Abstract
Hodgkin lymphoma represents unique clinicopathologic features because Hodgkin and Reed-Sternberg (H-RS) cells produce a variety of cytokines, express a variety of cytokine receptors, and are surrounded by numerous nonmalignant immunoreactive cells. We found that receptors for interleukin-4 (IL-4R) are highly expressed in H-RS cells. To target interleukin-4 receptor (IL-4R), we used a recombinant protein fusing circularly permuted human IL-4 and Pseudomonas exotoxin termed IL438-37-PE38KDEL, or IL-4 cytotoxin. The cytotoxic effect of IL-4 cytotoxin on H-RS cell lines was determined to be moderate to high in vitro. We developed an infiltrating model of Hodgkin disease (HD) by injecting an adherent population of HD-MyZ cells subcutaneously into the flanks of beige/nude/X-linked immunodeficient mice. The animal model exhibited spontaneous metastasis of H-RS cells to lymph nodes and dissemination to vital organs, including the lungs. Intraperitoneal or intratumoral treatment of these mice with IL-4 cytotoxin resulted in regression of the primary tumor mass and a decrease in the incidence of lymph node metastasis. Mice injected with HD-MyZ cells demonstrated 203% prolonged survival (mean survival, 63 days) compared with control (mean survival, 31 days) when they received systemic IL-4 cytotoxin treatment. Because numerous H-RS cell lines express receptors for IL-4, IL-4 cytotoxin may be a unique agent for the treatment of Hodgkin lymphoma.
Introduction
Hodgkin lymphoma is caused by Hodgkin and Reed-Sternberg (H-RS) cells, and it has unique biologic features among hematologic malignancies. H-RS cells express a variety of cytokine/cytokine receptors, including interleukin-3 (IL-3), IL-5, IL-6, IL-7, IL-9, IL-10, granulocyte macrophage–colony-stimulating factor (GM-CSF), and transforming growth factor-β (TGF-β).1-5 In recent years, it has been reported that H-RS cells express receptors for IL-13.6,7 H-RS cells also induce immunoreactive cell infiltration, which produces a variety of cytokines and chemokines.8-10 Genetic studies have demonstrated that H-RS cells are derived from a compartment of germinal center B cells.11 However, in spite of recent intensive studies, it is still unclear how this rare neoplastic cell originates, persists, and disseminates in a host.
Despite the poor understanding of the pathogenesis of Hodgkin lymphoma, recent studies have focused on the development of novel therapeutic agents for Hodgkin lymphoma. Among them, various immunotoxins and monoclonal antibody approaches are prominent.12-15 Because both classes of therapeutic agents target unique or overexpressed antigens on tumor cells, emphasis has been placed on identifying these targets. Previous studies have identified overexpression of CD25 and CD30 lymphocyte-activation markers on H-RS cells, which are seldom expressed on most normal lymphoid cells.13-16 To target these antigens, Barth et al13 constructed anti-CD25 and anti-CD30 immunotoxins that showed promising results in various preclinical studies.13-15 Based on these data, clinical studies are ongoing to evaluate their safety, tolerability, and efficacy in patients with Hodgkin disease (HD).17,18
We have identified a tumor-associated target protein in the form of IL-4 receptor (IL-4R) on a variety of tumor cell lines.19-22 The structure of IL-4R has been extensively studied.23 IL-4Rs are known to exist in 3 different forms in different cell lines.23 Type 1 IL-4R consists of IL-4Rα and IL-2Rγ chains, which are predominantly expressed in immune cells. Type 2 IL-4R consists of IL-4Rα and IL-13Rα1 chains, and this type of IL-4R is predominantly expressed in solid tumor cells.24 In type 3 IL-4R, all 3 chains are present; however, the IL-4Rα chain may couple with the IL-2Rγ or IL-13Rα1 chain to induce a biologic response.24 It is unknown whether H-RS cells express IL-4R, nor is the structure of these receptors known.
To target IL-4 receptors, an IL-4 cytotoxin, IL438-37-PE38KDEL—a recombinant fusion protein consisting of human IL-4 and truncated Pseudomonas exotoxin—was expressed and purified.27 This agent was found to have potent antitumor activity in solid tumors expressing IL-4R in vitro and in vivo.19-22 The effectiveness of IL-4 cytotoxin in hematologic malignancies has not been explored extensively. It has been demonstrated that H-RS cells express IL-13R and that IL-13 acts as an autocrine growth factor for these cells.6,7 In addition, we and others have shown that IL-4 receptors share 2 chains with IL-13R (IL-4Rα and IL-13Rα1 chains).24-26 Based on these findings, we were interested in investigating whether IL-4 cytotoxin can mediate antitumor activity to H-RS cells in vitro and in vivo.
Various investigators have attempted to establish a suitable animal model of HD to study the therapeutic effect of novel agents. von Kalle et al28 demonstrated that unmanipulated HD-derived cell lines and primary biopsy tissue do not grow in athymic nude mice. However, HD-derived cell lines formed tumors in severe combined immunodeficient (SCID) mice.28 One of the H-RS cell lines, designated HD-MyZ, was established from the pleural effusion of a 29-year-old patient with nodular sclerosing HD.4 Interestingly, HD-MyZ xenotransplantation into SCID mice by intravenous or subcutaneous inoculation led to the development of disseminated tumors with infiltrative and destructive growth.4 This animal model may be of value for elucidating the pathogenesis and biologic function of HD and for testing new therapeutic agents.
In this study, we assessed the expression of IL-4R on H-RS cells and tested the cytotoxic activity of IL-4 cytotoxin on 6 H-RS cell lines in vitro. An infiltrating animal model of Hodgkin lymphoma was developed using HD-MyZ cells in beige/nude/X-linked BR immunodeficient mice. The antitumor effect of IL-4 cytotoxin on subcutaneous HD-MyZ tumor growth and lymph node metastasis was evaluated using this animal model. The effect of IL-4 cytotoxin on the survival of animals with disseminated HD was also evaluated.
Materials and methods
Recombinant proteins
IL-4 cytotoxin (IL438-37-PE38KDEL), containing circularly permuted IL-4 in which amino acids 38 to 129 were linked to amino acids 1 to 37 through a GGNGG linker and then were fused to truncated toxin PE38KDEL, consisting of amino acids 253 to 364 and 381 to 618 of Pseudomonas exotoxin followed by KDEL (an endoplasmic retaining sequence), was expressed in Escherichia coli and purified by a modified procedure as described previously.27 Purified IL438-37-PE38KDEL was provided by Neurocrine Biosciences (San Diego, CA).
Cell lines
Human Hodgkin/Reed-Sternberg cell lines (L1236, HD-MyZ, L428, L540, L591, and HD-LM2) were kindly provided by Dr Daniel Re of Universität Klinik Köln, Germany.29 Cells were cultured in RPMI 1640 medium containing 10% fetal bovine serum, 1 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1 mM l-glutamine, 100 μg/mL penicillin, and 100 μg/mL streptomycin (Biosource International, Camarillo, CA).
Radioreceptor binding assay
Recombinant human IL-4 was labeled with sodium iodide I 125 (125I) (Amersham-Pharmacia, Freiburg, Germany) using IODO-GEN reagent (Pierce, Rockford, IL), as previously described.30 The specific activity of the radiolabeled IL-4 was estimated to be 16.7 μCi (0.618 MBq)/μg protein. For binding experiments, 5 × 105 cells in 100 μL binding buffer (RPMI 1640 containing 0.2% human serum albumin and 10 mM HEPES) were incubated with 200 pM 125I–IL-4 with 0 to 10 nM unlabeled IL-4 at 4°C for 2 hours. Cell-bound 125I–IL-4 was separated from unbound 125I–IL-4 by centrifugation through a phthalate oil gradient, and radioactivity was determined with a gamma counter (Wallac, Gaithersburg, MD). Binding affinity and number of IL-4 receptors were calculated with the LIGAND program, as described previously.31
RT-PCR analysis
To detect the mRNA expression of IL-4R chains in H-RS cells, total RNA was isolated using Trizol reagent (Life Technologies, Grand Island, NY), and reverse transcription–polymerase chain reaction (RT-PCR) analysis was performed using specific primers.29
Flow cytometry
For the detection of cell surface IL-4Rα expression on H-RS cells, cells were fixed by paraformaldehyde and then stained with anti-CD124/IL-4R PE antibody (BD Biosciences, San Diego, CA) or immunoglobulin G1 (IgG1) isotype control. Expression level of IL-4Rα chain was analyzed by FACScan (Becton Dickinson, San Jose, CA).
Immunoblotting
To assess the expression of IL-13Rα1 and IL-2Rγ chains in H-RS cell lines, cells (1 × 106) were lysed in ice-cold lysis buffer containing 1% Triton X-100, 50 mM Tris-HCl pH 7.5, 150 mM NaCl, 1 μg/mL leupeptin, 1 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM Na3VO4. Cell lysates were electrophoresed through 4% to 20% gradient sodium dodecyl sulfate polyacrylamide gels. Proteins were transferred onto polyvinylidene difluoride (PVDF) membranes and were immunoblotted with either anti–IL-13Rα1 monoclonal antibody (R&D Systems, Minneapolis, MN) or anti–IL-2Rγ monoclonal antibody (BD Biosciences).
For an analysis of signal transducer and activator of transcription 6 (STAT6) phosphorylation in H-RS cells, cells (1 × 106) were incubated with 50 ng/mL IL-4 for 15 minutes at 37°C. The blotted membrane was blocked with phosphate-buffered saline (PBS) containing 5% skim milk at room temperature for 1 hour, and the membrane was reacted with antiphosphorylated STAT6 polyclonal antibody (Cell Signaling Technology, Beverly, MA) overnight. Immunoreactive signal was visualized by using enhanced chemiluminescence (ECL). After stripping, the membrane was incubated with anti-STAT6 antibody (s-20; Santa Cruz Biotechnology, Santa Cruz, CA) for the detection of STAT6.
Immunocytochemistry
HD-MyZ cells (2 × 104) were cultured in chambered glass slides (Nalge Nunc International, Naperville, IL) and were fixed with cold methanol/acetone (1:1, vol/vol). The slides were subjected to indirect immunocytochemical analysis using Vector ABC kit (Vector Laboratories, Burlingame, CA) with polyclonal anti–IL-4Rα antibody (c-20; Santa Cruz Biotechnology) and were developed with 3,3′-diaminobenzidine substrate (Vector Laboratories).
Protein synthesis inhibition assay
The cytotoxic activity of IL438-37-PE38KDEL was tested as previously described.32 Typically, 104 cells were cultured in leucine-free medium with or without various concentrations of IL438-37-PE38KDEL for 20 to 22 hours at 37°C. Then 1 μCi (0.037 MBq) [3H]-leucine (NEN Research Products, Boston, MA) was added to each well and was incubated for an additional 4 hours. Cells were harvested, and radioactivity incorporated into cells was measured by a beta plate counter (Wallac).
Clonogenic assay
In vitro cytotoxic activities of IL-4 cytotoxin on cells were also determined by colony-forming assay. Cells were plated in triplicate in 100-mm Petri dishes with 7 mL medium containing 10% FBS and were allowed to attach for 20 to 22 hours. Then cells were exposed to various concentrations of IL438-37-PE38KDEL (0-100 ng/mL) for 14 days at 37°C in a humidified incubator. The cells were washed, fixed, and stained with crystal violet (0.25% in 25% ethanol). Colonies consisting of more than 50 cells were scored. Colony survival percentage was determined from the number of colonies remaining in the treated groups divided by the number of control group colonies.
Animals, infiltrating mouse model of Hodgkin lymphoma, and survival studies
Four- to 5-week-old (20-22 g body weight) female beige/nude/X-linked BR-immunodeficient (NIH-III) mice were obtained from Frederick Cancer Center Animal Facilities (National Cancer Institute, Frederick, MD). The mice were housed in sterilized filter-topped cages and maintained in a pathogen-free animal facility. Animal care was in accordance with the guidelines of the NIH Animal Research Advisory Committee. Human H-RS cell lines (L1236 or HD-MyZ) were implanted by subcutaneous injection of 5 × 106 cells in 150 μL PBS into the abdominal surfaces of mice. After 5 days, when solid tumors were established, excipient (0.2% human serum albumin in PBS) or IL438-37-PE38KDEL was administered intraperitoneally (500 μL) or intratumorally (30 μL) using a 27-gauge needle. Tumor sizes were carefully measured with a Vernier caliper. Tumor volume was calculated using the formula (tumor volume) = (length) × (width)2/2. Each treatment group consisted of at least 5 mice.
Survival
For survival studies, mice were injected intravenously through the tail vein with 5 × 106 HD-MyZ cells in 600 μL PBS. To avoid backflow and leakage, injection duration was 20 minutes. Animals received IL438-37-PE38KDEL intraperitoneally for 5 successive days from day 7 of cell injection, and survival was observed.
Cell viability assay
Cells (2 × 105/well) seeded in 6-well plates were treated with IL438-37-PE38KDEL (1 ng/mL), 0.5 μg/mL hydrocortisone (Sigma-Aldrich, St Louis, MO), or both for 24 hours. Cell viability was assessed by trypan blue exclusion.
Histologic analysis
Subcutaneously developed tumors, swelling superficial axillary and iliac lymph nodes, and vital organs were harvested from experimental animals fixed with 10% formalin, and paraffin-embedded sections were stained with hematoxylin and eosin (H&E). H-RS cells or fluid samples aspirated from swelling lymph nodes were stained with Giemsa Diff-Quik Stain Set (Dade Behring, Deerfield, IL). Slides were visualized under an Olympus IX70 fluorescence microscope (Olympus Optical, Tokyo, Japan). Images were compiled from sets of 3 consecutive single optical sections using SPOT Insight version 3.2 software (Diagnostic Instruments, Sterling Heights, MI).
Statistical analysis
The statistical significance of tumor regression and various parameters was calculated by Student t test.
Results
Expression and structure of IL-4R in human H-RS cell lines
To determine the expression levels and binding affinities of IL-4R on human H-RS cell lines, radioreceptor binding assay was performed using HD-MyZ or L1236 cell lines. HD-MyZ and L1236 cells bound 125I–IL-4, and nonradiolabeled IL-4 was able to compete for the binding in a concentration-dependent manner. Assessment of the binding data by Scatchard analysis suggested that each cell line expressed a single receptor type with a Kd of 381 pM in the HD-MyZ cell line and 151 pM in the L1236 cell line. The number of IL-4Rs was calculated to be 1078 IL-4 molecules bound/cell in the HD-MyZ cell line and 4541 IL-4 molecules bound/cell in L1236 cell line (data not shown).
The structure of IL-4R in H-RS cells was assessed by RT-PCR, flow cytometry, immunohistochemical analysis, and Western blot analysis. As shown in Table 1, L428 and HD-LM2 cell lines expressed low levels (as evident by faint band intensity and assigned +) of IL-4Rα mRNA, whereas L1236, L540, L591, and HD-MyZ cell lines expressed high levels (as evident by strong band intensity and assigned ++) of IL-4Rα mRNA.
Cytotoxicity of IL-4 cytotoxin to Hodgkin/Reed-Sternberg cells
Cell line . | IC50, ng/mL . | No. experiments performed . | IL-4Rα mRNA expression status . |
|---|---|---|---|
| L428 | 120-200 | 5 | + |
| L1236 | 0.1-0.5 | 8 | ++ |
| L540 | 10-130 | 5 | ++ |
| L591 | 2-20 | 6 | ++ |
| HD-MyZ | 2-35 | 7 | ++ |
| HD-LM2 | More than 1000 | 3 | + |
Cell line . | IC50, ng/mL . | No. experiments performed . | IL-4Rα mRNA expression status . |
|---|---|---|---|
| L428 | 120-200 | 5 | + |
| L1236 | 0.1-0.5 | 8 | ++ |
| L540 | 10-130 | 5 | ++ |
| L591 | 2-20 | 6 | ++ |
| HD-MyZ | 2-35 | 7 | ++ |
| HD-LM2 | More than 1000 | 3 | + |
IC50 is the concentration of IL4 -PE38KDEL at which 50% inhibition of protein synthesis was observed compared with untreated cells.
IL-4Rα mRNA expression was confirmed by RT-PCR analysis, as previously described.29 Band intensity was assigned + for faint band and ++ for strong band.
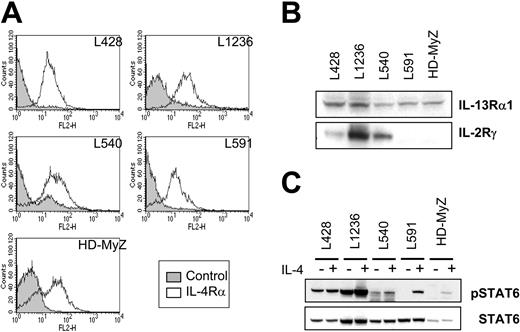
RT-PCR results were confirmed by flow cytometric analysis. As shown in Figure 1A, all H-RS cell lines examined expressed the IL-4Rα chain. We also performed immunocytochemical staining to assess IL-4Rα chain expression in HD-MyZ cells, which contained adherent cell populations. As shown by flow cytometry results, adherent populations of HD-MyZ cells were stained with antibody specific to the IL-4Rα chain (data not shown).
Expression of IL-4R subunits and STAT6 activation induced by IL-4 in human H-RS cell lines. (A) Expression of IL-4Rα chain on 5 H-RS cell lines was assessed by flow cytometry using phycoerythrin-conjugated anti–IL-4Rα monoclonal antibody (open curve). Staining with isotype-matched IgG served as control (shaded curve). (B) Cell lysates prepared from H-RS cell lines were subjected to immunoblotting analysis to assess the expression of IL-4R subunits. (C) Antibody to Tyr-641–phosphorylated STAT6 or whole STAT6 proteins was reacted with membranes with cell lysates from H-RS cells stimulated with or without IL-4.
Expression of IL-4R subunits and STAT6 activation induced by IL-4 in human H-RS cell lines. (A) Expression of IL-4Rα chain on 5 H-RS cell lines was assessed by flow cytometry using phycoerythrin-conjugated anti–IL-4Rα monoclonal antibody (open curve). Staining with isotype-matched IgG served as control (shaded curve). (B) Cell lysates prepared from H-RS cell lines were subjected to immunoblotting analysis to assess the expression of IL-4R subunits. (C) Antibody to Tyr-641–phosphorylated STAT6 or whole STAT6 proteins was reacted with membranes with cell lysates from H-RS cells stimulated with or without IL-4.
Because IL-4R may be composed of IL-13Rα1 or IL-2Rγ chains, or both, in addition to IL-4Rα, expression of IL-13Rα1 and IL-2Rγ chains in H-RS cells was analyzed by Western blot (Figure 1B). This analysis showed that the IL-13Rα1 chain is expressed in all 5 H-RS cell lines, whereas the IL-2Rγ chain is expressed in only 3 cell lines. These results indicate that H-RS cell express type 2 or type 3 IL-4R.
Function of IL-4R in H-RS cells
To assess whether type 2 or type 3 IL-4Rs in H-RS cells are biologically functional, phosphorylation of STAT6 protein was assessed in IL-4–treated H-RS cell lines by immunoblotting analysis. As evident in Figure 1C, type 3 IL-4R–positive cells (L428, L1236, and L540 cell lines) showed basal phosphorylation of STAT6 protein. When these cells were stimulated with IL-4, a further increase in the phosphorylation of STAT6 signal was detected. In contrast, type 2 IL-4Rs expressing H-RS cell lines (L591, HD-MyZ) did not show constitutive phosphorylation of STAT6 protein; however, when stimulated by IL-4, phosphorylation of STAT-6 protein was observed. These results suggest that IL-4 receptors expressed on H-RS cells are biologically functional.
Human H-RS cell lines are sensitive to IL-4 cytotoxin in vitro
Because H-RS cell lines express IL-4R, we evaluated whether IL-4 cytotoxin mediates cytotoxicity to these cell lines. Cytotoxicity was assessed by the inhibition of protein synthesis assay, which is shown to be directly proportional to cell death.32 As summarized in Table 1, IL-4 cytotoxin inhibited protein synthesis (detected by a decrease in 3[H]-leucine uptake) in these cell lines. The IC50 (the concentration of IL-4 cytotoxin causing 50% inhibition in protein synthesis) varied between cell lines (Table 1). Cell lines expressing high-density IL-4Rα mRNA (L1236, L540, L591, HD-MyZ) showed higher sensitivity than cell lines (L428, HD-LM2) expressing low-density IL-4Rα mRNA. L1236 cells were most sensitive to IL-4 cytotoxin, with an IC50 ranging between 0.1 and 0.5 ng/mL; the next most sensitive were HD-MyZ and L591 cell lines.
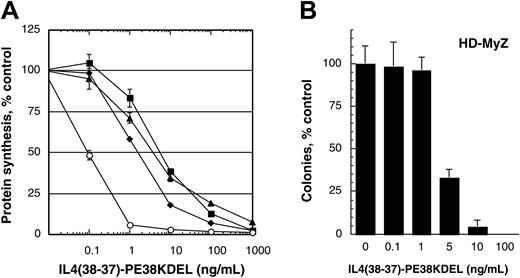
Because we aimed to use adherent populations of the HD-MyZ cell line in in vivo antitumor experiments, the sensitivity of adherent, suspension, and total populations of HD-MyZ cells to IL-4 cytotoxin was assessed in vitro. As shown in Figure 2A, the cytotoxic effect of IL-4 cytotoxin was similar in all 3 cell populations.
Cytotoxic effect of IL-4 cytotoxin on human H-RS cell lines. (A) L1236 (○) or HD-MyZ cells (▪, total population;  , suspension population; ▴, adherent population) were treated with various concentrations of IL438-37-PE38KDEL (0-1000 ng/mL), and protein synthesis inhibition was assessed. Results are presented as mean ± SD of quadruplicate determinations, and the assay was repeated 3 times. (B) Inhibition of HD-MyZ colony formation by IL-4 cytotoxin. HD-MyZ cells (adherent population) were allowed to adhere in Petri dishes and were cultured with various concentrations (0-100 ng/mL) of IL438-37-PE38KDEL for 14 days; then colonies consisting of at least 50 cells were scored after staining with crystal violet. Data were obtained from the mean of triplicate determinations. Data are means; bars, SD.
, suspension population; ▴, adherent population) were treated with various concentrations of IL438-37-PE38KDEL (0-1000 ng/mL), and protein synthesis inhibition was assessed. Results are presented as mean ± SD of quadruplicate determinations, and the assay was repeated 3 times. (B) Inhibition of HD-MyZ colony formation by IL-4 cytotoxin. HD-MyZ cells (adherent population) were allowed to adhere in Petri dishes and were cultured with various concentrations (0-100 ng/mL) of IL438-37-PE38KDEL for 14 days; then colonies consisting of at least 50 cells were scored after staining with crystal violet. Data were obtained from the mean of triplicate determinations. Data are means; bars, SD.
Cytotoxic effect of IL-4 cytotoxin on human H-RS cell lines. (A) L1236 (○) or HD-MyZ cells (▪, total population;  , suspension population; ▴, adherent population) were treated with various concentrations of IL438-37-PE38KDEL (0-1000 ng/mL), and protein synthesis inhibition was assessed. Results are presented as mean ± SD of quadruplicate determinations, and the assay was repeated 3 times. (B) Inhibition of HD-MyZ colony formation by IL-4 cytotoxin. HD-MyZ cells (adherent population) were allowed to adhere in Petri dishes and were cultured with various concentrations (0-100 ng/mL) of IL438-37-PE38KDEL for 14 days; then colonies consisting of at least 50 cells were scored after staining with crystal violet. Data were obtained from the mean of triplicate determinations. Data are means; bars, SD.
, suspension population; ▴, adherent population) were treated with various concentrations of IL438-37-PE38KDEL (0-1000 ng/mL), and protein synthesis inhibition was assessed. Results are presented as mean ± SD of quadruplicate determinations, and the assay was repeated 3 times. (B) Inhibition of HD-MyZ colony formation by IL-4 cytotoxin. HD-MyZ cells (adherent population) were allowed to adhere in Petri dishes and were cultured with various concentrations (0-100 ng/mL) of IL438-37-PE38KDEL for 14 days; then colonies consisting of at least 50 cells were scored after staining with crystal violet. Data were obtained from the mean of triplicate determinations. Data are means; bars, SD.
IL-4 cytotoxin inhibits colony formation of HD-MyZ cells
We next assessed the effect of IL-4 cytotoxin on the inhibition of colony formation of the HD-MyZ cell line. Five hundred cells were plated in Petri dishes and incubated with various concentrations of IL-4 cytotoxin. Colonies that formed after 14 days of culture were counted. As shown in Figure 2B, IL-4 cytotoxin inhibited colony formation in a concentration-dependent manner. At 5 ng/mL IL-4, cytotoxin colonies were inhibited by more than 50%, whereas at 100 ng/mL colony formation was completely inhibited. These results were consistent with the results obtained in the protein synthesis inhibition assays.
Antitumor activity of IL-4 cytotoxin and inhibition of lymphnode metastasis in a Hodgkin lymphoma animal model
To determine the antitumor activity of IL-4 cytotoxin in vivo, we developed a Hodgkin lymphoma tumor xenograft model by injecting the adherent population of HD-MyZ or L1236 cells subcutaneously into beige/nude/X-linked BR immunodeficient (NIH-III) mice. HD-MyZ cells consistently produced growing subcutaneous tumors causing central necrosis. We also tested the tumorigenic potential of other H-RS cell lines in NIH-III mice. L1236 cells formed tumors, but the tumor growth rate was much slower compared with HD-MyZ tumors. Other cell lines, including L428, L540, L591, and HD-LM2, were not tumorigenic in athymic nude mice or beige/nude/X-linked BR immunodeficient mice (data not shown).
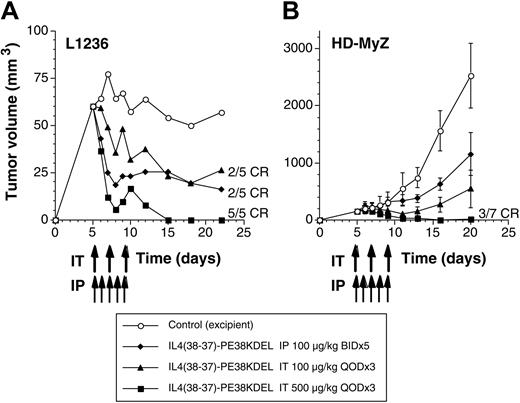
After implantation with HD-MyZ or L1236 tumor cells, animals were treated with different doses of IL-4 cytotoxin either by intraperitoneal (100 μg/kg twice a day for 5 days from day 5 through 9) or intratumoral (100 or 500 μg/kg once a day on days 5, 7, and 9) routes. As shown in Figure 3A, L1236 tumor cells formed a palpable mass within 5 days of implantation. When these animals were treated intraperitoneally with IL-4 cytotoxin, 2 of 5 mice showed complete regression of their established tumors. The remaining 3 animals showed tumor shrinkage. Better antitumor responses were observed using the intratumoral route of IL-4 cytotoxin administration in a dose-dependent manner. Intratumoral doses (500 μg/kg) resulted in complete regression of tumors in all mice by day 15, and these mice remained tumor free until the last day of the experiment (day 22). The tumor volume in other groups receiving IL-4 cytotoxin was significantly lower than the tumor volume in control untreated mice (P < .05 compared with control).
IL-4 cytotoxin mediates tumor regression of subcutaneous Hodgkin lymphoma. Beige/nude/X-linked BR immunodeficient (NIH-III) mice receiving subcutaneous injections of 5 × 106 L1236 (A) or HD-MyZ (B) cells at day 0 were treated with IL438-37-PE38KDEL by intraperitoneal (IP) (twice a day for 5 days;  ) or intratumoral (IT) routes (once a day on alternate days; total of 3 injections) as indicated by arrows (▴, 100 μg/kg; ▪, 500 μg/kg). Control mice are indicated by ○. Tumors were measured with a Vernier caliper, and tumor volume was calculated as described in “Materials and methods.” Each group consisted of 5 to 7 mice, and tumor volumes shown are mean ± SD. Experiments were repeated 2 times in the L1236 tumor model and 4 times in the HD-MyZ tumor model.
) or intratumoral (IT) routes (once a day on alternate days; total of 3 injections) as indicated by arrows (▴, 100 μg/kg; ▪, 500 μg/kg). Control mice are indicated by ○. Tumors were measured with a Vernier caliper, and tumor volume was calculated as described in “Materials and methods.” Each group consisted of 5 to 7 mice, and tumor volumes shown are mean ± SD. Experiments were repeated 2 times in the L1236 tumor model and 4 times in the HD-MyZ tumor model.
IL-4 cytotoxin mediates tumor regression of subcutaneous Hodgkin lymphoma. Beige/nude/X-linked BR immunodeficient (NIH-III) mice receiving subcutaneous injections of 5 × 106 L1236 (A) or HD-MyZ (B) cells at day 0 were treated with IL438-37-PE38KDEL by intraperitoneal (IP) (twice a day for 5 days;  ) or intratumoral (IT) routes (once a day on alternate days; total of 3 injections) as indicated by arrows (▴, 100 μg/kg; ▪, 500 μg/kg). Control mice are indicated by ○. Tumors were measured with a Vernier caliper, and tumor volume was calculated as described in “Materials and methods.” Each group consisted of 5 to 7 mice, and tumor volumes shown are mean ± SD. Experiments were repeated 2 times in the L1236 tumor model and 4 times in the HD-MyZ tumor model.
) or intratumoral (IT) routes (once a day on alternate days; total of 3 injections) as indicated by arrows (▴, 100 μg/kg; ▪, 500 μg/kg). Control mice are indicated by ○. Tumors were measured with a Vernier caliper, and tumor volume was calculated as described in “Materials and methods.” Each group consisted of 5 to 7 mice, and tumor volumes shown are mean ± SD. Experiments were repeated 2 times in the L1236 tumor model and 4 times in the HD-MyZ tumor model.
In contrast to L1236 tumors, HD-MyZ tumors grew rapidly after an initial stable tumor growth for the first 10 days, and then tumors grew linearly (Figure 3B). However, animals treated with 100 μg/kg IL-4 cytotoxin intraperitoneally showed tumor stasis; 1 mouse showed complete regression. Overall, on the last day of follow-up, the tumor volume in IL-4 cytotoxin–treated mice was significantly lower (1155 mm3) than it was in control mice (2530 mm3) (P < .005). In an additional experiment, we evaluated the antitumor activity of IL-4 cytotoxin given intraperitoneally at a dose of 50, 100, or 200 μg/kg. IL-4 cytotoxin exhibited antitumor activity in a dose-dependent manner (data not shown). Similarly, animals receiving intratumoral IL-4 cytotoxin showed antitumor response in a dose-dependent manner. At the highest dose (500 μg/kg), 3 of 7 mice showed complete regression of tumors and remained tumor free throughout the experimental period. The reduction of tumor volume by the end of experiment (day 20) was 78% (549 mm3; P < .001) in the 100 μg/kg group and 99% (18 mm3; P < .0001) in the 500 μg/kg group, when compared with control tumors (2530 mm3). These results suggest that IL-4 cytotoxin mediates potent antitumor activity against Hodgkin lymphoma tumors expressing IL-4R.
Interestingly, HD-MyZ tumor–bearing beige/nude/X-linked BR immunodeficient mice developed infiltrating features as subcutaneous tumors grew. Given that this metastatic clinical feature was consistently observed, we tested whether IL-4 cytotoxin could affect the regression of these nodes. Beige/nude/X-linked BR immunodeficient mice were injected subcutaneously with HD-MyZ cells (5 × 106), and 5 days later mice were treated with intraperitoneal (50, 100, or 200 μg/kg doses) or intratumoral (100 or 500 μg/kg doses) IL-4 cytotoxin. The treatment schedule was identical to that of antitumor experiments shown in Figure 3; however, the mice were observed not only for subcutaneous tumors but also for lymph node swelling. As shown in Table 2, on the last day of the experiment (day 20-25 after tumor implantation), 7 of 21 control mice exhibited swelling of the axillary lymph nodes, and 14 of 21 control mice exhibited swelling of the iliac lymph nodes. IL-4 cytotoxin treatment of mice by intraperitoneal or intratumoral injection resulted in the inhibition of lymph node swelling. All 3 intraperitoneal doses of IL-4 cytotoxin inhibited axillary and iliac lymph node swelling. At the 200 μg/kg dose, greater inhibition of iliac lymph node swelling was observed. Only 1 of 12 mice showed axillary lymph node swelling, and 3 of 12 mice showed iliac lymph node swelling. Intratumoral IL-4 cytotoxin administration also inhibited lymph node swelling. At the 500 μg/kg dose, 100% inhibition of axillary and iliac lymph node swelling in all HD-MyZ tumor-bearing animals was observed (n = 15). Interestingly, the 100 μg/kg intraperitoneal dose of IL-4 cytotoxin showed superior activity in inhibiting lymph node swelling than the 100 μg/kg intratumoral dose. These results suggest that IL-4 cytotoxin treatment mediates antitumor effects not only on subcutaneous primary tumors but also on infiltrating disease to lymph nodes.
Superficial lymph node swelling in beige/nude/X-linked BR immunodeficient mice bearing subcutaneous HD-MyZ tumors
Treatment . | Axillary lymph node . | Iliac lymph node . |
|---|---|---|
| Control | 7 of 21* | 14 of 21† |
| IL438-37-PE38KDEL, μg/kg | ||
| IP 50 | 0 of 12 | 5 of 12 |
| IP 100 | 3 of 14 | 9 of 14 |
| IP 200 | 1 of 12 | 3 of 12 |
| IT 100 | 5 of 15 | 14 of 15 |
| IT 500 | 0 of 15 | 0 of 15 |
Treatment . | Axillary lymph node . | Iliac lymph node . |
|---|---|---|
| Control | 7 of 21* | 14 of 21† |
| IL438-37-PE38KDEL, μg/kg | ||
| IP 50 | 0 of 12 | 5 of 12 |
| IP 100 | 3 of 14 | 9 of 14 |
| IP 200 | 1 of 12 | 3 of 12 |
| IT 100 | 5 of 15 | 14 of 15 |
| IT 500 | 0 of 15 | 0 of 15 |
IP indicates intraperitoneal; IT, intratumoral.
Presence of lymph node swelling was assigned by palpation
Mice were from 2 or 3 individual experiments
Histologic assessment of infiltrating Hodgkin lymphoma in animals receiving IL-4 cytotoxin treatment
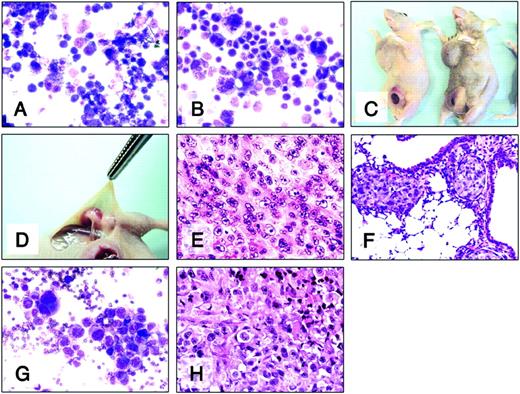
Because subcutaneous injection of H-RS tumor cells resulted in local and infiltrative disease to lymph nodes, we examined whether tumor deposits were also present in vital organs. As described in Figure 4 and Table 2, mice were injected with HD-MyZ tumor cells. Twenty days later tumors, lymph nodes, and vital organs were harvested for histologic evaluation using H&E or Giemsa staining. Figure 4A-B shows the morphology of H-RS cells (A, L1236; B, HD-MyZ) in vitro when injected subcutaneously into beige/nude/X-linked BR immunodeficient mice. The histologic features of Reed-Sternberg cells, such as abundant cytoplasm, and multilobed or multinucleated nuclei with amphophilic owl-eyed nucleoli were observed. These animals developed axillary and iliac lymph node swelling as primary subcutaneous tumors enlarged (Figure 4C-D). Treatment of these animals with IL-4 cytotoxin resulted in the inhibition of primary tumor nodule and lymph node swelling.
Effect of IL-4 cytotoxin on infiltrating Hodgkin lymphoma. H-RS cells (Giemsa staining; A, L1236; B, HD-MyZ) were injected subcutaneously into beige/nude/X-linked BR immunodeficient mice. (C) Representative image of mice developing subcutaneous primary HD-MyZ tumors and axillary and iliac lymph node swellings. (D) Swelling was not detectable in iliac lymph nodes of mice receiving IL438-37-PE38KDEL intraperitoneally (200 μg/kg dose). H&E staining of subcutaneous HD-MyZ tumor (E), lung (F), and axillary lymph node (H) harvested from untreated mice. (G) Giemsa staining of aspirated fluids from axillary lymph node of untreated mouse. Original magnifications, × 200 (A-B, E-G) and × 400 (H).
Effect of IL-4 cytotoxin on infiltrating Hodgkin lymphoma. H-RS cells (Giemsa staining; A, L1236; B, HD-MyZ) were injected subcutaneously into beige/nude/X-linked BR immunodeficient mice. (C) Representative image of mice developing subcutaneous primary HD-MyZ tumors and axillary and iliac lymph node swellings. (D) Swelling was not detectable in iliac lymph nodes of mice receiving IL438-37-PE38KDEL intraperitoneally (200 μg/kg dose). H&E staining of subcutaneous HD-MyZ tumor (E), lung (F), and axillary lymph node (H) harvested from untreated mice. (G) Giemsa staining of aspirated fluids from axillary lymph node of untreated mouse. Original magnifications, × 200 (A-B, E-G) and × 400 (H).
The histology of primary subcutaneous HD-MyZ tumors also exhibited features of H-RS cells surrounded by connective tissue with high vascularity (Figure 4E; control mouse). In addition to lymph nodes, HD-MyZ tumor cells were also detected in lungs (Figure 4F) and in some kidneys harvested from untreated mice (data not shown). However, other organs—including heart, liver, and spleen—did not contain these cells. Giemsa staining of aspirated fluids taken from swollen lymph nodes of control mice showed infiltrating H-RS cells (Figure 4G). H&E staining results confirmed the presence of H-RS cells in swollen lymph nodes (Figure 4H; control mouse). These data suggest that subcutaneous injection of HD-MyZ cells into beige/nude/X-linked BR immunodeficient mice develops into infiltrating Hodgkin lymphoma, and treatment of these mice with IL-4 cytotoxin successfully inhibits lymph node metastasis.
Treatment with IL-4 cytotoxin prolongs survival of animals with metastatic Hodgkin lymphoma
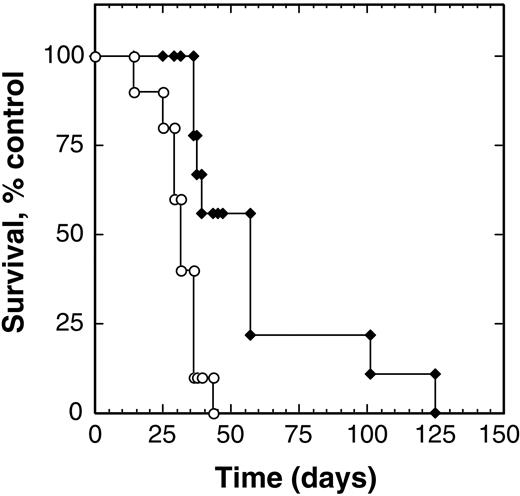
We observed that intravenous injection of HD-MyZ cells also led to the development of metastatic disease. Control animals (n = 10) developed signs of progressive disease within 4 weeks, with a mean survival time of 31 days after tumor injection (range, 14-43 days). When treated with 100 μg/kg per dose IL-4 cytotoxin twice a day for 5 days from day 7 after tumor injection, these mice exhibited prolonged survival (Figure 5). The mean survival time of treated animals (n = 7) was 62.9 days (range, 36-125 days). Overall survival of IL-4–cytotoxin treated animals was 203% greater than that of control animals. These results suggest that IL-4 cytotoxin prolongs survival of the Hodgkin lymphoma–bearing hosts and inhibits primary Hodgkin tumor growth and lymph node metastasis.
IL-4 cytotoxin prolongs survival of animals bearing systemic Hodgkin disease. Beige/nude/X-linked BR immunodeficient mice injected intravenously with HD-MyZ cells (5 × 106/injection) were treated intraperitoneally with excipient (○) or IL438-37-PE38KDEL (100 μg/kg;  ) twice a day through days 7 to 11, and survival of the animals was observed. Each group contained 7 to 9 animals, and the experiment was performed twice with identical results.
) twice a day through days 7 to 11, and survival of the animals was observed. Each group contained 7 to 9 animals, and the experiment was performed twice with identical results.
IL-4 cytotoxin prolongs survival of animals bearing systemic Hodgkin disease. Beige/nude/X-linked BR immunodeficient mice injected intravenously with HD-MyZ cells (5 × 106/injection) were treated intraperitoneally with excipient (○) or IL438-37-PE38KDEL (100 μg/kg;  ) twice a day through days 7 to 11, and survival of the animals was observed. Each group contained 7 to 9 animals, and the experiment was performed twice with identical results.
) twice a day through days 7 to 11, and survival of the animals was observed. Each group contained 7 to 9 animals, and the experiment was performed twice with identical results.
Combination effect of IL-4 cytotoxin and hydrocortisone
We also assessed whether IL-4 cytotoxin combines with hydrocortisone, a conventional chemotherapeutic agent, in mediating cytotoxicity to H-RS cells in vitro. Low doses of IL-4 cytotoxin (1 ng/mL) or hydrocortisone (0.5 μg/mL) mediated moderate growth inhibition of HD-MyZ cells; however, when cells were cultured with IL-4 cytotoxin (1 ng/mL) and hydrocortisone (0.5 μg/mL), a modest additive effect on the inhibition of cell viability was observed (data not shown). Because it is reported that corticosteroid (methylprednisolone) inhibits IL-4 signaling in human primary immune cells,33 we preincubated HD-MyZ cells with hydrocortisone for 24 hours and followed this with IL-4 cytotoxin treatment. Pretreatment with hydrocortisone did not affect the sensitivity of HD-MyZ cells to IL-4 cytotoxin (data not shown). These results suggest that IL-4 cytotoxin may be useful in combination with hydrocortisone, but it does not show a synergistic effect in vitro.
Discussion
In this study, we demonstrate that IL-4 receptors are overexpressed on H-RS cells and that these cells are highly sensitive to the cytotoxic effect of recombinant IL-4 cytotoxin in vitro. The combined use of IL-4 cytotoxin and hydrocortisone did not exhibit a synergistic effect on H-RS cell viability in vitro. IL-4 cytotoxin mediated antitumor activity in HD-MyZ tumor-bearing mice, as assessed by the inhibition of established subcutaneous tumor growth, lymph node metastasis, and prolonged survival of HD-bearing beige/nude/X-linked BR immunodeficient mice. These results are consistent with those of previous studies, which documented that IL-4 cytotoxin can mediate remarkable antitumor activity in several animal models of solid human tumors expressing IL-4R.19-22,34
We used HD-MyZ tumors subcutaneously xenografted into mice to evaluate the antitumor effects of IL-4 cytotoxin. These tumors in beige/nude/X-linked BR immunodeficient mice exhibited an infiltrative metastatic feature. In contrast, L428, L591, L540, and HD-LM2 failed to consistently grow in athymic nude mice. This observation is consistent with the previous report by von Kalle et al.28 It has been reported that L1236 cells form tumors in SCID mice when these cells are injected subcutaneously4,35 ; however, L1236 tumor growth was slower in beige/nude/X-linked BR immunodeficient mice. Because of their consistent growth and infiltrative nature, we used HD-MyZ cells for antitumor studies.
With the HD-MyZ HD model, IL-4 cytotoxin demonstrated potent antitumor activity against primary and metastatic disease. Intraperitoneal and intratumoral routes of IL-4 cytotoxin administration mediated antitumor effects, but the intratumoral route was superior to the intraperitoneal route in mediating tumor regression. This observation was consistent with our previous findings that intratumoral administration distributes the drug efficiently in the tumor nodule.21 In sharp contrast to observations in subcutaneous tumors, the intraperitoneal route of IL-4 cytotoxin administration was superior to the intratumoral route in inhibiting lymph node metastasis of HD-MyZ cells. Although intraperitoneal injection of IL-4 cytotoxin distributes less drug to the primary tumor nodule, systemic distribution can kill primary and infiltrating H-RS cells. Perhaps a combination of intratumoral and intraperitoneal routes of IL-4 cytotoxin administration may offer a maximum effect in the animal model of HD.
We also demonstrated the remarkable antitumor effect of IL-4 cytotoxin in systemic HD tumors, which were established by the intravenous administration of HD-MyZ cells. This model generated disease in lymph nodes and in several vital organs. As a result, 100% of the animals died; mean survival time was 31 days. Treatment with IL-4 cytotoxin improved the survival of these animals 203% compared with survival in untreated mice. These results suggest that IL-4 cytotoxin not only causes the regression of established tumors and metastasized lymph nodes, it prolongs the survival of the Hodgkin lymphoma–bearing host. Because the HD-MyZ model simulates the clinical features of the disease, it is possible that IL-4 cytotoxin will mediate similar antitumor effects in the clinic.
Although IL-4 cytotoxin mediated remarkable antitumor effects in vivo, no visible toxicity caused by IL-4 cytotoxin was observed. Specifically, no features—such as weight loss, ruffled fur, or inactivity—indicating toxicity were seen in mice receiving IL-4 cytotoxin treatment. It has been determined that IL-4 cytotoxin produces no impact on the murine immune system because human IL-4R in IL-4 cytotoxin does not bind murine cells.36 In addition, IL-4 expression levels in malignant cells are considerably higher than in normal immune cells.34 Nevertheless, IL-4 cytotoxin may mediate toxicity in patients in whom IL-4 binds to IL-4R–positive normal cells.
The significance of the overexpression of IL-4R in H-RS cells is unknown. Previously, we demonstrated that a variety of solid tumor cell lines overexpress IL-4R.19-22 Because IL-4R binds IL-4, which leads to signaling through the STAT6 pathway,24-26,31 it is possible that IL-4 is produced by H-RS cells and that it serves as an autocrine growth factor through the IL-4/IL-4R pathway. Newcom et al37 demonstrated that the L428 H-RS cell line produces IL-4; however, exogenous anti–IL-4 had no effect on the sustained proliferation of these cells. In contrast, it has been shown that most H-RS cells rarely produce IL-4.7,8 On the other hand, an IL-4–related cytokine, IL-13, has been shown to be produced by H-RS cells, and IL-13 serves as an autocrine growth factor. Given that IL-4R shares 2 chains (IL-4Rα and IL-13Rα1) with IL-13R to mediate STAT6 signaling, it is possible that IL-13 uses IL-4R for the autocrine growth of H-RS cells. More recently, Trieu et al38 demonstrated that soluble IL-13Rα2 decoy receptor inhibits L1236 Hodgkin lymphoma growth. In addition, blood serum levels of IL-13 were detectable in only 10% of HD patients, and it has been speculated that IL-13 may be locally elevated in tumor sites in other HD patients. Therefore, basal systemic IL-4 levels and IL-4R/IL-13R sharing components could also be responsible for the proliferation of H-RS cells. Further study is required to determine the precise function of IL-4R in H-RS cells.
Prepublished online as Blood First Edition Paper, December 30, 2004; DOI 10.1182/blood-2004-08-3216.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr S. Rafat Husain and Dr Shinichiro Tsunoda for critical reading of this manuscript. We also thank all members of the Laboratory of Molecular Tumor Biology, Center for Biologics Evaluation and Research/Food and Drug Administration, for helpful suggestions. The views presented in this article do not necessary reflect those of the Food and Drug Administration.
Dr Koji Kawakami is currently at the Department of Advanced Clinical Science and Therapeutics, Graduate School of Medicine, University of Tokyo, Japan.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal