Induction of cytotoxic T lymphocytes (CTLs) for treatment of relapsed leukemia after allogeneic stem-cell transplantation is hindered by the laborious and time-consuming procedure of generating dendritic cells for antigen presentation. Artificial antigen-presenting cells (aAPCs) offer the advantage of being readily available in sufficient numbers, thus allowing for a highly standardized in vitro induction of CTLs. We generated aAPCs coated with anti-CD28 antibody (Ab) and either high-density (HD) or low-density (LD) major histocompatibility complex (MHC) class I molecules loaded with HA-1H, a nonapeptide derived from the hematopoiesis-restricted minor histocompatibility antigen HA-1. HD- and LD-aAPCs potently induced HA-1H–specific CD8+ CTLs from untouched CD8+ T cells of healthy donors. CTLs were subsequently purified by magnetic-activated cell sorting. HD- as well as LD-aAPC–induced CTLs exerted high HA-1H–specific cytotoxicity, resembled Tc1 effector memory cells, survived a long time in vitro, and were expanded by a factor varying between 8.2 × 104 and 51 × 104. The T-cell receptor (TCR) repertoire of HA-1H tetramer–positive CTLs was oligoclonal with a prominent usage of Vβ6. The TCR repertoire of tetramer-positive CTLs was distinct from and more restricted than that of tetramer-negative cells. These findings indicate that aAPCs are attractive tools for the ex vivo generation of HA-1H–specific CTLs suitable for immunotherapy of relapsed leukemia.
Introduction
Because CD8+ T cells are considered to be key effectors against malignant diseases, adoptive transfer of in vitro–generated CD8+ cytotoxic T cells that recognize antigens of the respective tumor has been suggested as a therapeutic approach to induce tumor regression in vivo. Among the various antigens that can serve as targets for antigen-specific T cells, minor histocompatibility antigens (mHags)1 have the advantage of not being subject to self-tolerance and might therefore represent attractive targets after human leukocyte antigen (HLA)–matched and mHag-mismatched allogeneic stem-cell transplantation.2 Several other findings pinpoint the mHags HA-1 and HA-2 as promising targets for adoptive immunotherapy in leukemias. (1) HA-1 and HA-2 are expressed solely on hematopoietic and leukemic cells.3,4 Cytotoxic HA-1– or HA-2–specific T cells are thus expected to elicit graft-versus-leukemia reactions with little or no graft-versus-host disease.3 (2) The efficient lysis of leukemic cells in vitro has been demonstrated using HA-1– or HA-2–specific T cells.5,6 (3) In vivo, the appearance of HA-1– and HA-2–specific CD8+ T cells after donor lymphocyte infusion (DLI) has been associated with complete remission of relapsed leukemia.7
Until very recently, in vitro priming of cytotoxic T lymphocytes (CTLs) was performed using dendritic cells (DCs) that were differentiated from donor peripheral blood monocytes or CD34+ hematopoietic progenitors. However, the generation of DCs is expensive, laborious, time consuming, and limited in terms of the number of DCs that can be generated. Moreover, care needs to be taken to generate only fully matured DCs, because immature DCs have been shown to induce tolerance.8 Artificial antigen-presenting cells (aAPCs)9 might overcome these shortcomings because they are rapidly available and allow the establishment of highly reproducible conditions for the generation of cytotoxic T cells. In fact, controlling the density of major histocompatibility complex (MHC) has been shown to determine the avidity of cytotoxic T cells.10 This is of particular interest because cytotoxic T cells of high avidity are expected to promote a more efficient lysis of leukemic cells with a relatively low density of presented peptides. aAPCs coated with HLA/mHag complexes CD80 and CD54 have been shown to stimulate HA-1– and HA-2–specific CTL clones and to expand HA-1–specific T cells in polyclonal HA-1–specific CTL lines in vitro.11 Here, we show that aAPCs coated with HLA/HA-1 complexes and costimulatory anti-CD28 antibody (Ab) can be used for the potent primary in vitro induction of cytotoxic, expandable, and long-lasting HA-1–specific CD8+ effector memory T cells from healthy blood donors.
Materials and methods
Donor and patient characteristics
Forty-seven HLA-A*0201–positive donors were genotyped for the allelic expression of HA-1 by reverse transcriptase–polymerase chain reaction (RT-PCR). The detected frequency of 65% HA-1HH/HA-1HR individuals is consistent with data published previously.12,13 HLA-A*0201/HA-1RR individuals were chosen as donors for the generation of T cells. The selection criteria for donors were preclusion of pregnancy if female, an age range of 18 to 50 years, no history of blood transfusions, and no HIV infection. Donor no. 1 (D1) and D4 were female; D2, D3, and D5 male. The mother of D1 was genotyped HA-1RR.
Primary leukemic blasts from 3 patients with childhood acute lymphoblastic leukemia (ALL) (patient no. 1 [P1]: B-ALL, 72% leukemic blasts, HLA-A*0201/HA-1H; P2: T-ALL, 96% leukemic blasts, HLA-A*0201/HA-1H; P3: B-ALL, 86% leukemic blasts, HLA-A*0201 negative) were obtained at the time of diagnosis or relapse. Mononuclear cells were separated by density centrifugation, frozen for storage, and thawed prior to experiments. Leukemic blasts were negative for the BCR-ABL, MLL/AF4, TEL/AML1 rearrangements. Blasts of P1 showed hyperdiploidy in most analyzed mitoses, whereas blasts of P2 exhibited a normal karyotype.
The study protocol was approved by the Clinical Ethics Committee of the University of Tübingen, and the study was performed according to the principles of the Declaration of Helsinki. Informed consent was obtained from all donors and patients according to the institutional guidelines.
Peptides, recombinant MHC molecules, fluorescent tetramers, and MHC-coated microspheres (aAPCs)
The HA-1H peptide VLHDDLLEA and the HIV peptide ILKEPVHGV that served as a control peptide in this study were synthesized by the Hartwell Center at St Jude Children's Research Hospital, Memphis, TN, and by Stefan Stevanovic, Department of Immunology, Institute for Cell Biology, University of Tübingen, Germany, respectively, using standard Fmoc chemistry. The biotinylated recombinant HLA-A*0201 molecules and fluorescent MHC I tetramers were produced as described previously.14 The costimulatory mouse immunoglogulin G2a (IgG2a) anti–human CD28 Ab 9.3 15 was biotinylated using sulfo-N-hydroxysuccinimidobiotin as recommended by the manufacturer (Perbio Science, Bonn, Germany). For generation of aAPCs, 5.6 μm–diameter streptavidin-coated polystyrene particles with a binding capacity of 0.064 μg biotin–fluorescein isothiocyanate (FITC) per milligram of microsphere (Bangs Laboratories, Fishers, IN) were resuspended at 2 × 106 particles per milliliter in buffer containing biotinylated MHC I and anti-CD28 Ab at indicated concentrations and incubated at room temperature for 30 minutes.
Ag-specific in vitro induction of human CD8+ T cells from HLA-A*0201/HA-1RR donors
Peripheral blood mononuclear cells (PBMCs) were isolated from fresh and heparinized peripheral blood using standard gradient separation. “Untouched” CD8+ T cells were isolated by negative depletion using magnetic-activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany).
In vitro stimulations were started with a total of 1 × 107 to 2 × 107 CD8+ T cells per donor. In 24-well plates, 5 × 106 responder cells plus 1 × 106 beads were set up per well in 1.5 mL T-cell medium consisting of RPMI 1640 containing 25 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) (Life Technologies/Invitrogen, Karlsruhe, Germany) supplemented preferentially with 10% heat-inactivated autologous serum or human AB serum (CC Pro, Neustadt/Weinstrasse, Germany), 2 mM l-glutamine, 50 U/mL penicillin, 50 μg/mL streptomycin, and 20 μg/mL gentamicin (all BioWhittaker/Cambrex, Verviers, Belgium). Unless stated otherwise, 5 ng/mL human interleukin-12 (IL-12) p70 (R&D Systems, Minneapolis, MN) was added with microspheres. After 3 to 4 days of coincubation at 37°C, fresh medium and 20 U/mL human IL-2 (R&D Systems) was added, and cells were incubated for 3 to 4 days. This stimulation cycle was repeated 3 times.
Ag-specific T-cell enrichment and expansion
On day 35, aAPC-induced cells were fluorescence-activated cell sorter (FACS) sorted on a FACSVantage after staining with phycoerythrin (PE) tetramers and CD8-FITC mAb clone SK1 (BD Biosciences, Heidelberg, Germany).
As indicated, aAPC-stimulated cells were alternatively or additionally enriched with MACS technology (Miltenyi Biotec) by tetramer PE staining and anti-PE bead sort.
For in vitro expansion, sorted cells were cultured in the presence of irradiated Epstein-Barr virus (EBV)–transformed LG2 cells (100 Gy) and allogeneic feeder cells (50 Gy), 150 U/mL IL-2, and 5 ng/mL IL-7 for 1 week. Afterward, cells were grown in the presence of irradiated allogeneic feeder cells, 150 U/mL IL-2, and 5 ng/mL IL-7 for another week and thereafter in the presence of 150 U/mL IL-2, 5 ng/mL IL-7, and 10 ng/mL IL-15 only.
The EBV-transformed B-cell line LG2 (HLA-A*0201 negative) was a kind gift from Pierre van der Bruggen, Ludwig Institute for Cancer Research, Brussels, Belgium.
Flow cytometry and cytokine assays
Cells were stained using standard methods and analyzed on a FACSCalibur flow cytometer equipped with a 488 nm argon-ion laser and 635 nm red diode laser using Cell Quest 3.3 Software (BD Biosciences). Tetrameric analyses were performed by double stainings with PE-labeled MHC tetramers and Ab CD8-allophycocyanin clone SK1 (BD Biosciences). FITC-labeled Abs used were CD3, CD11b, CD18, CD27, CD28, CD62L, CD95 (all from BD Biosciences); PE-labeled Abs were CD3, CD8 (both from BD Biosciences), anti–HLA-A, -B, and -C (all from Dako Cytomation, Hamburg, Germany); peridinin chlorophyll protein (PerCP)–labeled Ab was CD3 (BD Biosciences); and allophycocyanin-labeled Abs were CD3, CD8 (both from BD Biosciences), CD45RA, CD45RO, and cytotoxic T-lymphocyte antigen-4 (CTLA-4) (from BD Pharmingen). As secondary antibodies, FITC-labeled goat antimouse or PerCP-labeled rat antimouse (both BD Biosciences) were used after incubation with unlabeled CD95L (BD Pharmingen) and unlabeled CD45RA, respectively. Biotinylated CCR7 was detected by FITC-labeled streptavidin (both BD Biosciences). The appropriate isotype controls were from BD Biosciences and Dako Cytomation. Cytokine release was detected using the BD Cytometric bead array Human Th1/Th2 Cytokine Kit (BD Biosciences). The experimental conditions were adjusted as described in detail by the manual of the manufacturer. LCL721 is an HLA-A*0201/HA-1HR EBV-transformed cell line.
T-cell receptor (TCR) repertoire analysis
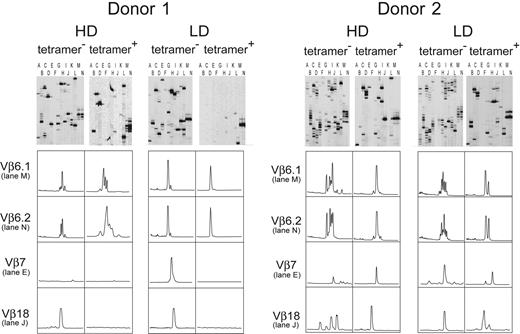
The T-cell receptor (TCR) repertoire diversity of HA-1–specific CTLs was determined by complementarity-determining region 3 (CDR3)–size spectratyping as described previously.16-18 Briefly, RNA was isolated and reverse transcribed to cDNA using standard procedures. The cDNA was amplified with sense primers from all 24 Vβ families (26 primers) and a fluorescence-labeled antisense primer (IRD800) Cβ-region antisense primer. Products were denatured, separated on a denaturing polyacrylamide gel, and visualized using the LiCor4200 device (LiCor, Bad Homburg, Germany). We used the classification of TCRVβ families according to the nomenclature described by Arden et al.19 In Figure 5, lane A refers to Vβ2/Vβ12 (shorter amplicons/longer amplicons), lane B to Vβ13/Vβ3, lane C to Vβ5.1/Vβ1, lane D to Vβ4/Vβ5.3, lane E to Vβ8/Vβ7, lane F to Vβ9/Vβ14, lane G to Vβ20/Vβ10, lane H to Vβ11/Vβ19, lane I to Vβ16/Vβ21, lane J to Vβ18/Vβ23, lane K to Vβ17/Vβ15, lane L to Vβ24/Vβ22, lane M to Vβ6.1, and lane N to Vβ6.2.
Generation of EBV-transformed B-cell lines
After separation by density centrifugation, 1 × 107 PBMCs of 3 donors (2 HLA-A*0201/HA-1RR and 1 HLA-A*0201/HA-1HH) and 1 × 107 cells of the diagnostic sample of P1 were incubated with 400 μL EBV-containing culture supernatant (ATCC no. CRL 1612; American Type Culture Collection, Manassas, VA) for 30 to 60 minutes in a 37°C water bath. Subsequently, 4 mL RPMI 1640 supplemented with 20% heat-inactivated fetal bovine serum, 1 μg/mL cyclosporin A (Sandoz, Basel, Switzerland), 2 mmol l-glutamine (Gibco, Karlsruhe, Germany) was added. Cells were seeded at 5 × 105 per well into a 96-well round-bottom microtiter plate and expanded as soon as proliferating clusters of immortalized B lymphocytes were detected.
Karyotype of EBV-transformed B-cell line of P1 as determined by G-banding (after 10 weeks of cultivation) was normal without any evidence for translocations (data not shown).
Cytotoxicity assay
Cytotoxicity was assessed by a time-resolved fluorometric assay employing bis(acetoxymethyl) 2,2′:6′,2′′-terpyridine-6,6′′-dicarboxylate (BATDA) as a fluorescence-enhancing ligand20 and taking 104 target cells per well. Percentage of specific lysis was calculated as follows: (experimental release – spontaneous release)/(total release – spontaneous release) × 100. The assay was performed as described previously in detail.21
Results
Potent primary in vitro induction of HA-1–specific CTLs by aAPCs
We investigated whether microspheres coated with HLA-A*0201/HA-1H complexes and costimulatory anti-CD28 Ab are capable of inducing HA-1–specific CTLs in vitro. Because the density of MHC molecules on the surface of the microspheres might influence the extent of the expansion, the avidity of HA-1–specific T cells, and the ability to recognize the respective Ag,10 we generated MHC class I high-density (HD) aAPCs coated with anti-CD28 Ab and 10 nM HLA-A*0201/HA-1H as well as MHC class I low-density (LD) aAPCs coated with anti-CD28 Ab and 100 pM HLA-A*0201/HA-1H (Supplemental Figure S1, available at the Blood website; see the Supplemental Figures link at the top of the online article). CD8+ T lymphocytes were negatively enriched from 5 HLA-A*0201/HA-1RR healthy blood donors (referred to here as D1 to D5) and stimulated every 6 to 8 days with either HD-aAPCs or LD-aAPCs. During the course of expansion, percentages of HA-1–specific T cells were monitored by tetrameric analysis in 4 donors (D2 to D5). We did not determine the expansion of HA-1–specific CTLs for D1 because for this donor we were aiming for the highest cell number possible for further experiments. Staining with MHC I tetramers loaded with the HIV peptide served as a control in these HIV-negative donors. In all 4 donors tested, aAPCs potently induced HA-1H–specific CTLs (Table 1; Supplemental Figure S2). As detailed in “Materials and methods,” these 4 donors have no history of a prior pregnancy or blood transfusion. The mother of D1 was genotyped as HA-1RR excluding a priming of HA-1–specific T cells in utero that has been described.22 Therefore, the induction of HA-1H–specific CTLs can be attributed to the activation of naive T cells by aAPCs.
In vitro induction of HA-1-specific CD8+ CTLs of 4 different donors by MHC I high-density (HD) aAPCs and MHC I low-density (LD) aAPCs
. | HD-aAPCs . | LD-aAPCs . |
|---|---|---|
| D2 | 2.8 (d35) | 6.4 (d35) |
| D3 | 13.4 (d41) | NA |
| D4 | 11.5 (d39) | 1.7 (d34) |
| D5 | 3.6 (d24) | 1.9 (d32) |
. | HD-aAPCs . | LD-aAPCs . |
|---|---|---|
| D2 | 2.8 (d35) | 6.4 (d35) |
| D3 | 13.4 (d41) | NA |
| D4 | 11.5 (d39) | 1.7 (d34) |
| D5 | 3.6 (d24) | 1.9 (d32) |
Maximum percentages of HA-1 tetramer-positive cells among CD8+ lymphocytes and respective days of in vitro induction are given.
NA indicates not assessed.
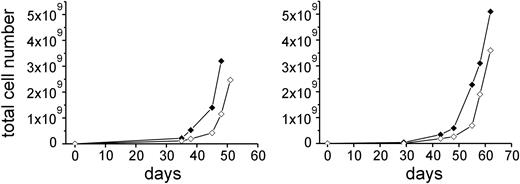
Expansion of CTLs. In vitro expansion of purified HA-1–specific CD8+ CTLs of D1 (left) and D2 (right) by HD-aAPCs (♦) and by LD-aAPCs (⋄). The number of days given refers to the days of expansion after the first sort. Cell numbers after first sort were 6.3 × 103 HD-aAPC– and 1.07 × 104 LD-aAPC–induced CTLs for D1 and 6.2 × 104 HD-aAPC– and 1.3 × 104 LD-aAPC–induced CTLs for D2.
Expansion of CTLs. In vitro expansion of purified HA-1–specific CD8+ CTLs of D1 (left) and D2 (right) by HD-aAPCs (♦) and by LD-aAPCs (⋄). The number of days given refers to the days of expansion after the first sort. Cell numbers after first sort were 6.3 × 103 HD-aAPC– and 1.07 × 104 LD-aAPC–induced CTLs for D1 and 6.2 × 104 HD-aAPC– and 1.3 × 104 LD-aAPC–induced CTLs for D2.
Purification and expansion of HA-1–specific CTLs
The adoptive transfer of high numbers of HA-1–specific T cells is likely to be essential for an efficient antileukemic response in vivo. However, cotransfusion of a substantial number of non-HA-1–specific T cells implies a higher risk of GVHD than the transfer of pure HA-1–specific T cells. We therefore investigated whether HA-1–specific CTLs can be purified and expanded in vitro to a degree that would allow a later clinical application. On day 35 of in vitro induction, we stained HD- and LD-aAPC–induced CTLs of D1 and D2 by HA-1 tetramers and sorted them by fluorescence-activated cell sorting (FACS). After 1 week of in vitro expansion, cells were restained with HA-1 PE tetramers and purified by anti-PE–coated beads and MACS. The purity of tetramer-positive cells was more than 99% in HD- and LD-aAPC–induced CTLs of D1 and D2. Subsequently, cells were further expanded in vitro. Forty-eight and 62 days after the first sorting, respectively, HA-1–specific CTLs of D1 and D2 had expanded by a factor ranging between 8.2 × 104 and 51 × 104 (Figure 1). The purity of expanded cells was still more than 99% by tetramer analysis.
Cytotoxic activity of aAPC-induced HA-1–specific CTLs is specific for HA-1
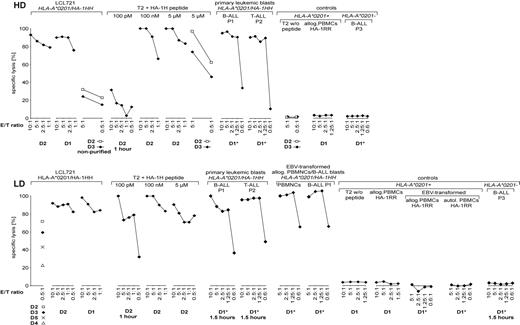
HD- and LD-aAPC–induced CTLs were purified on day 35 by MACS after tetramer staining and incubation with anti-PE beads (with the exception of D1 and D2, which were purified by FACS prior to MACS). The purity obtained was more than 99% of tetramer-positive cells. Cytotoxicity assays were performed after in vitro expansion for 2 weeks. The main findings of the cytotoxicity assays performed were as follows (Figure 2): First, purified HD- and LD-aAPC–induced CTLs demonstrated a high cytotoxicity against HLA-A*0201/HA-1HH primary leukemic blasts as well as PBMCs and EBV-transformed leukemic blasts and cell lines. Second, no alloreactivity of purified HD- and LD-aAPC–induced CTLs against HLA-A*0201/HA-1RR PBMCs, against T2 cells not loaded with HA-1H peptide, or HLA-A*0201–negative targets was observed. Third, only target cells loaded with a very low (100 pM) amount of HA-1 peptide were lysed more efficiently by LD-aAPCs as compared with HD-aAPC–induced CTLs.
aAPC-induced HA-1–specific CTLs are of Tc1 effector memory type
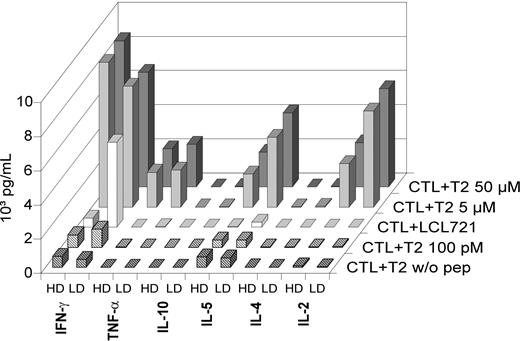
The cytokine secretion pattern was determined in HD-aAPC– and LD-aAPC–induced CTLs of D2. Three days after the final MACS purification step on day 35, CTLs were coincubated with LCL721 cells or T2 cells loaded with different amounts of HA-1H peptide (Figure 3). High amounts of interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α), IL-2, and IL-5, but no IL-4 or IL-10, were detected in the supernatants. Notably, after coincubation with LCL721 cells that endogenously express HA-1, LD-aAPC–induced CTLs secreted higher amounts of IFN-γ than HD-aAPC–induced CTLs.
Cytotoxic activity of HD-aAPC– and LD-aAPC–induced CD8+ CTLs of 5 donors. HD-aAPC– (top) and LD-aAPC– (bottom) induced CTLs were purified on day 35 (d35) after in vitro priming to more than 99% HA-1 tetramer–positive cells (unless indicated otherwise). *Assays with frozen and thawed aAPC-induced CTLs. Effector-target (E/T) ratios are given on the horizontal axis. Incubation time of assays was 3 hours (except where indicated).
Cytotoxic activity of HD-aAPC– and LD-aAPC–induced CD8+ CTLs of 5 donors. HD-aAPC– (top) and LD-aAPC– (bottom) induced CTLs were purified on day 35 (d35) after in vitro priming to more than 99% HA-1 tetramer–positive cells (unless indicated otherwise). *Assays with frozen and thawed aAPC-induced CTLs. Effector-target (E/T) ratios are given on the horizontal axis. Incubation time of assays was 3 hours (except where indicated).
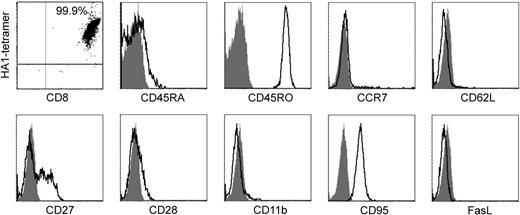
The phenotypic characterization of LD-aAPC–induced CTLs of 2 donors (D1 and D2) was performed after MACS purification and 2 weeks of in vitro expansion. There was no surface expression of CD45RA and CCR7 (Figure 4), a feature that has been described as distinctive for human effector memory T cells (TEM).23 The expression pattern of CD45RO+, CD62L–, CD27+/–, CD28–, CD11b–, CD95+, and Fas-L– further supports the assignment of the generated CTLs to TEM.24 Because the phenotypes of the CTLs from both donors were essentially identical, only one phenotypic analysis is shown in Figure 4. The memory type of in vitro–induced CTLs is supported by their life span in vitro. HD- and LD-aAPC–induced CTLs of D1 and D2 were cultivated with excellent viability for 165 and 159 days, respectively. TCR repertoire analysis by complementarity-determining region 3 (CDR3)–size spectratyping reveals oligoclonality of aAPC-induced HA-1–specific CTLs.
Cytokine secretion pattern of HD-aAPC– and LD-aAPC–induced CD8+ CTLs of D2. CTLs were coincubated with LCL721 cells or T2 cells loaded with different amounts of HA-1H peptide and the amount of cytokines determined in the cell supernatants. All cytokines were below 6 pg/mL in controls used (medium, medium with CTLs, and medium with T2 cells not loaded with peptide).
Cytokine secretion pattern of HD-aAPC– and LD-aAPC–induced CD8+ CTLs of D2. CTLs were coincubated with LCL721 cells or T2 cells loaded with different amounts of HA-1H peptide and the amount of cytokines determined in the cell supernatants. All cytokines were below 6 pg/mL in controls used (medium, medium with CTLs, and medium with T2 cells not loaded with peptide).
CDR3-size spectratyping analyses of all 24 Vβ families were performed with HD- and LD-aAPC–induced CTLs from D1 and D2 (Figure 5). Prior to RNA extraction, aAPC-induced CTLs were sorted into tetramer-positive and tetramer-negative cells on day 35 of induction by MACS. Both fractions of HD-aAPC– and LD-aAPC–induced CTLs of D1 and D2 showed a severely skewed TCR repertoire. Tetramer-positive CTLs exhibited a more restricted repertoire compared with tetramer-negative CTLs. Furthermore, in D1 at least, repertoire complexity in tetramer-positive LD-aAPC–induced CTLs was less diverse than that of tetramer-positive HD-aAPC–induced CTLs. Although usage of Vβ families varied between HD- and LD-aAPC–induced CTLs in both donors, Vβ6 using CDR3s were detected consistently in HD- as well as LD-aAPC–induced CTLs from both donors. Interestingly, in tetramer-positive LD-aAPCs of D1, the Vβ6 family represented 99.6% of the overall repertoire as determined by quantitative real-time PCR (data not shown). These data strongly suggest a monoclonal expansion of the latter population.
Phenotypic characterization of CD8+ LD-aAPC–induced CTLs from D2 after MACS purification of tetramer-positive cells and 2 weeks of in vitro expansion. The dot plot shows staining with HA-1 tetramer PE and CD8 APC Ab. The percentage of CD8+ HA-1 tetramer–positive cells is given in the right upper quadrant. A tetramer loaded with the HIV peptide was used as a control (not shown). Histograms show expressed antigens (open curves) and the respective controls (shaded curves). CD62L indicates CD62 ligand; FasL, Fas ligand.
Phenotypic characterization of CD8+ LD-aAPC–induced CTLs from D2 after MACS purification of tetramer-positive cells and 2 weeks of in vitro expansion. The dot plot shows staining with HA-1 tetramer PE and CD8 APC Ab. The percentage of CD8+ HA-1 tetramer–positive cells is given in the right upper quadrant. A tetramer loaded with the HIV peptide was used as a control (not shown). Histograms show expressed antigens (open curves) and the respective controls (shaded curves). CD62L indicates CD62 ligand; FasL, Fas ligand.
TCR repertoires of tetramer-sorted HD- and LD-aAPC–induced HA-1–specific CTLs. CDR3-size spectratyping was applied to analyze the TCR repertoire of HD-aAPC– and LD-aAPC––induced CTLs from D1 (left) and D2 (right). The upper panels show the respective spectratyping gels with the 24 Vβ families distributed on 14 lanes. The amplicons displayed on each lane are detailed in “Materials and methods.” Lower panels show histograms of selected Vβ families as representative examples of skewed TCR repertoires of HD- and LD-aAPC–induced CTLs. Although usage of Vβ families varied between HD- and LD-aAPC–induced CTLs in both donors, Vβ6 using CDR3s was detected consistently in HD- as well as in LD-aAPC–induced CTLs from both donors.
TCR repertoires of tetramer-sorted HD- and LD-aAPC–induced HA-1–specific CTLs. CDR3-size spectratyping was applied to analyze the TCR repertoire of HD-aAPC– and LD-aAPC––induced CTLs from D1 (left) and D2 (right). The upper panels show the respective spectratyping gels with the 24 Vβ families distributed on 14 lanes. The amplicons displayed on each lane are detailed in “Materials and methods.” Lower panels show histograms of selected Vβ families as representative examples of skewed TCR repertoires of HD- and LD-aAPC–induced CTLs. Although usage of Vβ families varied between HD- and LD-aAPC–induced CTLs in both donors, Vβ6 using CDR3s was detected consistently in HD- as well as in LD-aAPC–induced CTLs from both donors.
Discussion
The main objective of this study was to investigate whether aAPCs coated with HLA/HA-1 complexes and costimulatory anti-CD28 Ab can be used for the primary in vitro induction of HA-1 CTLs that are suitable for adoptive transfer after allogeneic stem-cell transplantation. For the generation of aAPCs we relied on preformed MHC I/peptide complexes that are coupled to streptavidin-coated microspheres via the biotinylated MHC. This system allows an exact control of the MHC density on the surface of the microspheres. In our study, MHC high-density and MHC low-density aAPCs reliably induced HA-1–specific CTLs in vitro in the presence of IL-12. This is in line with an earlier report that demonstrated that LD-aAPCs are also effective for in vitro priming as long as costimulation via CD28 and IL-12 was provided.10 Recently, evidence has been raised that HA-1–specific T cells can already be primed in utero in HLA-A*0201/HA-1RR fetus after the penetration of maternal cells of HA-1–mismatched mothers.22 The mother of D1 (the only mother from whom we were able to obtain blood) was genotyped as HA-1RR (data not shown), which argues that the aAPCs used in this study are capable of a primary in vitro induction of HA-1–specific CTLs.
To investigate properties of aAPC-induced CTLs, HA-1–specific T cells might either be purified or cloned. However, MACS purification of HA-1 tetramer–positive CTLs seems especially suitable for a later clinical application, because it is a reliable and quite easy to perform method that permits the generation of high numbers of HA-1–specific CTLs in a short time. Therefore, we decided to purify HA-1 tetramer–positive CTLs by MACS. Purified HD- and LD-aAPC–induced CTLs showed high cytotoxic activities against various targets endogenously expressing HA-1H as well as T2 cells loaded with varying amounts (100 nM to 5 μM) of HA-1 peptide. In particular, they were able to lyse primary leukemic blasts. No undesired alloreactivity of aAPC-induced CTLs could be detected. These findings are relevant for the adoptive transfer of aAPC-induced CTLs in respect of dissecting a graft-versus-leukemia effect from GVHD. Nevertheless, the GVHD risk of highly purified HA-1–specific CTLs in vivo is difficult to predict.25 The lysis of tissue-infiltrating host-derived APCs by HA-1–specific CTLs might be beneficial26 or, via the recruitment of allogeneic bystander T cells into the tissues, detrimental.3,25 The number of CTLs and the time points of administration with the lowest risk of GVHD induction therefore need to be determined in clinical trials. However, 2 findings indicate that LD-aAPC–induced CTLs might elicit a superior antileukemic response in vivo. When compared with HD-aAPC–induced CTLs, LD-aAPC–induced CTLs demonstrated a stronger cytotoxic activity against target cells loaded with a very low (100 pM) amount of HA-1 peptide. This is consistent with the notion that LD-aAPCs predominantly prime high-avidity CTLs that require low amounts of antigenic peptide for recognition.10 Furthermore, LD-aAPC–induced CTLs secreted higher levels of IFN-γ after coincubation with LCL721 cells.
Our analysis of the cytokine secretion pattern and the immunophenotypes suggests that HD- and LD-aAPC–induced CTLs were of Tc1 effector memory (TEM) type. TEM are thought to provide protective memory by migrating to peripheral tissues and exerting immediate effector function.23,27,28 Reactive memory is thought to be mediated by central memory T cells (TCM) that home to T-cell areas of secondary lymphoid organs and display little effector function but may proliferate and differentiate into effector cells after secondary antigenic stimulation.27,28 Immunophenotypically, human TEM can be distinguished from TCM by loss of the chemokine receptor CCR7,23,28 which together with L-selectin (CD62L) controls the first steps of T-cell homing to lymph nodes. Neither CCR7 nor CD62L could be detected on aAPC-induced CTLs. The other phenotypic markers used further support the assumption that the aAPC-induced CTLs represent a T-cell population with effector and memory properties.24 The observed lifetime in vitro is likewise consistent with a generation of memory cells. Until very recently, the expansion potential has been thought to decrease from human TCM to TEM Therefore, the high expandability of TEM in our study was an unexpected finding. However, a recent investigation in mice demonstrated that TEM mounted recall responses that were equal to or even greater than those mounted by TCM.29 Whether this holds true for human TEM is to date unknown. Thus, it needs to be determined whether the adoptive transfer of aAPC-induced TEM indeed transfers a powerful part of immunologic memory.
Two findings emerged from the TCR repertoire analyses that are of significance for the clinical application of aAPC-induced CTLs. First, despite a constant use of Vβ6 in tetramer-positive HD- and LD-aAPC–induced CTLs, the overall TCR repertoire was oligoclonal. An exclusive30,31 or restricted32 usage of Vβ6 by HA-1–specific CTLs has been reported previously. It seems reasonable to assume that the transfer of oligoclonal instead of monoclonal HA-1–specific CTLs is advantageous for an antileukemic response, because deletion of one or even several CTL clones will not result in abrogation of the graft-versus-leukemia (GvL) effect. Second, despite some overlapping, the TCR repertoires of tetramer-positive and -negative HD- and LD-aAPC–induced CTLs were largely distinct from each other. Although some HA-1–specific CTLs might have escaped tetramer staining by internalization of their TCRs, this is certainly not true for most tetramer-negative cells. Therefore, high purification of tetramer-positive cells eliminates T cells that do not specifically recognize the HA-1 peptide and thus might trigger GVHD after an adoptive transfer.
In summary, we show that aAPCs coated with HLA-A*0201/HA-1H complexes and costimulatory anti-CD28 Ab are suitable for the primary in vitro induction of HA-1–specific CD8+ CTLs. Moreover, the generated CTLs revealed properties that suggest an efficient and long-lasting antileukemic effect in vivo. HA-1–specific CTLs had a high cytotoxic capacity against primary leukemic blasts and other HA-1H targets, were of oligoclonal nature, could be expanded up to 510 000-fold, and were long-lived in vitro, which fits well with their Tc1 effector-memory-cell phenotype. Thus, the use of aAPCs for the in vitro generation of HA-1–specific CTLs appears to be an attractive tool for enhancing the antileukemic immune response after allogeneic stem-cell transplantation.
Prepublished online as Blood First Edition Paper, February 24, 2005; DOI 10.1182/blood-2004-07-2940.
Funded by grants from the Deutsche José Carreras Leukämie Stiftung, the Reinhold-Beitlich-Stiftung, and the fortüne-program of the University of Tübingen.
K.S. and G.K. contributed equally to this study.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Pierre van der Bruggen for providing LG2 cells and Lynne Yakes for thoroughly proofreading this manuscript.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal