Abstract
Activating Flt3 mutations occur in about 30% of patients with acute myeloid leukemia (AML), often as in-frame internal tandem duplication (ITD) at the juxtamembrane domain of the receptor. These mutations transform hematopoietic cell lines and primary mouse bone marrow. Here, we analyzed the interaction between oncogenic Flt3-ITD mutations and the Wingless-type (Wnt) signaling pathway in the myeloid progenitor cell line 32D. Microarray analyses revealed higher mRNA expression of Frizzled-4, a receptor for Wnt ligands in 32D/Flt3-ITD cells. Findings were verified by quantitative realtime reverse transcription–polymerase chain reaction (RT-PCR) and on the protein level. Compared with 32D/Flt3-WT (wild-type) cells, 32D/Flt3-ITD cells also showed greatly enhanced β-catenin protein levels, irrespective of their exposure to Wnt3a, a ligand inducing the canonical Wnt signal transduction pathway. In addition, 5 of 7 AML samples with Flt3-ITD mutations expressed high β-catenin protein levels, whereas patients with wild-type Flt3 did not. Also, Flt3-ITD induced enhanced T-cell factor (TCF)–dependent transcriptional activity and the induction of the Wnt target gene c-myc. In the presence of Flt3-WT or Flt3-ITD signaling, Wnt3a slightly increased 32D cell proliferation. However, transfection experiments with dominant-negative (dn) TCF4 revealed a strong dependence of Flt3-ITD–mediated clonogenic growth on TCF activity. Taken together, our results indicate that Flt3-ITD and Wnt-dependent signaling pathways synergize in myeloid transformation.
Introduction
The type III receptor tyrosine kinase (RTK) Flt3 plays an important role in survival and proliferation of hematopoietic progenitor cells and of CD14+ monocytes.1-3 Somatic mutations of Flt3 consisting of internal tandem duplications (ITDs) occur in 30% of patients with acute myeloid leukemia (AML) and are associated with a poor prognosis.4,5 They result in Flt3-ligand (FL)–independent kinase activation of Flt3.6,7 Previously, we and others have shown that Flt3-ITD mediates proliferation and survival, blocks myeloid differentiation, and induces leukemic transformation in hematopoietic progenitor cell lines and primary mouse bone marrow.8-11 Downstream signaling events of Flt3-ITD include the activation of Erk (extracellular signal regulated protein kinase) and Akt (a PI3K [phosphatidylinositol 3-kinase] target) as well as aberrant activation of STAT5 (signal transducer and activator of transcription 5).12-14 In addition to the ITD mutations, activating point mutations, insertions, or deletions in the activation loop of Flt3 have been described that most often involve codons 835/836 and 840/841. The most common of these tyrosine kinase domain (TKD) mutations is the substitution D835Y. TKD mutations occur in approximately 7% of patients with AML.15-18 Together, activating mutations of Flt3 are the most frequent genetic alteration in AML and represent a promising therapeutic target.
The Wingless-type (Wnt) signaling pathway is evolutionary highly conserved and serves important functions in cell fate decisions during embryonal development and in the adult organism.19,20 Recently, the Wnt signaling pathway has also been implicated in self-renewal and proliferation of hematopoietic stem cells.21 Deregulation of its activity is associated with a variety of human cancers, and several molecules downstream of Wnt act as either tumor suppressors or protooncogenes.22 The extracellular Wnt proteins mediate their signaling through binding to Frizzled receptors and the associated low-density lipoprotein (LDL) receptor-related proteins 5 or 6 (LRP-5/6), resulting in the activation of an intracellular cascade that regulates the stability of the transcriptional coactivator β-catenin.23-25 In the absence of Wnt ligand, β-catenin is recruited to a multiprotein complex consisting of adenomatous polyposis coli (APC), Axin, and the serine/threonine kinase glycogen synthase kinase 3β (GSK3β).26,27 In this complex, β-catenin is phosphorylated and marked for ubiquitination and proteasomal degradation. Upon Wnt binding to Frizzled and LRP-5/6, the complex is destabilized by a mechanism that involves the protein Dishevelled.23-25,28-31 Unphosphorylated β-catenin accumulates in the cytoplasm, translocates into the nucleus, and serves as a transcriptional coactivator for T-cell factor–lymphoid enhancer factor (TCF/LEF) transcription factors. Among the TCF/LEF target genes are known oncogenes such as c-myc or cyclin D1 that are thought to mediate the oncogenic function of inappropriate Wnt signal activation.32,33
Recently, we described for the first time a role for the Wnt signaling pathway in the pathogenesis of AML.34 Being interested in the evaluation of cooperative and converging molecular events on the way to AML, we previously performed microarray experiments analyzing Flt3-ITD target genes.35 We noticed that Wnt signaling regulators were modified in their expression by the presence of Flt3-ITD. Here, we analyzed the synergism of Flt3-ITD and the activation of the Wnt signaling pathway in leukemic transformation. Flt3-ITD mutations led to alterations in the expression of several Wnt regulators. They induced the accumulation of β-catenin and TCF-dependent transcriptional activity and highly sensitized the cells for β-catenin induction by Wnt3a. Finally, Flt3-ITD–mediated clonogenic growth highly depended on TCF-mediated transcriptional activity. Our results indicate that Flt3-ITD mediate their leukemogenic effects in part through the activation of the Wnt signaling pathway, possibly defining this signal system as a converging point of leukemogenic events elicited by Flt3 mutations and leukemia-associated fusion proteins.
Materials and methods
Antibodies and reagents
Recombinant human Flt3-ligand (FL) and recombinant murine interleukin-3 (IL-3) were purchased from PeproTech (Rocky Hill, NJ). Anti–β-Catenin mouse monoclonal antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA); anti–phospho-Akt (Ser 473) rabbit polyclonal and anti–total Akt rabbit polyclonal were purchased from Cell Signaling Technology (Beverly, MA). The anti–phospho-STAT5 mouse monoclonal antibody was obtained from Upstate Biotechnologies (Lake Placid, NY), and the anti–mouse Frizzled-4 antibody was from RD Biosystems (Wiesbaden, Germany). Horseradish peroxidase–coupled goat antirabbit and goat antimouse antibodies were purchased from Jackson Immuno Laboratories (West Grove, PA). The anti–mouse actin monoclonal antibody was obtained from Sigma (Taufkirchen, Germany). The Flt3-specific inhibitor was kindly provided by Novartis (Basel, Switzerland).
Patient samples
All samples were collected from patients enrolled in a treatment optimization trial in Germany.36 Written informed consent was obtained from all individuals. The use of the human material for scientific purposes was approved by the human ethics committees of the participating institution.
Cell lines and protein purification
The IL-3–dependent murine myeloid progenitor cell line 32Dcl3 was cultured in RPMI 1640 supplemented with 10% WEHI (Walter and Eliza Hall Institute)–conditioned medium as a source of IL-3. Wnt3a-conditioned medium was prepared from confluent cultures of Wnt3a-producing L cells (stably transfected with Wnt3a cDNA; ATCC, Manassas, VA) or control L cells (ATCC), grown in supplemented Dulbecco modified Eagle medium (DMEM). Culture supernatants were collected after 3 to 4 days. Purified mouse Dickkopf 1 (Dkk-1), containing a histidine (His)– and a Five NH2-terminally deleted epitope-tagged (Flag)–tag sequence was kindly provided by Dr Cati Logan and Dr Calvin Kuo, Stanford, California.37
RNA isolation, generation of cDNA, and real-time RT-PCR
Total RNA was isolated using TRIZOL reagent (Invitrogen, Karlsruhe, Germany) according to the manufacturer's recommendations. In brief, 1 μg total RNA was used for reverse transcription (RT). The cDNA was diluted to 200 μL with ddH20, and 2.5 μL was used for each polymerase chain reaction (PCR) reaction. The quantitation of mRNA levels was carried out using a real-time fluorescence detection method as described before.34,38 Relative gene expression levels were calculated using standard curves generated by the serial dilutions of cDNA from 32D cells. All samples were independently analyzed at least twice for each gene. The housekeeping gene GAPDH served as an additional control for the cDNA quality.
Western blot analyses
For preparation of whole-cell lysates, cells were washed once in ice-cold phosphate-buffered saline (PBS) and lysed for 30 minutes on ice in a buffer containing 150 mM NaCl, 1% NP40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 50 mM Tris (tris(hydroxymethyl)aminomethane) (pH 8.0) with proteinase inhibitors (Complete; Boehringer Mannheim, Mannheim, Germany) and 1 mM sodium orthovanadate. Cell lysates were clarified at 20 000g for 15 minutes. After adjustment of protein concentrations, the lysates were boiled in SDS sample buffer for 5 minutes and separated by SDS–polyacrylamide gel electrophoresis (PAGE). Samples for detection of Frizzled-4 protein were not boiled. Gels were blotted on a polyvinylidene diflouride (PVDF) membrane (Immobilon P; Millipore, Bedford, MA) and stained with the indicated antibody. Antibody binding was detected with a horseradish peroxidase (HRP)–coupled secondary antibody followed by chemoluminescence detection (ECL Plus; Amersham Pharmacia, Uppsala, Sweden).
For densitometry analyses, chemiluminescence was detected using a high-sensitivity CCD camera (INTAS, Göttingen, Germany), and the bands were quantified using the GelPro Analyser software (INTAS) according to the manufacturer's instructions.
3H-thymidine incorporation
A total of 1 × 104 cells per well were starved from IL-3 for 12 hours in a total volume of 200 μL. Subsequently, Wnt3a-conditioned medium or control-conditioned medium were added to a final concentration of 50% to the wells, and cells were exposed to either 20 ng/mL FL or 1 ng/mL IL-3 or cultured without cytokines for 6 hours. Subsequently, 1 μCi (0.037 MBq) 3H-thymidine was added, and cells were cultured for 12 hours under the same conditions. After freezing and thawing, cells were harvested on glass-fiber filters, and the β-emission of the bound DNA was detected in a scintillation counter. Experiments were repeated at least 3 times. Each data point represents the mean and the standard deviation of triplicates from one representative experiment. Statistical significance was calculated by the Student t test.
Clonal growth in methylcellulose
Flt3-ITD– or Flt3-WT (wild-type)–expressing 32Dcl3 cells (1 × 105) were electroporated with 10 μg pcDNA3.1 or pcDNA3.1-dnTCF4 expression vectors. The following day, 103 cells/dish were seeded in triplicate 35 × 10–mm dishes containing Iscoves modified Dulbecco medium (IMDM; Life Technologies, Grand Island, NY), 1% methylcellulose, 20% fetal calf serum (FCS), 0.5 mg/mL G418, and the indicated growth factors or conditioned media. Colonies (> 50 cells) were counted on day 10 to 14. The data shown represent results of 1 of at least 3 independent experiments per construct.
Transfection and luciferase assays
The effects of Flt3-WT or Flt3-ITD on TCF/LEF-dependent transcriptional activity were analyzed in SW387 colon carcinoma cells using the Topflash/Fopflash system containing either optimized TCF binding sites (TOP) or mutated sites (FOP) controlling the expression of a luciferase reporter gene. Cells were cotransfected in 6-well plates with 1.5 μg DNA per construct using the Superfect (Invitrogen) reagent according to the manufacturer's instructions and a Renilla luciferase vector (SV40-pRL) used for normalization purposes. Transfected cells were harvested and analyzed after 24 hours. Luciferase assays were performed with the dual reporter luciferase assay kit (Promega, Mannheim, Germany). For all cotransfection studies, firefly luciferase activity was normalized using Renilla luciferase activity. The normalized values were compared with the control (Flt3-WT FOP = 1).
The c-myc promoter-activation assays were performed in the murine hematopoietic progenitor cell line 32D either carrying Flt3-WT or Flt3-ITD. Cells were electroporated with 10 μg of either a c-myc reporter construct containing 2 TCF binding sites (del-2) or a deletion mutant lacking the 2 binding sites (del-4),39 together with 1 μg Renilla luciferase vector (SV40-pRLNull). Electroporation was performed by a single pulse of 330 V and 960 μF. Cells were cultured overnight in control-conditioned medium without cytokines, with FL at 30 ng/mL or with IL-3 at 1 ng/mL and subsequently harvested and analyzed for luciferase activity. To identify the TCF-dependent c-myc promoter activation, the ratio between the normalized values for del-2/del-4 is shown.
Results
Recently, we identified genes that were differentially expressed in the myeloid progenitor cell line 32Dcl3 upon expression of Flt3-ITD.35 We noted that one of the differentially expressed genes was Frizzled-4, one of the Wnt receptors. Its mRNA expression was significantly up-regulated in Flt3-ITD–bearing cells by the factor 8.5 (Figure 1A). These data prompted us to further analyze the consequences of Flt3-ITD expression for Wnt signaling and the cooperation of these 2 pathways in myeloid transformation.
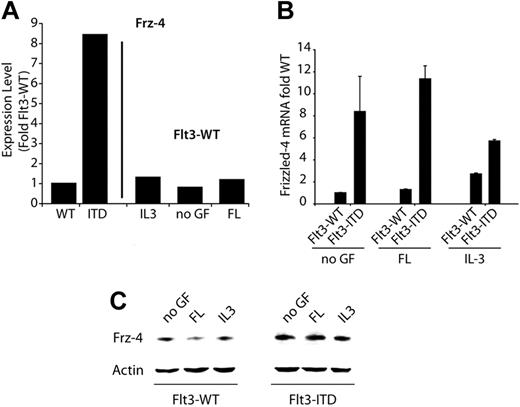
Frizzled-4 is regulated by activating Flt3 mutations. (A) Bar graph representing the relative Frizzled-4 mRNA expression levels as analyzed by microarray analyses.35 Expression levels in Flt3-WT, either stimulated with FL or left untreated, were set as 1. (B) Quantitation of Frizzled-4 mRNA levels by real-time RT-PCR. The 32D cells stably transfected with either wild-type Flt3 (Flt3-WT) or Flt3-ITD were depleted from IL-3 and cultured for 36 hours in FL (Flt3-WT) or without cytokines (Flt3-ITD). Then, the indicated growth factors were added for an additional 12 hours. Samples were taken for RNA preparation, and real-time RT-PCR analyses were performed. The mean of the Flt3-WT level was set as 1. Each bar represents the mean of 2 independent experiments ± SD. (C) Detection of Frizzled-4 protein levels. 32D/Flt3-WT or 32D/Flt3-ITD cells were cultured for 12 hours in the absence of growth factors. Subsequently, the indicated growth factors were added for 6 hours. Frizzled-4 protein levels were determined by Western blot analyses.
Frizzled-4 is regulated by activating Flt3 mutations. (A) Bar graph representing the relative Frizzled-4 mRNA expression levels as analyzed by microarray analyses.35 Expression levels in Flt3-WT, either stimulated with FL or left untreated, were set as 1. (B) Quantitation of Frizzled-4 mRNA levels by real-time RT-PCR. The 32D cells stably transfected with either wild-type Flt3 (Flt3-WT) or Flt3-ITD were depleted from IL-3 and cultured for 36 hours in FL (Flt3-WT) or without cytokines (Flt3-ITD). Then, the indicated growth factors were added for an additional 12 hours. Samples were taken for RNA preparation, and real-time RT-PCR analyses were performed. The mean of the Flt3-WT level was set as 1. Each bar represents the mean of 2 independent experiments ± SD. (C) Detection of Frizzled-4 protein levels. 32D/Flt3-WT or 32D/Flt3-ITD cells were cultured for 12 hours in the absence of growth factors. Subsequently, the indicated growth factors were added for 6 hours. Frizzled-4 protein levels were determined by Western blot analyses.
ITD mutations increase Frizzled-4 expression
First, we analyzed the modulation of Frizzled-4 in more detail, using real-time RT-PCR and Western blot analyses. We determined Frizzled-4 mRNA expression levels in 32D cells stably transfected with Flt3-WT or Flt3-ITD at different conditions and found significantly higher amounts of Frizzled-4 mRNA in 32D/Flt3-ITD cells (Figure 1B). Exposure of the cells to FL did not significantly influence the levels of Frizzled-4, while exposure to IL-3 induced a slight inhibition of Frizzled-4 mRNA in 32D/Flt3-ITD cells. In contrast, IL-3, but not FL, induced the expression of Frizzled-4 mRNA expression up to 3-fold in 32D/Flt3-WT cells. The results were confirmed on the protein level by Western blot analyses, whereby Flt3-ITD led to an induction of Frizzled-4 protein (Figure 1C). Taken together, AML-typical Flt3 mutations induce the expression of Frizzled-4 on the mRNA and protein level, mimicking the effects of IL-3.
Flt3-ITD activates canonical Wnt signal transduction
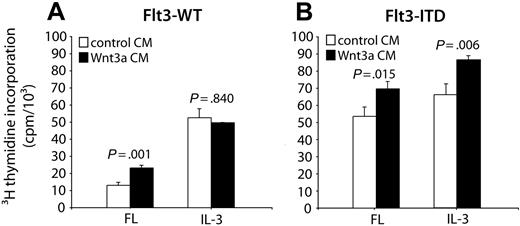
Growth of the murine myeloid progenitor cell line 32Dcl3 depends on the presence of IL-3. Expression of Flt3-WT in these cells allows IL-3 to be substituted by FL, whereas Flt3-ITD enables factor-independent proliferation.14,40 To analyze the effects of the canonical Wnt signal transduction pathway on 32D cell proliferation, we used conditioned medium of a cell line that was transfected with a Wnt3a expression construct. In the absence of exogenously added growth factors, Wnt3a-conditioned medium was not able to support 32D cell proliferation (data not shown). However, when we treated 32D/Flt3-WT and 32D/Flt3-ITD cells with IL-3 or FL in combination with Wnt3a-containing medium and analyzed their DNA synthesis by 3H-thymidine incorporation, we observed that Wnt3a-conditioned medium enhanced 32D cell proliferation (Figure 2A-B). The increase in proliferation was about 20% to 25% and statistically significant. Interestingly, we only observed stimulation of 32D cell proliferation, if the cells were concurrently stimulated by Flt3 signals, either wild type or ITD. In 32D/Flt3-WT cells incubated with IL-3 that grew in the absence of Flt3 activation, Wnt3a-conditioned medium did not have any effect. These experiments provided evidence that Wnt activation synergized with Flt3, but not with IL-3, signal transduction events to enhance 32D cell proliferation.
Wnt3a enhances proliferation of 32D/Flt3 cells. The 32D/Flt3-WT (A) and 32D/Flt3-ITD (B) cells were starved from growth factors for 12 hours and subsequently exposed to a combination of FL or IL-3 and Wnt3a-conditioned medium (CM; ▪) or a control-conditioned medium (□) for 6 hours. DNA synthesis was analyzed in triplicate by 3H-thymidine incorporation over 12 hours. Each bar corresponds to the mean incorporated radioactivity ± SD. The significance of differences between Wnt3a CM and control CM was calculated according to the Student t test.
Wnt3a enhances proliferation of 32D/Flt3 cells. The 32D/Flt3-WT (A) and 32D/Flt3-ITD (B) cells were starved from growth factors for 12 hours and subsequently exposed to a combination of FL or IL-3 and Wnt3a-conditioned medium (CM; ▪) or a control-conditioned medium (□) for 6 hours. DNA synthesis was analyzed in triplicate by 3H-thymidine incorporation over 12 hours. Each bar corresponds to the mean incorporated radioactivity ± SD. The significance of differences between Wnt3a CM and control CM was calculated according to the Student t test.
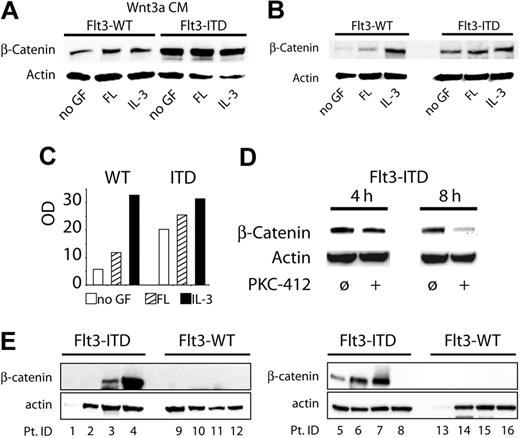
Next, we analyzed the influence of Flt3 activation on β-catenin stability as the central signal transducer of the canonical Wnt pathway. We incubated 32D/Flt3-WT and 32D/Flt3-ITD cells with control-conditioned or Wnt3a-conditioned medium in combination with FL or IL-3 and assessed cellular β-catenin protein levels by Western blot analyses. Interestingly, we found a consistent increase in basal β-catenin protein levels in 32D/Flt3-ITD cells in comparison to unstimulated 32D/Flt3-WT cells (Figure 3B). Stimulation of 32D/Flt3-WT cells with FL induced a slight β-catenin induction. Incubation with IL-3 resulted in a β-catenin induction that was comparable to that in Flt3-ITD cells.
Flt3 activates the canonical Wnt signaling pathway. (A-B) 32D cells stably expressing the indicated Flt3 constructs were starved from growth factors (GF) for 12 hours, exposed to the indicated cytokines for 6 hours with control-conditioned medium or Wnt3a-conditioned medium, and lysed, and the indicated protein was analyzed by Western blot analyses. (A) Expression of β-catenin in the presence of Wnt3a. (B) Expression of β-catenin in the absence of Wnt3a. (C) Densitometry of β-catenin. The bar diagram indicates the intensity of the β-catenin bands shown in Figure 3B. □ indicates no GF; ▨, FL; ▪, IL-3. For densitometry analyses we used an INTAS camera (Epichem3 Darkroom) and the GelPro Analyser (1D-Gel ToolBar) software. (D) 32D/Flt3-ITD cells (in the absence of exogenous growth factors) were exposed to 300 nM PKC412 for the indicated periods. The cells were lysed and subjected to Western blot analyses for β-catenin. (E) Expression of β-catenin in patients with ITD-negative and ITD-positive AML. Pt. ID indicates patient identification number.
Flt3 activates the canonical Wnt signaling pathway. (A-B) 32D cells stably expressing the indicated Flt3 constructs were starved from growth factors (GF) for 12 hours, exposed to the indicated cytokines for 6 hours with control-conditioned medium or Wnt3a-conditioned medium, and lysed, and the indicated protein was analyzed by Western blot analyses. (A) Expression of β-catenin in the presence of Wnt3a. (B) Expression of β-catenin in the absence of Wnt3a. (C) Densitometry of β-catenin. The bar diagram indicates the intensity of the β-catenin bands shown in Figure 3B. □ indicates no GF; ▨, FL; ▪, IL-3. For densitometry analyses we used an INTAS camera (Epichem3 Darkroom) and the GelPro Analyser (1D-Gel ToolBar) software. (D) 32D/Flt3-ITD cells (in the absence of exogenous growth factors) were exposed to 300 nM PKC412 for the indicated periods. The cells were lysed and subjected to Western blot analyses for β-catenin. (E) Expression of β-catenin in patients with ITD-negative and ITD-positive AML. Pt. ID indicates patient identification number.
When the cells were incubated with Wnt3a-conditioned medium, basal levels of β-catenin were increased under all conditions (Figure 3A). Interestingly, the increase of β-catenin protein levels in Flt3-ITD cells was much more prominent than in Flt3-WT cells. The observed pattern of the Wnt3a-induced increase of β-catenin levels nicely reflected the effects of Wnt3a on cellular proliferation as shown in Figure 2A-B: Wnt3a could not further increase the levels of β-catenin in 32D/Flt3-WT cells under the influence of IL-3 and, thus, did not have any effect on cellular proliferation under these conditions.
To rule out nonspecific induction of β-catenin protein levels after clonal selection in 32D/Flt3-ITD cells, we analyzed 3 independent bulk cultures of these cells for their β-catenin content, with similar results. We also analyzed whether inhibition of Flt3-ITD activity by PKC412, a known inhibitor of Flt3 activity, could inhibit Flt3-ITD–induced β-catenin levels (Figure 3D). We incubated Flt3-ITD–positive cells (in the absence of exogenous growth factors) with 300 nM PKC412. After 8 hours, we observed a significant decrease of β-catenin protein levels. We then analyzed the β-catenin expression levels in primary AML blasts from 16 patients, 8 with Flt3-ITD and 8 with Flt3-WT status. In the Flt3-ITD–positive samples, Western blot analyses revealed detectable protein levels for β-catenin in 5 of 7 evaluable cases (Figure 3E). In contrast, none of the 7 evaluable Flt3-WT samples contained β-catenin levels that were detectable in Western blots. Two of the 16 samples (1 of each group) were not evaluable because of low protein content as indicated by low actin expression.
Taken together, our results demonstrate that Flt3-ITD induced a basal activation of the canonical Wnt signaling cascade. Furthermore, these mutations also sensitized myeloid progenitor cells for Wnt3a-mediated stabilization of β-catenin. Activation of Wnt signaling by exogenous addition of Wnt3a in 32D cells caused a small, but consistent, increase in cellular proliferation. Primary blasts with Flt3-ITD mutations express high levels of β-catenin.
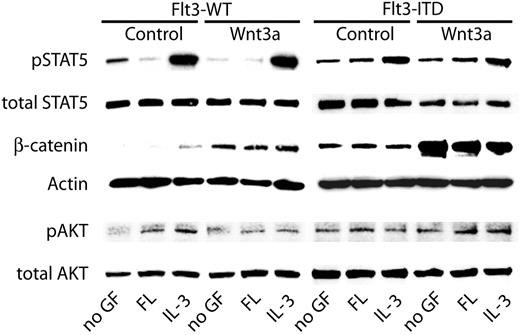
Wnt3a does not influence known Flt3-ITD–mediated signal transduction events
Flt3-ITD–induced signaling pathways have been studied extensively. Strong and constitutive phosphorylation of STAT5 has been observed in Flt3-ITD–transfected 32D cells as well as in primary AML blasts.13,14,41 In contrast, ligand-activated Flt3-WT induced only weak STAT5 phosphorylation without activation of its transcriptional activity.14 Furthermore, Flt3-ITD has been shown to induce constitutive Akt and Erk activation. To investigate the influence of the canonical Wnt signaling cascade on Flt3-ITD–dependent signal transduction, we analyzed the phosphorylation of STAT5 and Akt in 32D/Flt3-WT and 32D/Flt3-ITD cells under the influence of FL or IL-3 in combination with Wnt3a with activation-specific antibodies (Figure 4). As reported previously, expression of Flt3-WT or activation of this receptor with FL did not induce significant STAT5 phosphorylation, which was highly expressed in these cells and functional as indicated by its strong phosphorylation upon IL-3 stimulation (lanes 1-3). In contrast, expression of Flt3-ITD caused constitutive phosphorylation of STAT5 (lanes 7-9). Incubation of the cells with Wnt3a-conditioned medium did not change the STAT5 activation pattern. Similarly, Akt activation that we observed in response to ligand-activated Flt3-WT as well as to Flt3-ITD was not influenced by Wnt3a-conditioned medium. The pattern of β-catenin induction in this experiment was similar to the one described in “Flt3-ITD activates canonical Wnt signal transduction,” showing that the Wnt3a-conditioned medium we used was functional. Thus, Wnt3a does not seem to have a significant influence on known Flt3-ITD–mediated signaling events.
Influence of Flt3-ITD on Wnt signaling pathways. 32D cells stably transfected with Flt3-WT or with Flt3-ITD were grown for 12 hours in the absence of IL-3. Subsequently, cells were stimulated with FL, IL-3, or left without cytokine for 6 hours. Additionally, either 50% of Wnt3a-conditioned medium or control-conditioned medium was added. Lysates were subjected to Western blot analyses with the indicated primary antibodies.
Influence of Flt3-ITD on Wnt signaling pathways. 32D cells stably transfected with Flt3-WT or with Flt3-ITD were grown for 12 hours in the absence of IL-3. Subsequently, cells were stimulated with FL, IL-3, or left without cytokine for 6 hours. Additionally, either 50% of Wnt3a-conditioned medium or control-conditioned medium was added. Lysates were subjected to Western blot analyses with the indicated primary antibodies.
Flt3-ITD mutations activate TCF-containing promoters
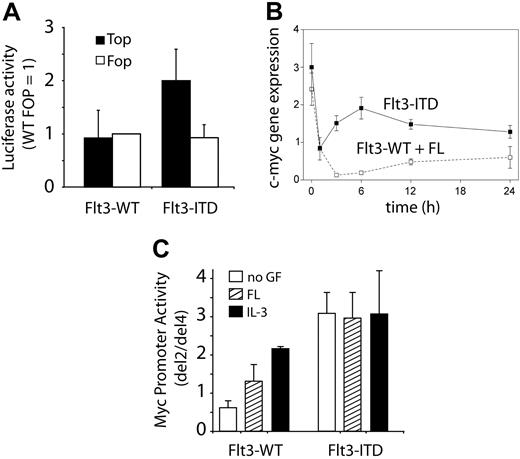
Stabilization of β-catenin leads to enhanced complex formation of the protein with members of the TCF/LEF transcription factor family in the nucleus, increasing their transcriptional activity on regulatory elements of known Wnt target genes such as cyclin D1 or c-myc. To analyze the changes in transcriptional activity induced by Flt3-ITD, we performed promoter activation studies using the Topflash/Fopflash system, containing either optimized TCF binding sites (TOP) or mutated sites (FOP). Figure 5A indicates that SW387 cells cotransfected with Flt3-ITD and the TOP promoter construct show a significant increase in luciferase activity. These effects were not observed with Flt3-WT or the FOP promoter as a negative control.
Activation of TCF-dependent transcriptional activity by Flt3-ITD. (A) Activation of TCF-dependent promoters. SW387 cells were transiently transfected with wild-type (Top; ▪) or mutant (Fop; □) TCF-response reporter constructs. Cells were either cotransfected with Flt3-ITD or Flt3-WT. TCF reporter–specific luciferase expression was detected with a dual luciferase assay as described in detail in “Materials and methods.” The results of 2 independent experiments (mean and SD) are shown. (B) Induction of c-myc mRNA by Flt3-ITD mutations. 32D/Flt3-WT (□) and 32D/Flt3-ITD cells (▪) were grown in IL-3–containing medium. At time point 0, cells were washed twice and resuspended in serum-free medium with FL. At the indicated time points, samples were taken for RNA preparation and subsequent analyses of c-myc mRNA expression. The graph represents the relative c-myc mRNA expression (mean of independent experiments ± SD). (C) The effects of Flt3-ITD on the c-myc promoter were analyzed in 32D/Flt3-WT and 32D/Flt3-ITD cells. The ratio of normalized luciferase activity between del2 and del4 is shown. The TCF-binding elements contained in del2 are deleted in del4. □ indicates no GF; ▨, FL; and ▪, IL-3. The results of 2 independent experiments (mean and SD) are shown.
Activation of TCF-dependent transcriptional activity by Flt3-ITD. (A) Activation of TCF-dependent promoters. SW387 cells were transiently transfected with wild-type (Top; ▪) or mutant (Fop; □) TCF-response reporter constructs. Cells were either cotransfected with Flt3-ITD or Flt3-WT. TCF reporter–specific luciferase expression was detected with a dual luciferase assay as described in detail in “Materials and methods.” The results of 2 independent experiments (mean and SD) are shown. (B) Induction of c-myc mRNA by Flt3-ITD mutations. 32D/Flt3-WT (□) and 32D/Flt3-ITD cells (▪) were grown in IL-3–containing medium. At time point 0, cells were washed twice and resuspended in serum-free medium with FL. At the indicated time points, samples were taken for RNA preparation and subsequent analyses of c-myc mRNA expression. The graph represents the relative c-myc mRNA expression (mean of independent experiments ± SD). (C) The effects of Flt3-ITD on the c-myc promoter were analyzed in 32D/Flt3-WT and 32D/Flt3-ITD cells. The ratio of normalized luciferase activity between del2 and del4 is shown. The TCF-binding elements contained in del2 are deleted in del4. □ indicates no GF; ▨, FL; and ▪, IL-3. The results of 2 independent experiments (mean and SD) are shown.
C-myc is a well-characterized Wnt target gene. Its regulation by β-catenin–dependent TCF transcription factors has been attributed to 2 TCF-binding elements (TBEs) in the c-myc promoter sequence.39 To gain functionally significant information on the TCF-dependent Flt3-ITD effects on this oncogene, we first analyzed the time course of c-myc mRNA expression by real-time RT-PCR following growth factor withdrawal (time point 0) from 32D/Flt3-WT and 32D/Flt3-ITD cells (Figure 5B). While c-myc expression was already higher in 32D/Flt3-ITD cells, it also surpassed the expression in FL-exposed Flt3-WT cells after IL-3 withdrawal. Next, we performed c-myc promoter activation studies in the hematopoietic progenitor cell lines 32D/Flt3-WT and 32D/Flt3-ITD. In these cells, we compared the activity of a c-myc promoter construct that contained TCF-binding elements (del2) with the activity of a construct, where the TBEs were deleted (del4). This system reliably identifies TCF-dependent c-myc promoter activation.39 As shown in Figure 5C, the TCF-dependent c-myc promoter activity is low in 32D/Flt3-WT with or without FL and increases after IL-3 exposure. In 32D/Flt3-ITD cells, the TCF-dependent c-myc promoter activity is very high under all conditions. Taken together, these data indicate that the increased β-catenin levels observed in Flt3-ITD–positive cell lines correlate with increased TCF-dependent transcriptional activity followed by increased expression of the important transforming TCF target gene c-myc.
Dominant-negative TCF4 inhibits leukemic cell growth of Flt3-ITD mutations
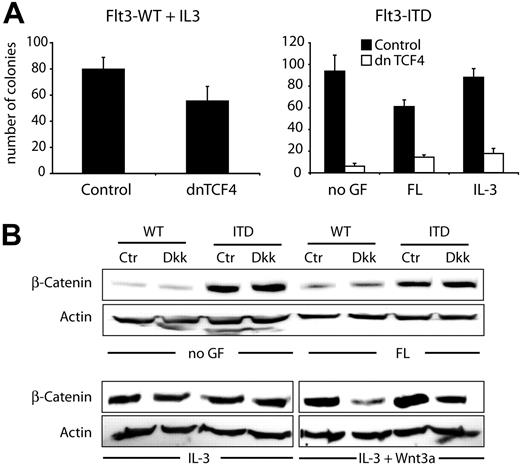
The findings described so far provided evidence that Flt3-ITD induced TCF/LEF-dependent transcriptional activity and sensitized cells for Wnt3a stimulation. We wondered whether this activation was important for Flt3-ITD–mediated proliferation and for its transforming activity. Therefore, we studied the consequences of TCF inhibition on clonal growth of 32D/Flt3-ITD and 32D/Flt3-WT cells. The cells were either electroporated with an expression vector containing a Neo selection cassette and dominant-negative (dn) TCF or with a control vector. Following transfection, we analyzed clonogenic growth in the presence of G418 with and without FL or IL-3 (Figure 6A). Coexpression of dnTCF4 with Flt3-ITD inhibited colony growth more than 80% regardless of the growth conditions, indicating that, in the presence of Flt3-ITD, 32D colony growth is highly dependent on TCF/LEF transcriptional activity. These data indicate that Flt3-ITD–dependent activation of TCF transcriptional activity is functionally relevant for the transforming activity of these receptor mutations.
Dominant-negative TCF4 inhibits leukemic cell growth of Flt3-ITD mutations. (A) Stable 32D/Flt3-ITD or 32D/Flt3-WT cells were either transfected with a dominant-negative TCF4 expression construct (dnTCF4; □) or with control vector (▪) as indicated. Cells were seeded in triplicates in colony assays in the presence of neomycin selection. Colony growth was evaluated on day 10. The bar graph shows the result of 1 of 3 independent experiments (mean and SD). (B) 32D cells stably transfected with Flt3-WT or with Flt3-ITD were grown for 12 hours in the absence of IL-3. Subsequently, cells were stimulated with FL or IL-3 or left without cytokine in the presence or absence of Dkk-1 protein for 6 hours. Either 50% of Wnt3a-conditioned medium or control-conditioned medium was added. Western blot analyses were performed with lysates from these cells using β-catenin or actin antibodies as indicated.
Dominant-negative TCF4 inhibits leukemic cell growth of Flt3-ITD mutations. (A) Stable 32D/Flt3-ITD or 32D/Flt3-WT cells were either transfected with a dominant-negative TCF4 expression construct (dnTCF4; □) or with control vector (▪) as indicated. Cells were seeded in triplicates in colony assays in the presence of neomycin selection. Colony growth was evaluated on day 10. The bar graph shows the result of 1 of 3 independent experiments (mean and SD). (B) 32D cells stably transfected with Flt3-WT or with Flt3-ITD were grown for 12 hours in the absence of IL-3. Subsequently, cells were stimulated with FL or IL-3 or left without cytokine in the presence or absence of Dkk-1 protein for 6 hours. Either 50% of Wnt3a-conditioned medium or control-conditioned medium was added. Western blot analyses were performed with lysates from these cells using β-catenin or actin antibodies as indicated.
Flt3-ITD–induced canonical Wnt signaling cannot be inhibited by Dkk-1
Flt3-ITD induced increased β-catenin levels and TCF/LEF-dependent transcriptional activity in the absence of exogenously added Wnt ligands. This could be due to secondary events such as autocrine production of Wnt proteins or other extracellular mediators of Wnt activation. To analyze this, we used purified Dkk-1, a potent inhibitor of Wnt ligands.42,43 Dkk-1 treatment of 32D/Flt3-ITD or 32D/Flt3-WT in the absence of Wnt3a-conditioned medium did not alter the stabilization of β-catenin (Figure 6B). However, in the presence of Wnt3a-conditioned medium, the addition of Dkk-1 to the medium resulted in a reduction of β-catenin protein levels in both cell lines. We conclude that Flt3-ITD provides a signal that is independent from extracellular Wnt ligand sources to increase β-catenin protein levels.
Discussion
Activating mutations in the tyrosine kinase receptor Flt3 have been detected in about 30% of patients with AML.44 The mechanisms of leukemic transformation by Flt3 mutations have not yet been completely identified. In this study, we analyzed the effects of Flt3-ITD activation on the Wnt signaling cascade, an evolutionary highly conserved signaling pathway that has recently been described to be targeted by AML fusion proteins and that serves important functions in hematopoietic stem cell self-renewal.21,34,45 Flt3-ITD induced the expression of Frizzled-4, a known Wnt receptor. The expression of Flt3-ITD induced increased baseline and Wnt3a-dependent β-catenin protein levels in a cell line model as well as the presence of Flt3-ITD in primary AML blasts induced higher β-catenin levels. The observed increases in β-catenin protein levels were functional, since they were associated with increased TCF/LEF transcriptional activity and TCF-dependent induction of c-myc, an important oncogene. Finally, we demonstrate that important biologic consequences of Flt3-ITD are mediated by TCF/LEF-dependent transcriptional activity.
The current study was stimulated by microarray experiments that we recently published, demonstrating that transcriptional up-regulation of plakoglobin expression is an important event in fusion protein–associated AML pathogenesis.34 Plakoglobin (γ-Catenin) is a close relative to β-catenin with redundant functions as a transcriptional coactivator for TCF/LEF transcription factors.
Previously, we published microarray data on differential gene expression induced by Flt3-WT versus Flt3-ITD. These data indicated that Frizzled-4, but none of the other 2 Frizzled receptors represented on the microarray (Frz-3 and Frz-6, data not shown), was significantly induced by Flt3-ITD.
Our data rule out that Flt3-ITD expression leads to functionally relevant secretion of Wnt ligands, since Dkk-1 did not influence the observed changes in β-catenin expression. Dkk-1 is a strong Wnt antagonist. The binding of Dkk-1 to LRP-5/6 and to the associated protein Kremen dissociates LRP-5/6 from Frizzled and, thus, prevents the formation of a functional Wnt receptor complex.42,43,46,47 The data shown in Figure 6B demonstrate that this interference had no influence on β-catenin protein levels in the absence of exogenous Wnt3a, making it unlikely that autocrine production of Wnt3a plays a major role in the observed activation.
For the same reasons, it is also unlikely that the observed changes in Frizzled-4 expression participate in the constitutive β-catenin stabilization induced by Flt3-ITD. Also, we did not find increased Frizzled-4 mRNA expression levels in Flt3-ITD–positive AML in comparison to Flt3-ITD–negative AML, although the receptor was significantly expressed in most AML samples (data not shown). Thus, the constitutive increase of β-catenin expression seems rather to be caused by a mechanism downstream of Wnt-Frizzled interactions that induces stabilization of β-catenin protein (Figure 7). In contrast to the constitutive β-catenin overexpression, the observed sensitization to exogenous Wnt3a might be attributed to increased Frizzled-4 expression, since it is to some extent Dkk-1 sensitive (Figure 6B, last 2 lanes). Further analyses on β-catenin stability and on the Flt3-ITD–induced posttranslational modifications of β-catenin may shed light on this open question.
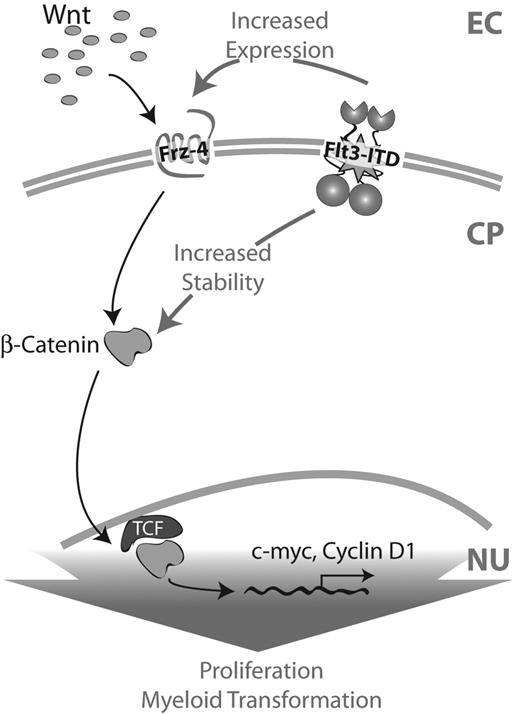
Schematic representation of cooperation between Wnt signaling and ITD mutations. In the presence of Wnt ligands the intracellular cascade of canonical Wnt signaling is activated via Frizzled receptors. This results in the stabilization of β-catenin protein in the cytoplasm and, thus, a higher translocation of β-catenin into the nucleus. There it forms a complex with TCF transcription factor and activates the transcription of Wnt target genes such as cyclin D1 or c-myc. In the presence of ITD mutations, β-catenin protein is stabilized independently of Wnt ligand–induced signaling. Additionally, the receptor Frizzled-4 is up-regulated. As a consequence, the canonical Wnt signaling cascade is also activated, resulting in a higher activation of β-catenin/TCF-dependent promoters and, thus, higher expression levels of Wnt target genes. EC indicates extracellular; CP, cytoplasm; and NU, nucleus.
Schematic representation of cooperation between Wnt signaling and ITD mutations. In the presence of Wnt ligands the intracellular cascade of canonical Wnt signaling is activated via Frizzled receptors. This results in the stabilization of β-catenin protein in the cytoplasm and, thus, a higher translocation of β-catenin into the nucleus. There it forms a complex with TCF transcription factor and activates the transcription of Wnt target genes such as cyclin D1 or c-myc. In the presence of ITD mutations, β-catenin protein is stabilized independently of Wnt ligand–induced signaling. Additionally, the receptor Frizzled-4 is up-regulated. As a consequence, the canonical Wnt signaling cascade is also activated, resulting in a higher activation of β-catenin/TCF-dependent promoters and, thus, higher expression levels of Wnt target genes. EC indicates extracellular; CP, cytoplasm; and NU, nucleus.
While the molecular mechanisms of the Flt3-ITD–mediated Wnt signal activation are not entirely clear, we demonstrate in this study for the first time that Flt3-ITD leads to increased β-catenin protein levels in cell lines. This increase is highly consistent, and Flt3-ITD was associated with high β-catenin levels in primary AML blasts. Thus, β-catenin induction could be a transforming event in AML that is specifically induced by Flt3-ITD consequence.
Cytosolic β-catenin levels have been established as the central protein of the so-called canonical Wnt signaling cascade that serves important functions in embryonic development as well as in the pathogenesis of several epithelial cancers.19,20,22 Since β-catenin fulfills several additional cellular functions that are not directly connected to the canonical Wnt signaling pathway, it is important that we could demonstrate the functional consequences of increased β-catenin levels: the activation of TCF/LEF transcription factors.32,48
The effects of Flt3-ITD on Wnt signaling in 32D cells were partially recapitulated by incubation of this cell line with its appropriate growth factor IL-3, but remarkably not by the signal from wild-type Flt3 receptors. This observation nicely reflects our previously published results that the ITD mutations not only induce constitutive activation of Flt3, but also change the signal quality.35 In mouse stem cells, Flt3 has been associated with early steps of differentiation rather than self-renewal.49 Since Wnt-dependent signaling events play an important role in stem cell self-renewal, the differences in Wnt-activating capacity between Flt3-ITD and Flt3-WT could point to a role of the ITD mutations in maintaining the leukemic stem cell pool. Further analyses of the effects of Flt3-ITD in the stem cell compartment will be necessary to shed light on this question.
What are the possible consequences of Flt3-ITD–mediated β-catenin up-regulation in hematopoietic progenitor cells? First, our results demonstrate that important proliferative responses to the expression of Flt3-ITD (and to the exposure to IL-3) are mediated by TCF/LEF-dependent signaling. The results indicating only a small increase in cellular proliferation after exposure of the cells to Wnt3a are in line with the observation of a strong inhibition of clonogenic growth by dominant-negative TCF-4. Since basal levels of β-catenin are strongly induced by Flt3-ITD and the cells already depend on β-catenin transcriptional activity for their proliferation, additional stimulation with Wnt3a will have a strong proliferative effect. Results generated with 32D cells that were starved from IL-3 and stimulated with Wnt3a indicated that Wnt signal transduction is not sufficient for 32D cell proliferation (data not shown). However, the strong inhibition of 32D cell colony growth by dominant-negative TCF4 clearly shows the high degree of dependence of this response on Wnt activation.
Receptor tyrosine kinases induce cell-cycle progression and cellular proliferation through acceleration of G1/S transition.50 In a recent study, we analyzed the expression of these regulators in AML blasts and found an increase of c-myc in AML in comparison to normal bone marrow.34 A proportion of cases with high c-myc expression and low expression of p14ARF, a mediator of c-myc–induced apoptosis, displayed a particularly bad prognosis (H.S., C.M.-T., unpublished data, July 2004). Here, we demonstrate that Flt3-ITD mutations activate the c-myc promoter only in the presence of TCF-binding sites. This is followed by increased c-myc expression in Flt3-ITD–positive cell lines. Since c-myc is one of the most relevant Wnt target genes in other human cancers and a very important regulator of cell-cycle progression and apoptosis induction, its TCF/LEF-dependent transcription could be one possible explanation for the strong dependence of 32D cell proliferation on TCF activity.
Studies from embryonic development and epithelial tissues have demonstrated that Wnt signaling is of fundamental importance for cell fate decisions coupling cellular differentiation and proliferation.19 It has been shown that the Wnt signaling cascade and β-catenin play an important role in hematopoietic cell decisions governing the regulation of early stem cell self-renewal and differentiation.21,51,52 Similar to normal hematopoiesis, AML is organized in a hierarchy of progenitor cells that fail to differentiate and, thus, get arrested in the mitotic pool.53 Previously, we showed for the first time that activation of the Wnt signaling pathway is involved in fusion protein–mediated AML pathogenesis.34 These data provided here give evidence for the first time that the Wnt signaling cascade also participates in the transforming events emanating from the most prominent member of the other mutation class found in AML, Flt3-ITD. Thus, this pathway may well be a common signal integration system with essential importance in maintaining hematopoietic homeostasis that is disrupted by both mutation classes in AML. In the future, it will be important to delineate the role of Wnt signaling events in primary cell leukemia models and to further evaluate its potential role as drug target.
Prepublished online as Blood First Edition Paper, January 13, 2005; DOI 10.1182/blood-2004-07-2924.
Supported by grants from the Deutsche Forschungsgemeinschaft (SFB293, Se 600/3-1), the José-Carreras Leukemia Foundation (R03/19f), the Interdisciplinary Centre of Clinical Research Münster (IZKF Project No. Ser2/041/04; Mül 2/096/04), and the Innovative Medizinische Forschung (IMF Project No. Ti 120 335) at the University of Münster, and the Heisenberg grant from the Deutsche Forschungsgemeinschaft (MU 1328/3-1).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Marion Baas for excellent technical assistance. We also thank Anand Reddi for producing a stably transfected Dkk-1–expressing cell line and Dr Cati Logan and Dr Calvin Kuo for providing us with purified Dkk-1.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal