Abstract
Immunostimulatory DNA containing unmethylated cytosine-phosphate-guanosine (CpG) induces the development of T helper 1 (Th1) immune responses. The response of B cells to CpG stimulation involves increased proliferation, cytokine production, and costimulatory molecule expression. Similar effects have been observed following CpG stimulation of a variety of malignant B cells. Pediatric precursor B acute lymphoblastic leukemia (B-ALL) cells express low levels of costimulatory molecules and are generally poor stimulators of T-cell responses. In this study, we evaluated the impact of CpG stimulation on precursor B-ALL cell lines and pediatric patient-derived samples. The ability to respond to CpG oligodeoxynucleotides was determined by the level of Toll-like receptor 9 (TLR9) expression. In contrast to both nonleukemic B-cell precursors and mature B cells, the response of precursor B-ALL cells was characterized by increased CD40 expression but only small changes in CD86 levels and no induction of CD80 expression. CpG stimulation of ALL blasts produced increased levels of interleukin-6 (IL-6), IL-8, and IL-10 but no detectable IL-12p70 and led to a skewing of allogeneic T cells, with enhanced interferon γ (IFN-γ) production and reduced secretion of IL-5. These results demonstrate the functional relevance of CpG stimulation of precursor B-ALL cells and provide a rational basis for study of these agents for use in treatment of this disease.
Introduction
Immunostimulatory oligodeoxynucleotides (ODNs) containing unmethylated cytosine-phosphate-guanosine (CpG) dinucleotide motifs are strong activators of both the innate and adaptive immune systems and have been shown to act as powerful stimulators of T helper 1 (Th1) immune responses.1 Successes in preclinical models using CpG ODNs, combined with early indications that their use in human subjects is safe, have led to considerable interest in the clinical potential of these agents—for example, in the treatment of allergic disease and cancer, or as vaccine adjuvants.2
Recognition of CpG ODNs is mediated by Toll-like receptor 9 (TLR9).3,4 TLR9 is one of a growing family of membrane-bound receptors that recognize pathogen-associated molecular patterns, allowing the immune system to detect conserved structures as danger signals and provoke an immediate and robust immune response.5 The distribution of TLR9 is limited primarily to B cells, dendritic cells, and macrophages.6 In human B cells, ligation of TLR9 by CpG ODNs induces proliferation,7,8 proinflammatory cytokine secretion,9 and up-regulation of costimulatory molecules such as CD40 and CD86.8 CpG ODNs have also been shown to stimulate similar responses in a number of malignant B cells, including chronic lymphocytic leukemia (B-CLL), large cell lymphoma, and marginal zone lymphoma.10-12
Precursor B acute lymphoblastic leukemia (pre-B-ALL) is the most common malignancy in children. While good responses to therapy are achieved, relapses remain an important clinical challenge for which new treatment strategies are required. Pre-B-ALL cells are poor stimulators of T-cell–mediated immune responses as a result of low expression of costimulatory molecules, most importantly CD80 and CD86, by the leukemic blasts.13-15 The relevance of immune responses to pre-B-ALL is demonstrated by the critical role of the graft-versus-leukemia (GVL) effect for cure of relapsed disease after bone marrow transplantation.16
Despite the results from other leukemias and lymphomas, there have been no reports of the effects of CpG stimulation on precursor B leukemias. Although a limited study suggested a lack of TLR9 expression in pre-B-ALL cell lines,17 we undertook a more extensive investigation using both precursor B-lineage ALL cell lines and primary patient-derived ALL blasts. We report that 5 of 8 precursor B-ALL cell lines respond to CpG stimulation and that the magnitude of response correlates with the expression level of TLR9. The pattern of CpG-induced costimulatory molecule expression changes in precursor B-ALL cell lines and patient samples is distinct from the responses of nonleukemic precursor B cells and normal, mature B cells. Despite the reduced costimulatory molecule response, CpG stimulation of primary precursor B-ALL samples induced the release of proinflammatory cytokines and interleukin-10 (IL-10) and shifted allogeneic T-cell responses toward a Th1 pattern of cytokine production. These observations suggest that the use of CpG ODNs may contribute to the development of enhanced antileukemia immune responses in pre-B-ALL patients.
Materials and methods
Cells and tissue culture
The REH, RS4;11, and Sup-B15 cell lines were obtained from American Type Tissue Collection (Manassas, VA). The remaining lines, OP-1, 697, 380, KOPN57bi (KOPN), and Nalm6, were generously provided by Dr Dario Campana (St Jude Children's Research Hospital, Memphis, TN). All cell lines were cultured in RPMI medium with 10% fetal bovine serum (FBS, complete medium), except Sup-B15, which was maintained in Iscoves modified Dulbecco medium (DM) with 20% FBS. Pediatric precursor B-ALL patient samples, bone marrow, and peripheral blood were obtained from BC Children's Hospital (Vancouver, BC), with consent obtained under institutional review board (IRB) guidelines. To enrich for mononuclear cells, samples were layered over Ficoll-Paque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden) and centrifuged at 700g for 20 minutes in an IEC Centra GP8R centrifuge (IEC, Needham Heights, MA). Mononuclear cells at the interface were removed and washed 3 times with 2 mL RPMI 2% FBS and resuspended in complete media at 1 × 106 cell/mL. In all experiments involving CpG ODN stimulation, the cells were incubated with or without ODN at 6 μg/mL for 48 to 72 hours prior to analysis. The activity of the CpG ODNs used in this study have been extensively analyzed previously.8 All CpG ODNs, except 1760, were provided by Coley Pharmaceutical Group (Wellesley, MA) and had no detectable endotoxin by Limulus amebocyte lysate assay. CpG 1760 was provided by Dr Donald Macfarlane (Iowa City, IA). For CD40 ligand (CD40L)–mediated stimulation, precursor B-ALL cells were incubated on a monolayer of irradiated murine fibroblasts expressing human CD40 ligand (kindly provided by Dr Gordon Freeman, Dana-Farber Cancer Institute, Boston, MA) for 48 hours and the supernatants harvested for cytokine analysis.
Expansion of precursor B cells in NOD-SCID mice
Cells (5 × 106) from ALL patient bone marrow samples were injected intravenously into nonirradiated nonobese diabetic–severe combined immunodeficient (NOD-SCID) mice. Leukemia cell burden was measured every 2 weeks by phenotyping peripheral blood for human CD45+ cells. Mice were killed when peripheral blood contained more than 80% leukemic blasts. Leukemic blasts were purified from the spleens of killed animals by negative selection (mouse/human chimera isolation StemSep kit; Stem Cell Technologies, Vancouver, BC). The 2 ALL samples studied here were a t(11;19), CD19+, CD10- prepre-B-ALL (A199) and a t(1;19), CD19+, CD10+ late pre-B-ALL (A286).
Immunophenotyping
The 4-color flow cytometry was performed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). CD10 Cy-chrome and CD19 allophycocyanin (APC) were used as common reagents in all tubes to permit specific gating on the leukemic blast population. For analysis of cell surface molecules, all samples were labeled with directly conjugated fluorescent antibodies at 4°C for 20 minutes. The samples were then washed and resuspended in 1.5% paraformaldehyde and analyzed within 24 hours. All antibodies were obtained from BD Pharmingen (San Diego, CA). The relevant labeled isotype control antibodies were included in all experiments. All cells with a staining intensity higher than the upper limit obtained using the isotype control were considered positive. Analysis was performed using CellQuest software (Becton Dickinson) after gating on the specific population according to forward and side scatter and CD10/CD19 expression.
T-cell purification
Peripheral blood mononuclear cells (PBMCs) from healthy volunteers were isolated over Ficoll-Paque Plus as described for patient samples and resuspended at 5 × 107 cells/mL. T cells were isolated using StemSep with Human T Cell Enrichment Cocktail (Stem Cell Technologies). Purified T cells were resuspended at 2 × 106 cell/mL in complete media. T cells were more than 95% pure as determined by flow cytometry (data not shown).
Proliferation assays
Assays were set up in triplicate in complete media using round-bottom 96-well plates. For cell line proliferation, 5 × 104 cells/well were plated, and 3H-thymidine incorporation was measured after 24 hours. For T-cell stimulation by leukemic samples, effector-responder ratios of 3:1 were used and T-cell and leukemic blast controls included in each assay. T cells (105) were plated in each well. Plates were incubated for 96 hours at 37°C and then 3H-thymidine was added to each well at a final concentration of 1 μCi/mL (.037 MBq) and incubation was continued for a further 18 hours. DNA was then harvested, using a Mach III M harvester (Tomtec, Hamden, CT), and 3H-thymidine incorporation counted on a 1450 Microbeta liquid scintillation counter (Wallac Trilux, Turku, Finland) using Wallac 1450 Microbeta windows workstation software (version 2.70.004).
Cytokine analysis
Supernatant (50 μL) was removed from ALL cultures following stimulation with CpG ODNs or CD40L, or from each well of T-cell proliferation assay plates immediately prior to the addition of 3H-thymidine. The cytokine content of the supernatants was then determined by cytometric bead array (CBA; Becton Dickinson) following the manufacturer's recommended protocol.
Quantitative reverse-transcriptase–polymerase chain reaction (RT-PCR)
Total RNA was extracted from cells using Trizol reagent (Gibco, Rockville, MD). Following DNase I treatment, cDNA was synthesized using 1 μg total RNA. Real-time quantitative PCR was performed using the TaqMan system (Applied Biosystems, Foster City, CA). The expression level of TLR9 and internal reference TATA-box binding protein (TBP) was measured by PCR using TaqMan probes labeled with FAM and VIC, respectively. Probes and primers were obtained from Applied Biosystems: TLR9 (Toll-like receptor 9) forward primer, 5′-TGC AGC CGG AGA TGT TTG-3′; reverse primer, 5′-CGC GGT AGC TCC GTG AAT-3′; and probe, 6FAM-CAG TCA ATG GCT CCMGB NFQ used at a final concentration of 900 nM and 200 nM, respectively. Control reagents were used as per the Predeveloped TaqMan Assay Reagent for Human TBP (Applied Biosystems). Real-time PCR amplification and product detection were performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) as recommended by the manufacturer. The quantity of cDNA for each RNA sample was normalized to the quantity of TBP cDNA in each sample. Relative expression was determined using ΔΔCt (threshold cycle) method according to the manufacturer's protocol. Each assay includes a fixed standard, a no-template control, and a cDNA sample in triplicate.
Statistical analysis
Descriptive statistics were generated on all data using StatView for the Macintosh (Abacus Concepts, Berkeley, CA). Significance of observed changes was determined using paired t tests. Correlation coefficients (r) were calculated using Fisher r-to-z test. The alpha (P) value was set at .05, making all P values less than .05 statistically significant.
Results
Stimulation of B-cell precursor ALL cell lines by CpG ODNs
There were 8 human B-cell precursor ALL cell lines stimulated with CpG ODN 2006 (optimized for stimulation of human B cells8 ), or the non-CpG control ODN 2137 for 48 hours, and changes in expression of the costimulatory molecules CD40, CD80, and CD86 were measured. Overall, 5 of 8 ALL cell lines responded to CpG stimulation (Figure 1A). A wide range of CD40 responses was observed. There were 2 cell lines, RS4;11 and 380, that up-regulated CD40 expression on more than 90% of cells, while cell line KOPN increased CD40 levels on 60% of cells. Cell lines OP-1 and Sup-B15 demonstrated small but detectable (2%-10%) increases in CD40 expression. The remaining 3 lines did not increase CD40 expression. Changes in CD86 expression were observed only with the RS4;11, 380, and KOPN cells, but the increases were small (< 20% of cells). Small increases in CD80 expression were observed only on the highest responding cell lines, 380 (7%), RS4:11 (3%), and KOPN (2%) (data not shown). For all cell lines that showed a response to CpG 2006, a detectable but much reduced response to ODN 2137 was observed, indicating that non-CpG–mediated stimulation by the ODN accounted for some of the overall changes in costimulatory molecule expression. In contrast to the increases in costimulatory molecule expression, none of the cell lines demonstrated changes in proliferation rate in response to CpG ODN 2006 stimulation (Figure 1B).
Responses of precursor B-lineage ALL cell lines to CpG stimulation. (A) The cell lines were incubated for 48 hours in the absence (untreated) or presence of 6 μg/mL CpG ODN 2006 or control ODN 2137, then analyzed for costimulatory molecule expression. Quadrants were drawn using isotype control antibody–stained cells. The percentage of cells in each quadrant is shown. A representative of 3 independent experiments is shown. (B) Cell lines were cultured for 24 hours in absence (▦) or presence (▪) of CpG 2006 and proliferation measured by 3H-thymidine incorporation. The results are expressed as the average ± SE of 2 independent experiments. (C) RS4;11 (▦) or 697 (▪) cells were incubated for 48 hours in the presence or absence of the indicated CpG ODN, and analyzed for CD40 expression. Results are expressed as the change in the percentage of CD40-expressing cells following CpG treatment. A representative of 2 independent experiments is shown.
Responses of precursor B-lineage ALL cell lines to CpG stimulation. (A) The cell lines were incubated for 48 hours in the absence (untreated) or presence of 6 μg/mL CpG ODN 2006 or control ODN 2137, then analyzed for costimulatory molecule expression. Quadrants were drawn using isotype control antibody–stained cells. The percentage of cells in each quadrant is shown. A representative of 3 independent experiments is shown. (B) Cell lines were cultured for 24 hours in absence (▦) or presence (▪) of CpG 2006 and proliferation measured by 3H-thymidine incorporation. The results are expressed as the average ± SE of 2 independent experiments. (C) RS4;11 (▦) or 697 (▪) cells were incubated for 48 hours in the presence or absence of the indicated CpG ODN, and analyzed for CD40 expression. Results are expressed as the change in the percentage of CD40-expressing cells following CpG treatment. A representative of 2 independent experiments is shown.
The presence of different sequences flanking the CpG dinucleotide can result in discrete response profiles.18 To determine whether the pattern of responses observed with B-cell precursor ALL cell lines was the result of inefficient stimulation by CpG 2006, we tested a panel of CpG ODNs for stimulation of RS4;11 and 697 cells, cell lines shown with CpG 2006 to be highly responsive and nonresponsive, respectively. Analysis of CD40 expression revealed that RS4;11 cells responded to all CpG ODNs, with the exception of the nonstimulatory ODN 2041, with the highest responses observed with CpG ODN 2006 (Figure 1C). The low response obtained with CpG ODN 1982 is in agreement with previous results obtained with nonleukemic blood.18 Consistent with the results shown in Figure 1A, only CD40 was significantly up-regulated in response to the stimulatory CpG ODNs. No significant changes in CD80 or CD86 were observed (data not shown). None of the CpG ODNs stimulated an increase in costimulatory molecule expression by 697 cells (Figure 1C).
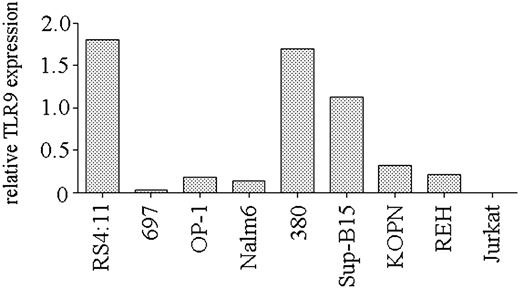
Expression of TLR9 is necessary for CpG ODN–mediated stimulation.3,4 To determine if the heterogeneity in the response of precursor B-ALL cell lines to CpG stimulation was the result of differing levels of TLR9 expression, we used real-time PCR to quantify TLR9 transcript levels in the cell lines. We observed a range of TLR9 expression levels in the panel of ALL cell lines (Figure 2). Correlation of TLR9 expression and the CD40 up-regulation in response to CpG ODN 2006 stimulation was significant (P = .03), indicating that the ability of B-cell precursor ALL cell lines to respond to CpG ODN stimulation is primarily determined by their level of expression of TLR9.
TLR9 expression by B-lineage ALL cell lines. Real-time PCR analysis of TLR9 expression by cell lines. Expression is shown as the relative level of TLR9 compared with that obtained from peripheral blood B cells from a healthy donor. A representative of 2 independent experiments is shown.
TLR9 expression by B-lineage ALL cell lines. Real-time PCR analysis of TLR9 expression by cell lines. Expression is shown as the relative level of TLR9 compared with that obtained from peripheral blood B cells from a healthy donor. A representative of 2 independent experiments is shown.
Response of primary pre-B-ALL cells to CpG ODNs
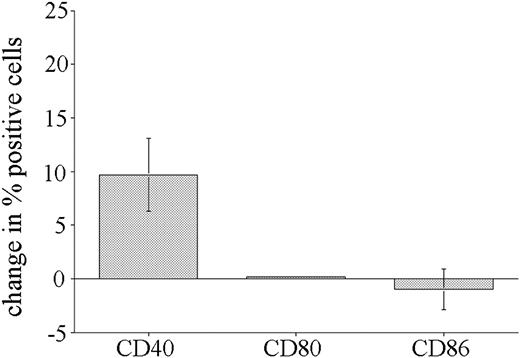
The results obtained using B-cell precursor ALL cell lines indicate that many cells respond to CpG ODN stimulation, but that the response differs from that reported for other B-cell types.7,8,10,12 To determine if this altered CpG ODN response was also seen with primary precursor B-ALL cells, we analyzed the effect of CpG stimulation on expression of CD40 (10 patients), CD80 (6 patients), and CD86 (6 patients) by leukemic blasts present in bone marrow biopsies from pediatric patients. In the flow cytometric analysis, leukemic blasts were selectively gated on the basis of their CD10+/CD19+ phenotype. Consistent with the results obtained using ALL cell lines, overall CD40 was the only molecule up-regulated by CpG stimulation of primary leukemic blasts (Figure 3). No change in CD80 expression and only small changes in CD86 expression in response to CpG stimulation were observed on primary B-ALL blasts.
Response of primary ALL blasts to CpG stimulation. Bone marrow biopsies from precursor B-ALL patients were cultured in the presence or absence of CpG ODN 2006 or 1760 for 72 hours and then analyzed for expression of CD40 (10 patients), CD80 (6 patients), and CD86 (6 patients). Specific gating on leukemic blasts was achieved by CD10 and CD19 costaining. The cumulative results expressed as the mean change (± SE) in the percentage of positive cells following CpG ODN treatment.
Response of primary ALL blasts to CpG stimulation. Bone marrow biopsies from precursor B-ALL patients were cultured in the presence or absence of CpG ODN 2006 or 1760 for 72 hours and then analyzed for expression of CD40 (10 patients), CD80 (6 patients), and CD86 (6 patients). Specific gating on leukemic blasts was achieved by CD10 and CD19 costaining. The cumulative results expressed as the mean change (± SE) in the percentage of positive cells following CpG ODN treatment.
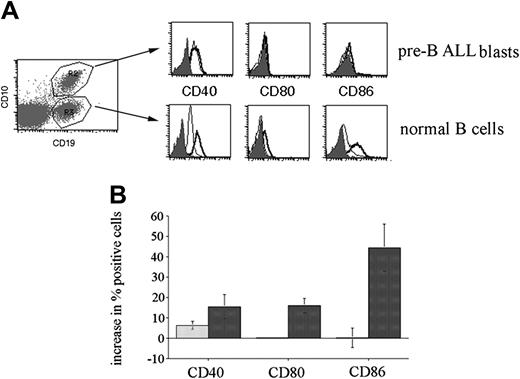
Next, we investigated whether there was a general change in CpG stimulatory activity in the presence of leukemia cells. To achieve this goal, we used peripheral blood obtained from patients with a significant peripheral leukemic blast count. Use of this blood permitted the flow cytometric analysis of the CpG ODN responses of both normal mature B-cell and leukemic blasts within the same sample. Pre-B-ALL blasts were selectively gated on the basis of CD10 and CD19 double positivity, while normal mature B cells were defined as CD19+, CD10-. Figure 4A shows a representative analysis of a patient peripheral blood sample stimulated with CpG 2006 for 72 hours. The overall response of 4 patient peripheral blood samples to stimulation, shown in Figure 4B, confirms that leukemic blasts respond differently than mature B cells to CpG stimulation, with smaller changes in CD40 and CD86 expression and no up-regulation in CD80 expression. These results show that the expected responses to CpG stimulation are observed in nonleukemic B cells present in these samples.
CpG stimulation of peripheral blood from pre-B-ALL patients. Peripheral blood mononuclear cells were cultured in the presence or absence of CpG ODN 2006 for 72 hours and then analyzed for costimulatory molecule expression by cellular subsets defined by CD10 and CD19 staining. (A) Analysis of a representative patient sample is shown. Isotype control staining is depicted by the solid histogram; untreated cells, by the thin line; and CpG-stimulated cells, by the thick line. R2 defines the CD10+/CD19+ pre-B-ALL cells and R3 defines the CD10-/CD19+ normal B-cell population. (B) Changes in expression of costimulatory molecules on CD10+/CD19+ ALL blasts are indicated by the light bars; changes on normal B cells, by the dark bars. The cumulative results obtained from the analysis of 4 patients, expressed as the mean change (± SE) in the percentage of positive cells in response to CpG stimulation.
CpG stimulation of peripheral blood from pre-B-ALL patients. Peripheral blood mononuclear cells were cultured in the presence or absence of CpG ODN 2006 for 72 hours and then analyzed for costimulatory molecule expression by cellular subsets defined by CD10 and CD19 staining. (A) Analysis of a representative patient sample is shown. Isotype control staining is depicted by the solid histogram; untreated cells, by the thin line; and CpG-stimulated cells, by the thick line. R2 defines the CD10+/CD19+ pre-B-ALL cells and R3 defines the CD10-/CD19+ normal B-cell population. (B) Changes in expression of costimulatory molecules on CD10+/CD19+ ALL blasts are indicated by the light bars; changes on normal B cells, by the dark bars. The cumulative results obtained from the analysis of 4 patients, expressed as the mean change (± SE) in the percentage of positive cells in response to CpG stimulation.
Response of normal precursor B cells to CpG ODNs
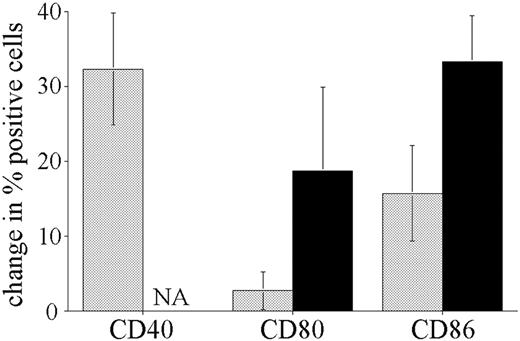
The difference in CpG ODN response of pre-B leukemic blasts compared with normal B cells may be the result of malignant transformation or simply be a reflection of the underlying phenotype of the precursor cells. To address this question, we stimulated bone marrow cells obtained from biopsies taken from nonleukemic individuals. The significantly higher number of normal B cells in nonleukemic marrow permitted comparison of CD19+/CD10+ pre-B and CD19+ mature B-cell responses to CpG stimulation, in the same manner as described for peripheral blood above. Figure 5 shows the results for the stimulation of 3 nonleukemic pediatric bone marrow biopsies. The response of nonleukemic pre-B cells differed from that observed with leukemic blasts, with larger increases in CD40 and CD86 and detectable up-regulation of CD80 expression. Although smaller in magnitude, this pattern of response is similar to that observed on normal mature B cells. Mature B cells present in the bone marrow samples responded to CpG ODNs with increases in CD40, CD80, and CD86 of similar magnitude to that seen for the normal B cells in the peripheral blood of leukemia patients.
Response of normal bone marrow resident CD10+/CD19+ B-cell precursors to CpG ODN stimulation. Mononuclear cells isolated from bone marrow samples obtained from 3 nonleukemic children were incubated with or without CpG ODN 2006 for 72 hours and analyzed in the same way as described for ALL patient peripheral blood. ▦ indicates CD10+/CD19+ cells; ▪, CD19+ B cells. The cumulative results for 3 patients are shown, expressed as the mean change (± SE) in the percentage of positive cells in response to CpG stimulation. All unstimulated CD19+ B cells were CD40+, so the increase in CD40+ cells was not assessable (NA).
Response of normal bone marrow resident CD10+/CD19+ B-cell precursors to CpG ODN stimulation. Mononuclear cells isolated from bone marrow samples obtained from 3 nonleukemic children were incubated with or without CpG ODN 2006 for 72 hours and analyzed in the same way as described for ALL patient peripheral blood. ▦ indicates CD10+/CD19+ cells; ▪, CD19+ B cells. The cumulative results for 3 patients are shown, expressed as the mean change (± SE) in the percentage of positive cells in response to CpG stimulation. All unstimulated CD19+ B cells were CD40+, so the increase in CD40+ cells was not assessable (NA).
Effect of CpG stimulation of purified primary precursor B-ALL blasts
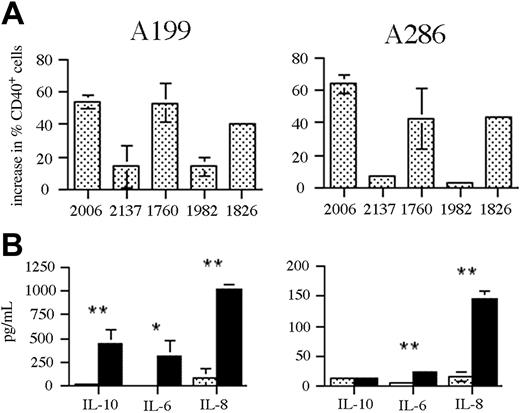
To examine the influence of CpG ODN stimulation on T-cell responses to ALL, we used precursor B-ALL blasts from 2 patients expanded in NOD-SCID mice prior to analysis. Passage of bone marrow cells through NOD-SCID mice allows the selective expansion of human leukemic blasts that can then be purified from contaminating mouse cells. Using this method, we obtained ALL blasts at more than 90% purity (as determined by human CD19 expression; data not shown), allowing the influence of CpG ODN–induced changes on ALL cells to be examined in the absence of other bone marrow cells. Stimulation of ALL samples A199 (pre-pre-B) and A286 (late pre-B) with a panel of CpG ODNs resulted in a significant increase in CD40 expression, greater than that seen with previous primary ALL samples (Figure 6A). The pattern of responses to the different CpG ODNs was the same as seen with the ALL cell lines. Despite the large increase in CD40 expression, there was no significant change in the expression of CD80 or CD86 following CpG ODN stimulation of either sample (data not shown). In addition to changes in costimulatory molecule expression, we investigated the effect of CpG ODN stimulation on cytokine production by the ALL blasts (Figure 6B). In both cases, significant increases in the production of IL-6 and IL-8 were observed following CpG ODN stimulation. In the case of sample A199, IL-10 was also significantly up-regulated.
Response of NOD-SCID expanded primary ALL to CpG stimulation. (A) Patient ALL samples A199 and A286 were stimulated with a panel of CpG ODNs for 48 hours and then analyzed by flow cytometry for changes in CD40 expression. The results are expressed as the change in percentage of CD40+ cells following stimulation with the indicated CpG ODNs. The results shown are the mean ± standard deviation for 3 independent experiments. (B) The ALL samples (A199, left; A286, right) were either untreated (▦) or stimulated with CpG 2006 (▪) for 48 hours and the culture supernatant was analyzed for cytokine content by CBA. The results shown are the means ± standard deviation for 3 independent experiments. * indicates P < .05; ** indicates P < .005.
Response of NOD-SCID expanded primary ALL to CpG stimulation. (A) Patient ALL samples A199 and A286 were stimulated with a panel of CpG ODNs for 48 hours and then analyzed by flow cytometry for changes in CD40 expression. The results are expressed as the change in percentage of CD40+ cells following stimulation with the indicated CpG ODNs. The results shown are the mean ± standard deviation for 3 independent experiments. (B) The ALL samples (A199, left; A286, right) were either untreated (▦) or stimulated with CpG 2006 (▪) for 48 hours and the culture supernatant was analyzed for cytokine content by CBA. The results shown are the means ± standard deviation for 3 independent experiments. * indicates P < .05; ** indicates P < .005.
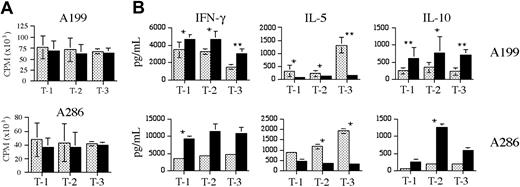
To determine the outcome of CpG ODN–induced changes on T-cell responses to ALL blasts, we compared the allogeneic T-cell response against CpG-treated or untreated A199 and A286 blasts, using T cells purified from 3 healthy donors. No significant change in T-cell proliferation was observed for any combination of donor T-cell and ALL sample (Figure 7A). Despite the lack of change in T-cell proliferative responses, statistically significant changes in T-cell cytokine production were observed (Figure 7B). For all 3 T-cell populations, increases in interferon γ (IFN-γ) and IL-10 and decreases in IL-5 production were observed in response to both CpG-stimulated ALL samples. We have observed similar changes in T-cell cytokine production in response to 3 of 4 CpG ODN–stimulated precursor B-ALL bone marrow biopsy samples, indicating that this Th1 skewing can occur in the presence of other bone marrow cells (data not shown).
Allogeneic T-cell responses to unstimulated or CpG-stimulated primary precursor B-ALL samples. A199 and A286 purified ALL cells were incubated without (▦) or with (▪) CpG 2006 for 48 hours, irradiated, and then incubated with healthy donor T cells at a ratio of 1:3 for 5 days. T-1, T-2, and T-3 represent 3 different T-cell donors. (A) The proliferative response of the allogeneic T cells is shown as the average count per minute (CPM) ± standard deviation for 3 independent experiments. The proliferation of unstimulated T cells alone and irradiated ALL cells alone was always less than 1000 CPM. (B) The levels of IFN-γ, IL-5, and IL-10 in the T-cell supernatants, as determined by CBA analysis. Results shown are the mean ± standard deviation for 3 independent experiments for A199, and a representative of 2 independent experiments for A286 (shown as mean ± standard deviation for 2 replicates). * indicates P < .05; ** indicates P < .005.
Allogeneic T-cell responses to unstimulated or CpG-stimulated primary precursor B-ALL samples. A199 and A286 purified ALL cells were incubated without (▦) or with (▪) CpG 2006 for 48 hours, irradiated, and then incubated with healthy donor T cells at a ratio of 1:3 for 5 days. T-1, T-2, and T-3 represent 3 different T-cell donors. (A) The proliferative response of the allogeneic T cells is shown as the average count per minute (CPM) ± standard deviation for 3 independent experiments. The proliferation of unstimulated T cells alone and irradiated ALL cells alone was always less than 1000 CPM. (B) The levels of IFN-γ, IL-5, and IL-10 in the T-cell supernatants, as determined by CBA analysis. Results shown are the mean ± standard deviation for 3 independent experiments for A199, and a representative of 2 independent experiments for A286 (shown as mean ± standard deviation for 2 replicates). * indicates P < .05; ** indicates P < .005.
It has been reported recently that stimulation of B cells with CD40 ligand and CpG ODNs together results in the synergistic production of several cytokines.19 To determine if such an event occurred with precursor B-ALL cells, we incubated A199 and A286 cells with CpG 2006, CD40 ligand–expressing fibroblasts, or both CpG 2006 and CD40 ligand fibroblasts, and measured the cytokine production after 48 hours. Figure 8 shows that for both ALL samples, the combination of CpG ODN and CD40 ligand stimulation resulted in profound increases in IL-6 production over the increases seen with individual stimulation. A similar effect was seen for IL-8 and IL-10 production by A199 but not by A286. For both ALL samples in all stimulation conditions, IL-12p70 levels were less than 20 pg/mL, the lower detection limit for CBA cytokine measurement (data not shown).
Synergistic response of ALL cells to stimulation by CpG ODN and CD40 ligand. Precursor B-ALL cells were incubated for 48 hours either unstimulated or in the presence of CpG 2006, CD40L-expressing fibroblasts, or both 2006 and CD40L. The cell supernatants were then analyzed for the presence of IL-6, IL-8, and IL-10. IL-12p70 was not detectable under any of the assay conditions. The results are shown as the mean ± standard deviation for 2 independent experiments.
Synergistic response of ALL cells to stimulation by CpG ODN and CD40 ligand. Precursor B-ALL cells were incubated for 48 hours either unstimulated or in the presence of CpG 2006, CD40L-expressing fibroblasts, or both 2006 and CD40L. The cell supernatants were then analyzed for the presence of IL-6, IL-8, and IL-10. IL-12p70 was not detectable under any of the assay conditions. The results are shown as the mean ± standard deviation for 2 independent experiments.
Discussion
The results of this study demonstrate the responsiveness of precursor B-ALL cells to stimulation by CpG ODNs. It has been reported previously that ALL cell lines do not express TLR9.17 This conclusion was based on the analysis of 2 lines, Nalm6 and 697. Consistent with this previous report, we observe no response to CpG ODNs by these 2 lines, and detect only very low levels of TLR9 expression by them. However, for the 6 additional lines analyzed in our study, 5 show detectable responses to CpG stimulation and TLR9 expression. For the 3 highest responding cell lines, detectable responses to the non-CpG ODN 2137 were also observed. This is consistent with previous findings showing that the phosphorothioate backbone of ODNs can mediate stimulatory activity.20 Together, these observations indicate that the expression level of TLR9 is the primary determinant of responsiveness to CpG stimulation by B-lineage ALL cells. From the limited panel of cell lines that we analyzed, it is not possible to define any characteristics common to the CpG responsive or nonresponsive groups. Interestingly, however, 3 of the lines, Sup-B15, KOPN57bi, and OP-1, are positive for the t(9;22)(q34;q11) translocation, and all 3 show detectable responses to CpG stimulation, albeit to differing degrees. It remains to be determined whether CpG responsiveness is characteristic of some precursor B-ALL subtypes and not others.
The results obtained from cell lines and primary ALL blasts, from both bone marrow and peripheral blood, show a consistent response to CpG stimulation. This response is characterized by a detectable increase in CD40 expression, but an absence of induction of CD80 expression and only a small change in CD86 expression. This pattern is in marked contrast to that observed with normal B cells, and differs from the response of nonleukemic B-cell precursors. These findings implicate the transformation of precursor B cells in the altered response to CpG ODNs. However, it is possible that leukemic ALL cells show a response characteristic of B cells at particular stages of differentiation, but that is masked in normal bone marrow by the response of pre-B cells at other stages. The similarity of the costimulatory molecule response of the 2 purified primary samples studied contrasts with their differing cytokine response and emphasizes that more variables than just TLR9 expression contribute to the outcome of precursor B-ALL interaction with CpG ODNs. Differential signaling of immature and mature B cells has been described in other systems.21,22 The distinct response to CpG ODNs may be influenced by alternative signaling components and/or target gene accessibility in B-cell development stages.
The response of B-lineage ALL cells is distinct from the responses of other malignant B-cell types, most notably in regard to CD80 and CD86 up-regulation. B-cell chronic lymphocytic leukemia, large cell lymphoma, mantle cell lymphoma, and marginal zone lymphoma have all been reported to up-regulate both CD80 and CD86 expression in response to CpG ODNs.12 Our data suggest that this difference from precursor B-ALL may reflect the degree of differentiation of the malignant B cell. Interestingly, differences between the responses of mature malignant B cells have also been observed, including a stronger proliferative response of marginal zone lymphoma cells compared with B-CLL cells.12 In addition, the lack of increased proliferation observed with B-lineage ALL cell lines is also observed with A20 murine lymphoma cells.23 Overall, these results emphasize that while most malignant B cells do respond to CpG ODNs, the nature of that response is determined by the malignant cell type involved. In addition to augmenting leukemia cell immunogenicity, CpG ODN stimulation has been shown to enhance the susceptibility of B-CLL cells to other therapeutic interventions, including antisense DNA and immunotoxins.24,25 Further investigation into the utility of other CpG-effected mechanisms in pre-B-ALL is required.
Studies using mouse models have shown that immune responses dominated by Th1 cytokine-producing T cells exert a more potent antitumor effect and are required for the generation of effective antitumor cytotoxic T lymphocytes (CTLs).26-29 In this context, the demonstration of altered T-cell cytokine production in response to CpG ODN–stimulated pre-B-ALL bone marrow is exciting. It has been reported that T cells from patients with ALL show a bias toward Th2 cytokine production,30,31 and we have shown that relapsed precursor B-ALL preferentially induces proliferation of IL-5–producing T cells.32 The ability of CpG ODN stimulation to enhance IFN-γ production, while simultaneously reducing IL-5 release by allogeneic T cells responding to ALL cells, may provide a strategy to skew responses back toward a Th1 profile and thus enhance anti-ALL immune responses. It has been shown that CpG ODNs are capable of switching an established Th2 response to a Th1 response in experimental models of allergic disease.33,34 Such ability may have important implications for the treatment of B-lineage ALL.
The ability of CpG ODN stimulation of ALL blasts to skew T-cell responses in this study is clear, but the mechanism underlying this switch is not. The production of IL-12p70 is thought to be a critical contributor to the development of Th1 responses. It has been shown that combined CD40 ligand and CpG ODN signaling results in synergistic production of IL-6, IL-10, and IL-12 by B cells, and that it is the IL-12 that mediates development of a Th1 response.19 While we observed a similar synergism in the production of IL-6 for both samples, and IL-8 and IL-10 for sample A199 (Figure 8), IL-12p70 production was undetectable in all conditions. It remains to be determined whether CpG ODN–stimulated ALL cells produce sufficient IL-12p70, although not detected in this study, to drive the Th1 response or whether another mechanism is involved. It is noteworthy that in the previous report,19 IL-12p70 concentrations of 30 pg/mL and less were sufficient to drive Th1 responses and that induction of Th1 responses in the absence of detectable IL-12 has been reported.35
Precursor B-ALL cells have been shown in several studies to be poor stimulators of T-cell responses and the lack of costimulatory molecule expression can lead to the generation of T-cell anergy.13,36 It was reported recently that CD40 activation of precursor B-ALL did not lead to the generation of efficient antigen-presenting cells and failed, in some cases, to prevent anergy induction.36 The inability of CpG ODN stimulation to significantly up-regulate CD80 or CD86 on ALL cells or augment allogeneic T-cell proliferative responses suggests that this strategy may likewise be unable to prevent T-cell anergy. However, for the many precursor B-ALL cells that express significant levels of CD86, as was the case for samples A199 and A286 used in this study, the generation of T-cell anergy is not well documented. In such cases, the ability to skew the stimulated T cells toward a Th1 response may be clinically useful.
Bone marrow transplantation is a curative therapy for relapsed pediatric ALL and its activity is mediated primarily through the GVL effect. The GVL effect against ALL correlates strongly with the development of graft-versus-host disease (GVHD) in patients.16 In addition, it has been reported that the loss of antihost alloreactivity may lead to leukemia relapse after bone marrow transplantation.37 The use of CpG ODNs in a bone marrow transplantation setting in mice has been shown to increase both GVHD and GVL.38 The results presented here, showing the skewing of allogeneic T responses against pre-B-ALL toward a Th1 profile, suggest that CpG ODNs may have use in posttransplantation settings where the controlled development of GVHD may significantly enhance the elimination of leukemia cells. In addition, leukemia-reactive T cells have been isolated from pre-B-ALL patients.39,40 The use of CpG ODNs may provide a strategy to enhance the activation of these T cells in vivo.
Prepublished online as Blood First Edition Paper, January 13, 2005; DOI 10.1182/blood-2004-06-2468.
Supported in part by a Children's Oncology Group Young Investigator award to G.S.D.R.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Drs Arthur Krieg and Donald Macfarlane for providing ODNs and for helpful suggestions and insight; Dr Gordon Freeman for providing CD40L-expressing fibroblasts; and Dr Dario Campana for providing ALL cell lines. Excellent technical assistance was provided by Stacey Grubb, Gregory Doho, and Sharon Bader.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal