Although glucocorticoids (GCs) have been described as acting mainly as anti-inflammatory and immunosuppressive drugs, they may also positively influence the immune system. In the present study, we demonstrate for the first time that hydrocortisone (HC), in synergy with interleukin-15 (IL-15), induces a dramatic increase in the expansion of peripheral blood–derived CD56+ cells, favoring the preferential outgrowth of classical natural killer (CD56+CD3– NK) over CD56+CD3+ natural killer T (NKT) cells. HC plus IL-15–driven CD56+ cells exhibited an increased potential for cytokine production with no impairment in their NK- and lymphokine-activated killer (LAK) activities. Elevated levels of GC-induced leucine zipper protein (GILZ) messenger RNA (mRNA) were detected in both NK and NKT cells cultured with HC and IL-15, in comparison to IL-15 alone. Phosphorylation status of signal transducer and activator of transcription 5 (STAT5) was not affected by the presence of HC in either of the populations. On the contrary, HC differentially affected the IL-2/IL-15R β- and γ-chain surface expression and the phosphorylation levels of extracellular signal-regulated kinases 1/2 (ERK1/2) in IL-15–activated NK and NKT cells. Our data ascribe a novel role to GCs on mature NK-cell expansion and function and open new perspectives for their use in cellular adoptive cancer immunotherapy.
Introduction
The most prominent effect of glucocorticoids (GCs) is immunosuppression and these hormones are widely used as anti-inflammatory agents.1 GCs suppress inflammation by down-regulating the expression of proinflammatory factors, such as cytokines and chemokines, or by up-regulating the production of anti-inflammatory molecules, such as lipocortin-1, an interleukin-1 (IL-1) receptor antagonist, and IL-10.2,3 The expression of the IL-10 receptor is also up-regulated by GCs, supporting its anti-inflammatory action.2 Another recognized effect of GCs is the induction of apoptosis. GCs have been demonstrated to induce lymphoid-cell apoptosis in different experimental systems, including hybridoma T cells, thymocytes, and peripheral blood lymphocytes.4-8 GCs suppress survival signals through (1) the direct suppression of survival genes,9 (2) the induction of inhibitors of survival factors, including IκB-α and the regulatory subunit of the phosphatidyl-inositol 3 (PI3) kinase,4,7 and (3) enhancement of the transcription levels of Bim, a member of the proapoptotic Bcl-2 family.10
Most of the effects of GCs are mediated by modulation of gene transcription via interaction with the GC receptor (GCR).11 GCR functions as a ligand-dependent transcription factor which, upon homodimerization, translocates into the nucleus where it regulates gene expression either directly, by binding to DNA, or indirectly, through protein-to-protein interaction with other transcription factors.12,13 Thus, GCs have been reported to inhibit functional activities of T and natural killer (NK) cells (including proliferation, cytotoxicity, and cytokine production), mostly by interfering with transactivation of several transcription factors such as NF-κB and activated protein-1 (AP-1).4,9,11,14,15
Although potent immunosuppressive properties have been assigned to GCs, recent reports have challenged this notion and showed that the GC-mediated effect on immune lymphocytes may depend on their activation state as well as the nature of stimulants used for activation.16,17 Consequently, GCs have also been demonstrated to have positive effects on the immune system and its function. Thus, GCs may synergise with cell-activation–induced pathways in rescuing T cells from programmed cell death. For example, whereas both GC or T-cell–receptor (TCR) activation induces apoptosis in thymocytes and T-cell hybridomas when applied alone, the simultaneous delivery of both signals results in enhanced survival of committed cells.18,19 GCs have also been demonstrated to positively influence the expression of the IL-7 receptor α-chain (IL-7Rα), which was associated with rescue from GC-induced apoptosis and enhanced IL-7–mediated signaling and function in T cells.20 A similar GC-mediated enhancing effect on the expression of IL-2Rα21,22 supports the positive modulatory role of GCs on the immune system's function. Given the accumulating evidence of a stress-induced enhancement of immune function,23-29 some direct immune stimulatory effects of GCs have been interpreted in the context of their adoptive actions in the acute phase of a stress response.30
A typical example for the dual action of GCs on the immune system is GC–induced leucine zipper protein (GILZ). GILZ was first isolated as a dexamethasone-responsive gene from a thymus substraction library.31 GILZ expression is induced by GCs in lymphoid cells31 and by IL-4, IL-10, and GCs in monocytes/macrophages.32 GILZ may exert its effects by interfering at various stages of the signal transduction pathways, including inhibition of NFκB activation and nuclear translocation,33 inhibition of AP-1,34 and also through inhibition of Raf-1 phosphorylation, with consequent inhibition of Raf mitogen–activated extracellular signal-regulated kinase (Raf-MEK-ERK) activation.35 The expression of GILZ is enhanced upon dexamethasone treatment and down-regulated by TCR triggering.33 Although overexpression of GILZ has been shown to inhibit T-cell activation pathways,31,36 the same molecule has also been demonstrated to protect T cells from IL-2 deprivation–induced apoptosis by down-regulating the expression of Bim,10 a proapoptotic member of the Bcl-2 family.37,38
NK cells are large granular lymphocytes that spontaneously lyse tumor and virally infected cells, produce a series of immunoregulatory cytokines and chemokines, and constitute an important component of the innate immune defense against various microorganisms and cancer.39,40 The majority of NK cells are characterized by the absence of CD3 expression, although a subpopulation of these cells express surface CD3 and are designated as NKT cells.40 NK cells constitutively express cytokine receptors, which, upon stimulation with their specific ligand, induce downstream activation pathways resulting in proliferation, cytotoxicity, and cytokine production.41 Resting NK cells also express the common IL-2/IL-15R β- and γ-chains and therefore expand in vitro in the presence of IL-2 or IL-15.41,42 However, due to the lack of abundant IL-2 during the early innate immune response,41 IL-15 is the more important physiologic ligand for NK-cell development, cytotoxicity, and cytokine production.42,43
Given the antiapoptotic role of GCs in lymphocytes undergoing activation,33 and taking into consideration previous reports44,45 demonstrating the capacity of GC to induce, at low concentrations, proliferation in human primary cells, along with the fact that lymphocytes in vivo develop and function under the pressure of physiologic levels of GCs, we sought to investigate the effect of IL-15 in combination with hydrocortisone (HC) on CD56+-cell proliferation and function. The results, in this report, demonstrate that the combination of IL-15/HC differentially affects NK and NKT-cell proliferation, by dramatically enhancing the expansion of CD56+CD3– cells only, although protecting both populations from apoptosis. The cytotoxic potential of HC-treated CD56+ cells remained unaffected. IL-15/HC-driven CD56+ cells were capable, upon costimulation with IL-12 and IL-18, of secreting increased amounts of IL-10, granulocyte macrophage–colony-stimulating factor (GM-CSF), interferon gamma (IFN-γ), and tumor necrosis factor alpha (TNF-α). To examine possible causes underlying the differential effect of HC on NK and NKT cells, we investigated IL-2/IL-15R β- and γ-chain surface expression, GILZ messenger RNA (mRNA) levels, signal transducer and activator of transcription 5 (STAT5), and ERK1/2 phosphorylation, since all of them are known to be involved in GC- and IL-15–induced pathways.35,42 Our results ascribe a prominent role to HC in combination with IL-15 for large-scale expansion and activation of NK cells to be used in the cellular adoptive immunotherapy of cancer.
Materials and methods
Peripheral blood CD56+-cell isolation
Samples of human peripheral blood (PB) were obtained from healthy volunteers. This study was approved by the Saint Savas Hospital Institutional Review Board (Athens, Greece), and informed consent was provided according to the Declaration of Helsinki. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Hypaque centrifugation using standard procedures. CD56+ cells were isolated from PBMCs using anti-CD56–coated microbeads and passed through 2 sequential large-scale (LS) columns (Miltenyi Biotec, Gladbach, Germany) according to the manufacturer's instructions. CD3+ and CD3– subpopulations were separated by cell sorting from the purified CD56+-cell fraction, with a Coulter Epics Altra cell sorter (Beckman Coulter, Fullerton, CA). The purity of the isolated populations was always more than 98%.
Cell lines
The human cell lines K562 (erythroleukemia) and Daudi (Burkitt lymphoma) were obtained from the American Type Culture Collection (Manassas, VA) and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mM l-glutamine, and 50 μg/mL gentamicin (all purchased from Life Technologies, Paisley, Scotland) at 37°C in a CO2 incubator.
Cell cultures
Isolated CD56+ cells were plated in 24-well plates at 1 × 106cells/mL in 1 mL alpha-minimum essential medium (alpha-MEM; Life Technologies) with 20% fetal calf serum (FCS), 2 mM l-glutamine, and 50 μg/mL gentamicin (complete medium), supplemented with recombinant human IL-15 (R&D Systems, Abington, United Kingdom) at 20 ng/mL or IL-2 (Proleukin; Chiron BV, Amsterdam, The Netherlands) at 1000 IU/mL and the indicated concentration of HC (Sigma, St Louis, MO). Every 3 to 4 days, half of the medium was discarded and replenished by fresh medium containing freshly added cytokine and HC, whereby cell density was adjusted to 0.5 × 106 cells/mL.
Cell-number counts were evaluated using a standard hemocytometer.
Assessment of viability and apoptosis
Viability was determined by trypan blue dye exclusion. Annexin V–phycoerythrin (PE) and the nucleic acid dye 7-amino-actinomycin D-(7-AAD; Pharmingen, San Diego, CA) were used for the estimation of cells undergoing apoptosis, in flow cytometric assays, according to the manufacturer's protocol.
Proliferation assays
In order to examine the effect of different HC concentrations on the proliferation rate, cells from the total CD56+-cell cultures, incubated for the indicated time period with IL-15 in the presence of the various concentrations of HC, were plated at 50 × 103 cells/well, in triplicate, in 96-well flat-bottomed plates and pulsed for 16 hours with 1 μCi/well [37 MBq/well] [3H]thymidine incorporation ([3H]TdR) (30 Ci/mmol to 40 Ci/mmol; Amersham, Cardiff, United Kingdom). Subsequently, cells were harvested and [3H]TdR uptake was measured in a microbeta counter (Wallac, PerkinElmer, Boston, MA). Results are expressed in counts per minute (cpm).
For the proliferation assays of sorted cells, CD56+CD3+ or CD56+CD3– cells were plated in triplicate, in 96-well round-bottomed plates, at 5 × 103 cells per 200 μL final volume of complete medium supplemented with IL-15, in the presence or absence of 10–4 M HC. Half of the medium was replaced with freshly prepared medium twice weekly. [3H]TdR (1 μCi/well) was added for the last 16 hours of the indicated period of cultures, followed by cell harvesting and measurement of [3H]TdR uptake.
Monoclonal antibodies and immunophenotyping
Anti-CD3 monoclonal antibody (mAb) conjugated with fluorescein isothiocyanate (FITC) was obtained from Becton Dickinson (Mountain View, CA). Anti-CD132 mAb conjugated with PE was purchased from Pharmingen (San Diego, CA). PE-conjugated anti-CD122 and phycoerythrin-cyanin 5.1 (PEcy5) anti-CD56 mAbs were obtained from Immunotech (Marseille, France). Cells were washed twice with ice-cold phosphate buffered saline (PBS)/1% bovine serum albumin (BSA) followed by incubation with the appropriate mAbs for 15 minutes at room temperature and fixed with 1% paraformaldehyde (PFA) in PBS. For the detection of phosphorylated molecules, anti-phosphoERK1/2 and anti-phosphoSTAT5, conjugated with PE and Alexa 647 respectively, were purchased from Becton Dickinson and used as recommended by the manufacturer, along with anti-CD3–FITC. Samples were analyzed using FACSCalibur (Becton Dickinson) and CellQuest analysis software (BD Biosciences, Franklin Lakes, NJ).
For the differential detection of CD122, CD132, phospho-ERK1/2, phospho-STAT5, and apoptosis in NK and NKT cells, gating on CD3– and CD3+ was applied in fluorescence-activated cell sorting (FACS) analysis.
The quantitation of immunofluorescence is based on the use of a series of beads which contain known amounts of molecules of equivalent soluble fluorochrome (MESF).46 In order to obtain MESF values, 4 MESF beads (Quantum R-PE; Sigma) with varying MESF values (ie, 1648, 4784, 23 777, 61 908) were mixed and used to calibrate the flow cytometer scale. The median relative linear fluorescence intensity (MRFI) was calculated using CellQuest software. A calibration curve was constructed and subsequent MRFI data from analyzed samples were converted to MESF using the QuickCal program (Sigma).
Cytotoxicity assay
Cytotoxic activity of cultured cells was determined in a standard 4-hour 51Cr-release assay against the NK-sensitive cell line K562 and the NK-resistant cell line Daudi, as previously described.47 In brief, target cells were labeled with 100 μCi [37 MBq] sodium [51Cr] chromate (Amersham) per 1 × 106 target cells for 1 hour. Effector cells were incubated with target cells at the indicated ratios. Spontaneous 51Cr release was measured by incubating target cells in the absence of effector cells. Maximum 51Cr release was determined by adding 1% Triton X-100 (Sigma). Spontaneous lysis did not exceed 10% of maximum release. The amount of 51Cr released was measured in a γ-counter (Packard, Downers Grove, IL) and percent lysis was calculated as follows: % specific lysis = (experimental 51Cr release – spontaneous 51Cr release) / (maximum 51Cr release – spontaneous 51Cr release) × 100.
Quantitation of cytokines in culture supernatants
For cytokine production determinations, cells recovered from cultures with IL-15, in the presence or absence of HC, were washed twice with Hanks balanced salt solution (HBSS; Life Technologies) and incubated for an additional 48 hours in fresh medium containing IL-15 (20 ng/mL), IL-12 (2 ng/mL; R&D), and IL-18 (100 ng/mL; R&D) and the corresponding HC concentration. Supernatants were collected by centrifugation and stored at –70°C until use. Cytokines (IFN-γ, GM-CSF, TNF-α, and IL-10) were quantitated using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Diaclone Research, Besançon, France) according to the manufacturer's instructions.
RNA extraction and reverse transcription
Cells were harvested at various time points of culture and adjusted to similar numbers (approximately 1 × 106 cells/sample). Total RNA was extracted using the NucleoSpin RNA II kit (Macherey-Nagel, Düren, Germany), according to the manufacturer's protocol. First-strand complementary DNA (cDNA) synthesis was performed using approximately 1 μg total RNA, random primers, and the SuperScript III RNase H (–) reverse transcriptase (Invitrogen, Paisley, Scotland) according to a preamplification system protocol, in a total volume of 20 μL.
RNA extraction from CD56+CD3– and CD56+CD3+ subpopulations
Total CD56+ cells were cultured in the presence of IL-15 without or with HC. Cells were harvested at the indicated time points, washed with cold PBS, and incubated in PFA 1% for 5 minutes at 4°C. Subsequently, cells were washed again with PBS and CD56+CD3+ cells were isolated using CD3 Dynabeads 450 (Dynal Biotech, Oslo, Norway), according to the manufacturer's instructions. Immediately after separation, total RNA was extracted and first-strand cDNA synthesis was performed following the same protocols as mentioned in the previous paragraph.
Relative quantitation of GILZ mRNA levels
To evaluate the relative levels of GILZ mRNA, we performed real-time polymerase chain reaction (PCR) analysis on a LightCycler system (Roche Diagnostics, Mannheim, Germany).
For the amplification of GILZ and β2-microglobulin (which was used as the housekeeping reference gene in all the experiments), we used LUX custom primers (Invitrogen), which are custom-synthesized oligonucleotides, labeled with a single fluorophore (6-carboxyfluorescein [FAM] for our experiments) close to the 3′ end in a hairpin structure. When the primer is incorporated into a double-stranded PCR product, the fluorophore is dequenched, resulting in a significant increase in fluorescent signal. The primers for GILZ were designed by us and were as follows: GILZFLUX, 5′-CAA GCA TCT CCT TCT TCT CTT CTC TGC CTG-3′ and GILZR, 5′-TGG CCT GTT CGA TCT TGT TGT-3′. As housekeeping gene we used the FAM-labeled Human B2M-Certified LUX Primer Set (Invitrogen).
The reactions were performed in a total volume of 20 μL using the Platinum qPCR SuperMix UDG (Invitrogen) according to the manufacturer's recommendations for LightCycler and 2 μL of the cDNA material prepared as described in “RNA extraction and reverse transcription.” The cycling protocol consisted of 50°C for 2 minutes and 95°C for 2 minutes (uracil-DNA glycosylase reaction) and 50 cycles of denaturation at 94°C for 5 seconds, annealing at 60°C for 10 seconds, plate read and extension at 72°C for 10 seconds. To confirm amplification specificity, we performed melting curve analysis at the end of each cycling program. To evaluate the relative amount of transcripts in each sample, the Ct value of the housekeeping gene was subtracted from the cycle threshold (Ct) of the target gene (=ΔCt). To estimate the levels of expression of GILZ in each sample we subtracted the ΔCt value of the day-0 sample (freshly isolated CD56+ cells) from the ΔCt value of each sample (: ΔΔCt) and the exported number was used in the equation 2–ΔΔCt. Thus, we estimated the fold of change of GILZ expression in each sample versus the expression in the sample D0.
Statistical analysis
Data were analyzed by Student t test. A P value of less than .05 was considered significant.
Results
CD56+-cell expansion with IL-15/HC
IL-15 induces the proliferation of CD56+ cells in a dose-dependent fashion with significant effects within a range of nanomolar concentrations.48 Thus, at the initiation of our experiments, we sought to investigate whether the presence of HC could have an influence on CD56+ cells in cultures supplemented with IL-15. As depicted in Figure 1A, the number of CD56+ cells increased significantly (P < .001) in the presence of IL-15 and at HC doses ranging from 10–4 M to 10–8 M. This increase in cell numbers correlated well with higher proliferation rates of total CD56+ cells in the presence of HC as indicated by the increased [3H]TdR incorporation (Figure 1B). In addition, CD56+ cells grown in cultures with IL-15/HC exhibited a more increased survival than control CD56+ cells expanded in the presence of IL-15 alone, which was detectable at later time points (Figure 1C). HC doses lower than 10–8 M did not have any effect on CD56+-cell proliferation (Figure 1A) and viability (Figure 1C). IL-2 had similar effect on CD56+-cell proliferation (78-fold expansion in the presence of 10–6 M HC compared with 14-fold with IL-2 alone, after 15 days of culture). HC alone did not support NK-cell growth in vitro (data not shown).
Cytotoxic potential of CD56+ cells expanded with IL-15/HC
Evidence has suggested the ability of GCs to inactivate NK cells and subsequently diminish their cytotoxic activities.49 Thus, it was important to know to what extent our IL-15/HC–expanded CD56+ cells could lyse tumor targets. As shown in Figure 2A-B, NK- and LAK-sensitive tumor targets were equally lysed by CD56+ cells stimulated in 20-day cultures, either by IL-15 alone or costimulated by HC at concentrations ranging from 10–4 M to 10–8 M. Similar levels of cytotoxicity by CD56+ cells were observed throughout a 20-day period of culture (Figure 2C-D). Similar results were obtained when IL-2 was used instead of IL-15 (data not shown). Nevertheless, HC alone, as expected, in the absence of IL-15, greatly inhibited the CD56+ cell–mediated cytolytic activity against K562 (85% inhibition at 10:1 effector-to-target ratio in CD56+ cells cultured for 20 hours in the presence of HC 10–6 M).
HC induces high proliferation in IL-15–activated CD56+ cells. Purified CD56+ cells were cultured with IL-15 in the absence or presence of HC at concentrations ranging from 10–10 M to 10–4 M for a period of 20 days. (A) Cell counts, (B) [3H]TdR incorporation, and (C) viability estimated by trypan blue dye exclusion were analyzed every 5 days. Results represent the mean plus or minus the standard deviation (SD) of 3 independent experiments using CD56+ cells from healthy donors.
HC induces high proliferation in IL-15–activated CD56+ cells. Purified CD56+ cells were cultured with IL-15 in the absence or presence of HC at concentrations ranging from 10–10 M to 10–4 M for a period of 20 days. (A) Cell counts, (B) [3H]TdR incorporation, and (C) viability estimated by trypan blue dye exclusion were analyzed every 5 days. Results represent the mean plus or minus the standard deviation (SD) of 3 independent experiments using CD56+ cells from healthy donors.
HC does not affect the killing capacity of CD56+ cells. Isolated CD56+ cells cultured for 20 days with IL-15 in the absence or presence of HC, at concentrations ranging from 10–8 M to 10–4 M, were tested for their cytolytic activity against (A) NK-sensitive (K562) and (B) LAK-sensitive (Daudi) targets in a 4-hour chromium release assay at different effector-to-target (E/T) ratios. NK (C) and LAK (D) activities of the cultured cells were tested throughout the whole culture period at the indicated time points (E/T ratio 1:1). Results from 4 (A and B) and 3 (C and D) healthy donors (mean ± SD) are presented.
HC does not affect the killing capacity of CD56+ cells. Isolated CD56+ cells cultured for 20 days with IL-15 in the absence or presence of HC, at concentrations ranging from 10–8 M to 10–4 M, were tested for their cytolytic activity against (A) NK-sensitive (K562) and (B) LAK-sensitive (Daudi) targets in a 4-hour chromium release assay at different effector-to-target (E/T) ratios. NK (C) and LAK (D) activities of the cultured cells were tested throughout the whole culture period at the indicated time points (E/T ratio 1:1). Results from 4 (A and B) and 3 (C and D) healthy donors (mean ± SD) are presented.
Potential of IL-15/HC–stimulated CD56+ cells to produce cytokines
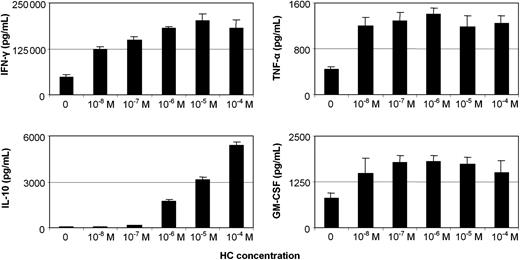
In the following set of experiments, we sought to investigate whether our CD56+ cells derived in response to IL-15 alone or IL-15/HC had the capacity to display distinct patterns of cytokine production. Cells were collected from 20-day cultures and cytokine secretion was quantitated in supernatants from an additional 2-day culture with IL-12 and IL-18.47 Independently of the HC concentration used, there was a significant increase (P < .001) in IFN-γ, TNF-α, and GM-CSF levels compared with IL-15 alone. IL-10 levels were also elevated, although in a dose-dependent manner (Figure 3). Doses lower than 10–6 M had no statistically significant effect on IL-10 secretion.
IL-15/HC favors outgrowth of CD56+CD3– cells
Human total CD56+ cells from 5 different donors were found to contain 52% to 88% CD56+CD3– and the remaining 12% to 48% were CD56+CD3+ NKT cells. As shown in Figure 4, during culture of CD56+ cells with IL-15/HC at several doses ranging from 10–8 M to 10–4 M, there was a steady increase both in the percentage and absolute number of the CD56+CD3– cells with a concomitant decrease of CD56+CD3+ cells. In contrast, IL-15 alone favored the expansion of CD56+CD3+ cells (Figure 4). This preferential growth of CD56+CD3– NK subpopulation with HC was dose- and time-dependent and could be detected as early as day 5, in the presence of even 10–6 M HC (Figure 4), whereas after day 20, cultures with 10–6 M to 10–4 M HC were overpopulated by CD56+CD3– cells.
To elucidate whether this differential effect correlated with differences in survival and/or proliferation, we examined the 2 populations for apoptosis and 3[H]TdR incorporation. HC protected both populations from apoptosis, since reduced percentages of cells undergoing apoptosis were observed in NK and NKT cells stimulated by HC plus IL-15, as opposed to IL-15 alone (Figure 5A). On the contrary, proliferation was differentially affected by the presence of HC in both CD56+ populations. Sorted CD3+ and CD3– cells were cultured with IL-15 in the absence or presence of 10–4 M HC and tested for their proliferative capacity. NK cells responded to HC with extremely high proliferation (Figure 5B), whereas NKT cells only marginally incorporated [3H]TdR under the same culture conditions (Figure 5B-C). It is noteworthy that in the absence of HC, NKT cells proliferated more rapidly than NK cells, which might explain the increased percentage of NKT cells observed in the cultures with IL-15 alone. In a manner similar to IL-15, IL-2 also favored the expansion of NK cells when combined with HC: starting with an initial population (ie, on day 0) consisting of 68% CD56+CD3–, we observed an increase of this subpopulation (88% NK cells) upon stimulation with IL-2 plus 10–6 MHCon day 15, whereas at the same time points cultures grown with IL-2 alone consisted of 48% CD56+CD3–.
Cytokine production capacity of IL-15/HC–activated CD56+ cells. Purified CD56+ cells cultured for 20 days with IL-15 in the absence or presence of HC, at concentrations ranging from 10–8 M to 10–4 M, were tested for their capacity to produce cytokines, after an additional 48-hour incubation with IL-12 and IL-18. The data represent the mean plus or minus SD from 3 independent experiments, using different healthy donors.
Cytokine production capacity of IL-15/HC–activated CD56+ cells. Purified CD56+ cells cultured for 20 days with IL-15 in the absence or presence of HC, at concentrations ranging from 10–8 M to 10–4 M, were tested for their capacity to produce cytokines, after an additional 48-hour incubation with IL-12 and IL-18. The data represent the mean plus or minus SD from 3 independent experiments, using different healthy donors.
GILZ expression in CD56+ cells cultured with IL-15/HC
GILZ is a GC-inducible gene that is involved in molecular pathways controlling lymphoid-cell activation and apoptosis.31 Having demonstrated that the proliferation status of IL-15–stimulated NK and NKT cells is differentially affected by HC, we sought to investigate whether this could be attributed to differences in the HC-induced GILZ mRNA levels among these 2 populations. As shown in Figure 6A, in total CD56+ cells GILZ mRNA levels were significantly increased in the presence of HC compared with IL-15 alone, even 24 hours after culture initiation. This increase was further potentiated, almost reaching a plateau after 20 days in culture with IL-15/HC.
HC favors the expansion of CD56+CD3– cells. Purified CD56+ cells were cultured with IL-15 in the absence or presence of HC, at concentrations ranging from 10–8 M to 10–4 M, for 20 days and the percentages of CD3– (▪) and CD3+ (□) cells were monitored at 5-day intervals. The results from one representative donor of 6 tested are presented. The percentage of CD3– cells within the CD56+ population of this donor on day 0 was 64%.
HC favors the expansion of CD56+CD3– cells. Purified CD56+ cells were cultured with IL-15 in the absence or presence of HC, at concentrations ranging from 10–8 M to 10–4 M, for 20 days and the percentages of CD3– (▪) and CD3+ (□) cells were monitored at 5-day intervals. The results from one representative donor of 6 tested are presented. The percentage of CD3– cells within the CD56+ population of this donor on day 0 was 64%.
To explore the levels of GILZ mRNA expression in the 2 distinct CD56+ (CD3– and CD3+) populations, we first took considerable care to exclude any additional signaling that might interfere with our results, by the anti-CD3 cross-linking of CD3 on CD56+CD3+ cells. For this, after culture termination we fixed total CD56+ cells just prior to isolating the 2 populations and extracting total RNA. As shown in Figure 6B, in the presence of HC high levels of GILZ mRNA expression were observed in all 3 groups, demonstrating that both NK and NKT cells behave in a very similar way, concerning GILZ expression, upon costimulation with HC.
HC induces enhanced expression of IL-2/IL-15Rβ– and γ-chains on CD56+ cells
IL-15 mediates its signals upon interaction with its specific receptor and particularly via the β- and γ-chains, both of which initiate signal transduction pathways.42 Furthermore, it has been previously shown50 that GCs may affect the expression of various cytokine receptors. Therefore, it was important to find out whether the differential effect of HC on the 2 cell populations involved changes in the expression of IL-2/IL15Rβ– and γ-chains. The number of cell-surface receptors results from the balance between their rates of synthesis, internalization, and recycling. Following interaction with their ligand (in this case IL-15), IL-2/IL15Rβ– and γ-chains are rapidly endocytosed and degraded.51 Thus, in order to be able to measure the number of receptor molecules on the cell surface, the ligand must be removed shortly before measurement, to reduce the rates of endocytosis. For this purpose, we set up CD56+-cell cultures in the presence of IL-15 alone or IL-15/HC at various doses and, after a period of 10 to 15 days, cells from both groups were harvested, washed to remove any excess IL-15 and HC, and analyzed 8 hours later for cell-surface expression of CD122 (β-chain) and CD132 (γ-chain) in NK and NKT subpopulations. The percent of positive cells for IL-15Rβ– and γ-chains was already high (> 80%) in cultures with IL-15 alone and this was not significantly changed upon treatment with various doses of HC (Figure 7). However, the number of binding sites for both IL-15Rβ– and γ-chains (Figure 7A-B) were significantly increased (P < .001) in CD56+CD3– after HC treatment. On the contrary, CD122 and CD132 expression remained unaffected in CD56+CD3+ cells.
HC effect on apoptosis and proliferation of IL-15–activated CD56+CD3– and CD56+CD3+ cells. (A) Total CD56+ cells were cultured with IL-15 in the absence or presence of 10–4 M HC for 7 days and analyzed by FACS, on CD3– and CD3+ gated cells, for 7AAD (dead cells) and annexin V (apoptotic or undergoing apoptosis cells) staining. Results from one representative donor of 3 tested are shown. Numbers in the quadrants represent percentage of positive cells. (B) Sorted CD56+CD3– and (C) CD56+CD3+ cells were cultured with IL-15 for a period of 20 days in the absence (♦) or presence (⋄) of 10–4 M HC. Proliferation of the 2 populations was estimated by [3H]TdR incorporation at the indicated time points. Results represent mean plus or minus SD of triplicate cultures from 2 different donors.
HC effect on apoptosis and proliferation of IL-15–activated CD56+CD3– and CD56+CD3+ cells. (A) Total CD56+ cells were cultured with IL-15 in the absence or presence of 10–4 M HC for 7 days and analyzed by FACS, on CD3– and CD3+ gated cells, for 7AAD (dead cells) and annexin V (apoptotic or undergoing apoptosis cells) staining. Results from one representative donor of 3 tested are shown. Numbers in the quadrants represent percentage of positive cells. (B) Sorted CD56+CD3– and (C) CD56+CD3+ cells were cultured with IL-15 for a period of 20 days in the absence (♦) or presence (⋄) of 10–4 M HC. Proliferation of the 2 populations was estimated by [3H]TdR incorporation at the indicated time points. Results represent mean plus or minus SD of triplicate cultures from 2 different donors.
IL-15/HC–activated CD56+ cells express high levels of GILZ mRNA. (A) Isolated CD56+ cells activated with IL-15 in the absence (•) or presence of HC at 10–6 M (▪) and/or 10–4 M (▴) were monitored for GILZ mRNA relative expression by real-time PCR. Total CD56+ cells were tested over a 35-day culture period. (B) Cells obtained from CD56+ cultures stimulated with IL-15 in the absence (open symbols) or presence of 10–4 M HC (filled symbols), at the indicated time points, were first fixed and then immunomagnetically separated into CD3– (circles) and CD3+ (triangles) fractions, as described in “Materials and methods.” Squares indicate total CD56+ population. All fixed populations were tested for GILZ mRNA levels. Data represent the mean plus or minus SD from 3 (A) and 2 (B) independently performed experiments.
IL-15/HC–activated CD56+ cells express high levels of GILZ mRNA. (A) Isolated CD56+ cells activated with IL-15 in the absence (•) or presence of HC at 10–6 M (▪) and/or 10–4 M (▴) were monitored for GILZ mRNA relative expression by real-time PCR. Total CD56+ cells were tested over a 35-day culture period. (B) Cells obtained from CD56+ cultures stimulated with IL-15 in the absence (open symbols) or presence of 10–4 M HC (filled symbols), at the indicated time points, were first fixed and then immunomagnetically separated into CD3– (circles) and CD3+ (triangles) fractions, as described in “Materials and methods.” Squares indicate total CD56+ population. All fixed populations were tested for GILZ mRNA levels. Data represent the mean plus or minus SD from 3 (A) and 2 (B) independently performed experiments.
HC enhances the expression of IL-2/IL15Rβ– and γ-chains in CD56+CD3– cells. Purified CD56+ cells activated with IL-15 in the absence or presence of HC, at concentrations of 10–6 M and 10–4 M, for 10 to 15 days, were extensively washed and cultured for an additional 8-hour period in the absence of IL-15 and HC and tested for surface β- (CD122) and γ-chain (CD132) expression by FACS analysis on gated CD3– and CD3+ cells. (A,B; left) □ indicates absence of HC; ▨, 10–6 M HC; and ▪, 10–4 M HC. (A) CD122+ and (B) CD132+ MESF values (mean ± SD from the pooled data with 3 different healthy donors). (A,B; right) Dotted curve indicates isotype control; filled curve, absence of HC; light gray curve, 10–6 M HC; and dark gray curve, 10–4 M HC. Histogram plots from a representative donor are presented.
HC enhances the expression of IL-2/IL15Rβ– and γ-chains in CD56+CD3– cells. Purified CD56+ cells activated with IL-15 in the absence or presence of HC, at concentrations of 10–6 M and 10–4 M, for 10 to 15 days, were extensively washed and cultured for an additional 8-hour period in the absence of IL-15 and HC and tested for surface β- (CD122) and γ-chain (CD132) expression by FACS analysis on gated CD3– and CD3+ cells. (A,B; left) □ indicates absence of HC; ▨, 10–6 M HC; and ▪, 10–4 M HC. (A) CD122+ and (B) CD132+ MESF values (mean ± SD from the pooled data with 3 different healthy donors). (A,B; right) Dotted curve indicates isotype control; filled curve, absence of HC; light gray curve, 10–6 M HC; and dark gray curve, 10–4 M HC. Histogram plots from a representative donor are presented.
ERK1/2 and STAT5 phosphorylation in CD56+ cells costimulated with HC
The observed differences in IL-2/IL15Rβ– and γ-chain levels in CD56+CD3– may explain the outgrowth of this population in the presence of HC. On the other hand, in CD56+CD3+ cells, IL-2/IL15Rβ– and γ-chains remain unaffected and this cannot account for the reduced proliferation of this population in the presence of HC.
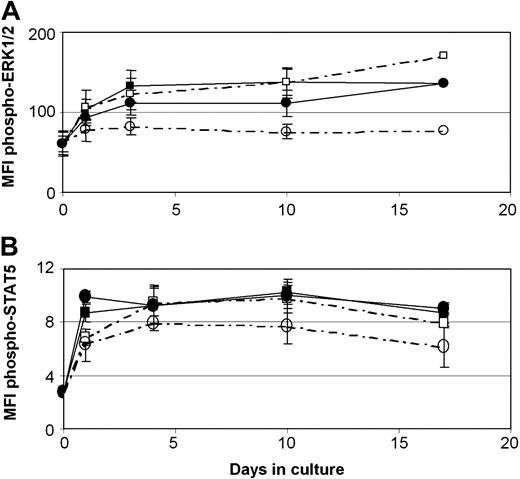
Taking into consideration previous reports indicating that GCs inhibit the Ras-Raf-MEK-ERK pathway4 and that dexamethasone prevents IL-2 signaling via STAT5,52 we sought to analyze the 2 cell populations for the presence of the activated, that is, phosphorylated, ERK1/2 and STAT5 proteins. As shown in Figure 8A, NKT cells responded to 10–4 M HC by inhibition of ERK1/2 phosphorylation, in contrast to NK cells, which displayed even increased levels of phosphorylated ERK1/2, compared with IL-15 alone. On the other hand, no statistically significant differences were observed in STAT5 phosphorylation, which was induced in both populations by IL-15, even in the presence of HC (Figure 8B), suggesting that none of the 2 populations becomes refractory to IL-15 signals when simultaneously treated with HC.
Discussion
In the present study, we show that the combination of HC with IL-15 dramatically enhances the expansion of CD56+ cells that also remain potent cytotoxic effectors, capable of efficiently lysing tumor targets throughout the entire culture period. Moreover, IL-15/HC–driven CD56+ cells exhibited an increased potential for cytokine production, which underlines their role as immunoregulatory cells.
ERK1/2 and STAT5 phosphorylation levels in CD56+ cells costimulated with HC. Total CD56+ cells were stimulated with IL-15 in the absence (filled symbols) or presence of 10–4 M HC (open symbols) for a 17-day period and tested at the indicated time points for (A) phospho-ERK1/2 and (B) phospho-STAT5 staining by FACS analysis on gated CD3– (squares) and CD3+ cells (circles). Mean fluorescence intensity (MFI) values (mean ± SD) from 3 different donors are presented.
ERK1/2 and STAT5 phosphorylation levels in CD56+ cells costimulated with HC. Total CD56+ cells were stimulated with IL-15 in the absence (filled symbols) or presence of 10–4 M HC (open symbols) for a 17-day period and tested at the indicated time points for (A) phospho-ERK1/2 and (B) phospho-STAT5 staining by FACS analysis on gated CD3– (squares) and CD3+ cells (circles). Mean fluorescence intensity (MFI) values (mean ± SD) from 3 different donors are presented.
The data presented herein also provide first evidence that NK and NKT cells activated by IL-15 are differentially affected by HC. Thymidine-incorporation measurements and apoptotic rate analysis showed that, whereas in NK cells HC induced accelerated proliferation, in NKT cells it imposed a proliferative impediment. Both populations, when costimulated with HC, exhibited elevated GILZ mRNA levels and unaffected phosphorylation of STAT5, indicating that both populations are responsive to HC and IL-15. In contrast, different alterations in IL-2/IL15Rβ– and γ-chain expression and ERK1/2 phosphorylation were observed. Namely, NK cells exhibited elevated IL-2/IL15Rβ– and γ-chain expression and higher phosphorylated ERK1/2 levels when costimulated with HC. IL-15 mediates its signals upon interaction with its specific receptor and particularly via the β- and γ-chains, both of which initiate signal transduction pathways.42 Enhanced signaling via the IL-15R may justify the NK-cell outgrowth in the presence of HC. Our data are in agreement with previous reports demonstrating up-regulation of various cytokine receptors by GCs20,22,50,53 and a synergism between GCs and growth factors to induce proliferation.54
On the other hand, the unaltered expression of IL-2/IL15Rβ– and γ-chains in NKT cells, by itself, cannot account for their reduced proliferation in the presence of HC. In these cells HC reduced ERK1/2 phosphorylation, but did not significantly affect the levels of phosphorylated STAT5. As both JAK-STAT5 phosphorylation and signaling, and shc activation of Ras-Raf-MEK-ERK cascade have been reported to act in concert to mediate maximal cell proliferation,55 it is likely that the inhibition of the later cascade by HC may explain reduced NKT-cell proliferation, although we cannot exclude the possibility that other pathways might also be involved. It has been shown recently that the development of GC resistance in CD4+ T cells is mediated by MEK and ERK, as the selective blockade of MEK/ERK signal transduction abolished resistance to GCs upon CD28 or IL-2 costimulation.52 Thus it would be of interest to monitor whether the HC effect on NKT cells could be reverted by increasing the amount of mitogen-activated protein kinase (MAPK) signaling in the presence of an additional costimulus.
GILZ has been reported to exert its negative effects on T-cell proliferation by interfering at various stages of the signal transduction pathways, including inhibition of NFκB activation and nuclear translocation,33 inhibition of activated protein-1 (AP-1),34 and also through inhibition of Raf-1 phosphorylation, with consequent inhibition of Raf-MEK-ERK activation.35 Our results show that increased GILZ expression might not be necessarily associated with negative effects on proliferation.
It is now well established that IL-15, alone or in concert with other cytokines, exerts multiple effects on NK-cell differentiation, expansion, and function, including cytotoxicity and cytokine production.56-58 Because IL-15 and IL-2 share common signaling components (ie, the β- and γ-chains of their specific receptors),42 accumulating evidence to date suggests that these cytokines, upon interaction with their specific receptors, induce similar immunomodulatory pathways. In accordance with this, we found that HC, apart from IL-15, also synergises with IL-2 for increased expansion of CD56+ cells with high cytotoxic potential, in a way similar to that described herein with IL-15.
HC did not affect the capacity of the IL-15–stimulated CD56+ cells to lyse either NK- or LAK-sensitive targets. This finding was consistent throughout the incubation period and was valid not only for K562 and Daudi targets, but also in the case of a wide spectrum of tumor-cell lines (data not shown). IL-2, IL-7, and IL-15, sharing the common γ-chain in their receptors, have been recently demonstrated to reverse the negative effects of GCs on PBMC proliferation and expression of adhesion molecules CD2 and LFA-1.59 The reversal of such GC-mediated inhibitory effects was thought to be mediated through a strong positive cytokine-dependent and γ-chain–triggered intracellular signaling. Given the observed increased expression of both IL-2/IL15Rβ– and γ-chains on CD56+ cells costimulated with HC, it is logical to assume that the exogenously added IL-15 may induce a more potent signal, thus restoring cytotoxic responses to normal levels (ie, those obtained with IL-15–stimulated CD56+ cells).
A similar result has been recently reported in T cells for dexamethasone-induced up-regulation in the expression of the IL-7Rα chain, resulting in enhanced IL-7–mediated signaling and function.20 In analogous reports,22,53 dexamethasone has been described as enhancing the expression of the high-affinity IL-2 receptor in cell lines, by specifically inducing transcription of the IL-2Rα gene.
A similar mechanism may also account for the increased potential of our IL-15/HC–stimulated CD56+ cells to produce, upon triggering, elevated amounts of the inflammatory cytokines IFN-γ, GM-CSF, and TNF-α, all of which are known to be suppressed by GCs.2 On the other hand, the dose-dependent increased production of IL-10 by HC-treated CD56+ cells, when triggered, may be a direct effect of GCs through binding of the GCR to its specific GRE sequence in the IL-10 promoter, as has been previously suggested for the constitutive production of IL-10 by dexamethasone-treated monocytes.60
The data from this report strongly emphasize the role of HC acting along with IL-15 as a costimulus of NK-cell activation and expansion. We have recently published47 that cord blood progenitors (CD34+ or CD14+ cells) differentiate into mature CD56+ cells in media containing physiologic doses of HC (10–6 M) and supplemented with IL-15 and flt3-ligand. These data may lead to a reconsideration of the previously prevalent view that the NK-cell switch from progenitor cells should take place in the absence of HC.61 Taking also into consideration the presence of active cortisol in many tissues, including peripheral blood and liver,62 the physiologic role of GCs on NK-cell biology has to be re-evaluated.
The large-scale expansion of appropriately activated CD56+ cells for cancer immunotherapy, alone or in combination with other cell types (ie, dendritic cells), remains a prerequisite for successful clinical application. The data presented herein suggesting that IL-15 (or IL-2) plus HC efficiently activates and expands NK cells in high numbers, may prove to be advantageous as a new modality for the cellular immunotherapy of cancer, since the cumbersome and time-consuming leukapheresis procedure would be avoided.
Prepublished online as Blood First Edition Paper, March 8, 2005; DOI 10.1182/blood-2004-08-3232.
Supported by a grant from the Regional Operational Program Attika No. 20, MIS code 59 605GR (M.P.) and a donation from Terry Fox Run.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

![Figure 1. HC induces high proliferation in IL-15–activated CD56+ cells. Purified CD56+ cells were cultured with IL-15 in the absence or presence of HC at concentrations ranging from 10–10 M to 10–4 M for a period of 20 days. (A) Cell counts, (B) [3H]TdR incorporation, and (C) viability estimated by trypan blue dye exclusion were analyzed every 5 days. Results represent the mean plus or minus the standard deviation (SD) of 3 independent experiments using CD56+ cells from healthy donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-08-3232/4/m_zh80130580810001.jpeg?Expires=1765969885&Signature=CVQ2~yaxRA4GfBzFjCcNPlShe193SUe5ZGndLLOK~zz796NSTJnXaPNriMJbNsRVlsBUCMRp84mkh7JwrjAb~MYIxNG-j4WIlk7zn~Nce~16b02mCZxMKDXYaXsiSp~Z-N2ysS80dSqYXs8TK5NKGOb7xVcArEKEaSoj9bilm9J5vf2U4DK0s1K8K0~GoeXBW-4QmP9gdYugc7NG-RVwsy3IxjoMGt6h3eqRaCX3jy~qTEQRoPw2Jfd5Z7RC1sSG6bxuRdiLfNgaG6MgNhhCOd2b7UAwiQF2t5XKQwLJUZ0jPEZXvAoj6j~CA7CmTZxSQnqCp2PkuBIoPyVTqvah9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Figure 5. HC effect on apoptosis and proliferation of IL-15–activated CD56+CD3– and CD56+CD3+ cells. (A) Total CD56+ cells were cultured with IL-15 in the absence or presence of 10–4 M HC for 7 days and analyzed by FACS, on CD3– and CD3+ gated cells, for 7AAD (dead cells) and annexin V (apoptotic or undergoing apoptosis cells) staining. Results from one representative donor of 3 tested are shown. Numbers in the quadrants represent percentage of positive cells. (B) Sorted CD56+CD3– and (C) CD56+CD3+ cells were cultured with IL-15 for a period of 20 days in the absence (♦) or presence (⋄) of 10–4 M HC. Proliferation of the 2 populations was estimated by [3H]TdR incorporation at the indicated time points. Results represent mean plus or minus SD of triplicate cultures from 2 different donors.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/106/1/10.1182_blood-2004-08-3232/4/m_zh80130580810005.jpeg?Expires=1765969885&Signature=2YNjCDr-VeagveZp5-ENbpjA7SXpe9d40lSOxFi2mJZvxBUDEdU0K8PmlzLZhRIn-38ESMGrUz1miihoNE8nIdXQ6r7KPptee14bqL0NjMxTmWxa~~EXU6yu7z10z54Gjb9ZvJAH4PlHd9KDW61ifOx4xm0lz-X4YeXmfmEsNU5PFzBVOpAW2baFa8DH1qhfjqvfXWH4eBmKAj4peRfer3rC-0odEYmN1qFSsMTElG1B6Y3hat0XiXIYnPRTl~GAt1meJHNx1Qp-I0WiMalzL9jpaEukge7Pw5TEKvQ3o4KJKUgWclGxa7epr2lPv~I4AurS8ijv28P0SOuP0Y-W5g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal