one week in the presence of CM without and with 50 ng/mL HB-EGF, which was added 2 hours before CM and left for the entire culture. Then both groups were cultured for 2 weeks with AM or OM, without HB-EGF and CM. MSCs treated with CM without HB-EGF underwent differentiation into chondrocytes, as suggested by morphology and Toluidine blue (A), while MSCs cultured with HB-EGF and CM did not (B). Adipogenic and osteogenic differentiation were not achieved with untreated MSCs (C,E), but only with HB-EGF–pretreated MSCs (D,F). The presence of HB-EGF in the preliminary culture with CM not only prevented MSC differentiation into chondrocytes, but also preserved their multilineage stem cell potential. The figure shows a representative case of 3 different experiments. Image visualization, acquisition, and processing were performed as for Figure 6.
We have observed that HB-EGF enhances ex vivo MSC proliferation via the interaction with its receptor HER-1, whose role in normal and neoplastic epithelial cell expansion has been clear for a long time. MSC differentiation potential is not affected by the enhancement of self-renewal, as the proliferative effect is not associated with spontaneous differentiation. The effect is highly controlled because surface HER-1 is down-regulated after interaction with HB-EGF; this occurs rapidly but reversibly, because HER-1 RNA is still synthesized and leads to the re-expression of surface HER-1 a few hours after HB-EGF removal from culture. By contrast, HER-1 expression is permanently lost during MSC differentiation into mesenchymal cell lineages. HB-EGF proliferative effect on MSCs is direct, dose dependent, long lasting, comparable with other growth factors such as bFGF, and HER-1 mediated, because it is completely prevented by antagonizing HB-EGF/HER-1 interaction with anti–HER-1 blocking antibodies.
Of utmost interest is the finding that HB-EGF inhibits MSC differentiation in a stronger manner than bFGF. Not only does HB-EGF prevent spontaneous MSC differentiation, but also makes the MSCs refractory to differentiating media for a significantly long time (1-2 weeks). This inhibitory effect is detectable only if HB-EGF is added to the culture immediately before the differentiation medium and left for the entire culture time. This suggests that the preliminary HER-1 triggering by HB-EGF is necessary to hamper the differentiation cascade, and that surface HER-1 is probably re-expressed during the culture after being down-regulated following HB-EGF interaction. This is consistent with the evidence that HER-1 RNA is still detectable in MSCs after HB-EGF addition (Figure 1C lane 3). It is likely that the molecular events that occur downstream of the engagement of HER-1 by HB-EGF may interfere at a very early level with those activated by the specific medium.
There is increasing evidence of the potential use of MSC infusion for clinical purposes, such as hematopoietic support, tissue repair, immunosuppressive cell therapy, and anticancer gene therapy.21-22,25-28,30 Thus, it is of great interest to study which factors may have a role in MSC expansion, maintenance of MSC stem cell plasticity, and interaction with normal and pathologic cells once the MSCs are recruited and included in proliferating tissues. The rapid ex vivo MSC expansion and down-regulation of the MSCs sensitivity to physiologic differentiation agents could represent a valid alternative to other factors, such as bFGF, with an advantage in terms of MSC differentiation potential, in situ recruitment, and proliferation, and therefore of in vivo transplantation efficiency. MSC differentiation potential and curative effect should be maintained anyway, as the differentiation block of MSCs is temporary, depending on the presence of HB-EGF.
The in vivo role of HER-1 expression by MSCs is unknown. The evidence that HER-1 phosphorylation occurs in MSCs in the presence of HB-EGF–producing cells suggests that HB-EGF/HER-1 signaling may be operational in vivo, as previously shown for epithelial cells.9 As HB-EGF is not produced by MSCs, it is likely that its effects are exerted in vivo through the interaction between MSCs and microenvironments. HER-1 expression by MSCs makes possible a potential interaction with a large number of HB-EGF–producing cells, such as monocytes,3 T cells,7 granulocyte-macrophage colony-stimulating factor (GM-CSF)–activated granulocytes,32 myeloid leukemia blasts,4 vascular SMCs,5 endothelial cells,7 and normal6 or neoplastic epithelial cells.7 Most of these HB-EGF–producing cell types normally interact with stromal tissues. In the case of tissue damage or neoplastic growth, these cells are recruited and take part in either inflammatory or regenerative phases. Stromal support is guaranteed by both the local tissue mesenchymal progenitors and the migration of BM MSCs.21-22,24-25,27-28 It has to be investigated whether the HB-EGF/HER-1 signaling may contribute in vivo to maintain a broad, proliferating pool of undifferentiated MSCs, thus ensuring the regenerative process or the efficient angiogenesis to neoplastic growth. The use of HB-EGF inhibitors could have a role in these conditions.
HB-EGF–producing cells activate HER-1 signaling in MSCs. (A) Coculture of MSCs with cells that normally produce HB-EGF, such as LP1 myeloma cell line (membrane HB-EGF positive, soluble HB-EGF producing, and membrane HER-1 and HER-4 negative) led to a significant HER-1 phosphorylation in MSCs, compared with that observed using human recombinant HB-EGF (50 ng/mL), which is prevented by the use of blocking antibodies against HB-EGF (B). As phosphorylation is the event that triggers all HER-1–dependent intracellular activities,9 this evidence suggests that HB-EGF/HER-1 signaling may be operational in vivo. SSC indicates side scatter; FSC, forward scatter. Error bars indicate means ± SD of 3 different experiments.
HB-EGF–producing cells activate HER-1 signaling in MSCs. (A) Coculture of MSCs with cells that normally produce HB-EGF, such as LP1 myeloma cell line (membrane HB-EGF positive, soluble HB-EGF producing, and membrane HER-1 and HER-4 negative) led to a significant HER-1 phosphorylation in MSCs, compared with that observed using human recombinant HB-EGF (50 ng/mL), which is prevented by the use of blocking antibodies against HB-EGF (B). As phosphorylation is the event that triggers all HER-1–dependent intracellular activities,9 this evidence suggests that HB-EGF/HER-1 signaling may be operational in vivo. SSC indicates side scatter; FSC, forward scatter. Error bars indicate means ± SD of 3 different experiments.
In conclusion, the effects of HB-EGF/HER-1 signaling on MSCs give the cue to understand better the biology and the role of MSCs. These findings suggest a unifying basis for the pleiotropic activity of HB-EGF in a number of other conditions, including the mesenchymal-epithelial transition.40 Moreover, this study supports the idea that some complex biologic phenomena, such as the microenvironment/neoplasia interaction, should be reconsidered when evaluating the role of broad-activity factors on stem cells.
Prepublished online as Blood First Edition Paper, March 8, 2005; DOI 10.1182/blood-2004-09-3645.
Supported by MIUR (Italian Ministry of University and Scientific Research, ex 60% funds), AIRC (Italian Association for Cancer Research), CNR (Italian National Research Centre), and Fondazione Cariverona.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Serena Pedron and Marco Chilosi, from the Department of Pathology, University of Verona, for their immunohistochemistry support.

![Figure 2. Effect of HB-EGF on MSC expansion. Enhancement of MSC proliferation was induced by HB-EGF in a dose-dependent fashion with concentrations between 5 to 50 ng/mL, starting from both 5 × 103 (A) and 104 (B) cells. MSC expansion with 5 ng/mL (▦), 25 ng/mL (▪), and 50 ng/mL (□) HB-EGF was assessed by MTT method at 72 hours and expressed as additional proliferation (the percentage of increase of MSC proliferation following HB-EGF addition [P1] compared with the spontaneous MSC proliferation that normally occurs without HB-EGF [P2]). In other words, (P1–P2)/P2 × 100. P < .01 refers to the difference between the spontaneous expansion without HB-EGF and the additional proliferation with 5, 25, and 50 ng/mL HB-EGF. The figure shows means ± SD of 5 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3645/4/m_zh80130580530002.jpeg?Expires=1767784379&Signature=3UKLZ7BLddlzPFvirxQucsMu9O7VcJJH~btYo0rDw4HQZpASOvNwK~9DrHZ6H9~Wsc2hajdvlQqXe1NPM38M7RGpyp-cF-OvFsz14aLj5ThuUNIiW-AwYVU6-4U3EYhaTR7mSiQ6-1awhzBXb~Hp5odfWK3i6vfqZToIyyF7o65O46kbFdiROTA0max0c8y9oLPJiqiUyqKociZuzzGB9SakSSe1ywVr-prsV~qMxuPKtclWg2zSRC5IFvu1CxKp6qkiNiJJkfNjwOc5tXuyUnuDTY~qB5PbiqSEKF4TP3EN2ojmQ7WKJnuNRRM4PcvXdYnqjEJIONM4e~hxcDChZw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Effect of blocking antibodies against HER-1 and HB-EGF on MSC proliferation. Starting from both 5 × 103 (A) and 104 (B) cells, the enhancement of MSC proliferation induced by 5 ng/mL (▦), 25 ng/mL (▪), or 50 ng/mL (□) HB-EGF (1) is completely abolished by the addition of blocking antibodies against HER-1 (2) and HB-EGF (3). No inhibitory effect is obtained by adding to HB-EGF the same concentration of indifferent goat immunoglobulins (4), the blocking antibody isotype. Proliferation rate is assessed by MTT method at 72 hours and expressed as additional proliferation (the percentage of increase of MSC proliferation following HB-EGF addition [P1] compared with the spontaneous MSC proliferation that normally occurs without HB-EGF [P2]). In other words, (P1–P2)/P2 × 100. P < .01 refers to the difference between the arms without blocking antibodies and the others. The figure shows means ± SD of 5 different experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-09-3645/4/m_zh80130580530005.jpeg?Expires=1767784379&Signature=pZyEvjFUsufUSJfgi8n2LW-54M~GzpmSHWxmdubmT4mKTnlqbDNyhg1DCDqqz98SRAnl7yB4ftg7FkPTaiDDAtf5eVwV5ZJoQtGvikUujR7w8o6aVANrzVR-1-tixGIuJD5KHgLUJimOpW1~WCjTdGk~p9OktAD40rhYZZGEXCEMv-bjkHdiiV9S7aKABw~ke4vdiwCbNaUPqnivadMjPERjXguPTpr0ZwkMeLBHs0u5vQoS-i9SJNRWIEjGyDkWeKRbNHzwUkS-qdlFjUr8yMRnjqHHHBT3pGTjq8Vc5gktKORfvJoB6J1UP6T-zricZMvqVT3XcYuyR2eODs9RtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal