Abstract
In vitro studies suggest that Ras activation is necessary for erythroid cell development. However, genetic inactivation of the Ras isoforms H-Ras, N-Ras, and K-Ras in mice reportedly did not affect adult or fetal erythropoiesis, though K-Ras-/- embryos were anemic. Given these discrepancies, we performed a more detailed analysis of fetal erythropoiesis in K-Ras-/- embryos. Day-13.5 K-Ras-/- embryos were pale with a marked reduction of mature erythrocytes in their fetal livers. The frequency and number of both early (erythroid burst-forming unit [BFU-E]) and late erythroid progenitors (erythroid colony-forming unit [CFU-E]) were reduced in K-Ras-/- fetal livers compared with wild-type controls and displayed a delay in terminal erythroid cell maturation. Further, K-Ras-/- hematopoietic progenitors had reduced proliferation in response to erythropoietin and Kit ligand compared with control cells. Thus, these studies identify K-Ras as a unique Ras isoform that is essential for regulating fetal erythropoiesis in vivo.
Introduction
Ras activation following binding of erythropoietin (EPO) and kit-ligand (KitL) to their receptors is essential for the differentiation, proliferation, and survival of erythroid progenitors in vitro.1-10 In mammals there exist 3 homologous Ras proteins, H-Ras, N-Ras, and K-Ras (reviewed in Scheele et al,11 Ehrhardt et al,12 and Rebollo and Martinez13 ). While H-Ras-/-, N-Ras-/-, and doubly mutant H-Ras-/-; N-Ras-/- knockout mice have no overt hematopoietic cell phenotypes,14,15 K-Ras-/- mice die between days 12.5 and 14 of gestation with fetal liver defects and evidence of anemia.16,17 However, when a heterogenous population of K-Ras-/- fetal liver cells was plated in methylcellulose assays, only a slight decrease in erythroid progenitor cell frequency was observed.16 Based on these results, the anemia observed in K-Ras-/- embryos was attributed to a defect in the fetal liver microenvironment. Thus, despite in vitro studies supporting a role for Ras in regulating erythroid cell function, in vivo studies have not identified a Ras isoform essential for erythropoiesis. Given these discrepancies, we performed a more detailed analysis of erythropoiesis in K-Ras-/- embryos by testing erythroid cell differentiation with a newly developed flow cytometric assay18 and the erythroid colony-forming ability of a purified population of hematopoietic progenitor cells.
Study design
Mice and fetal hematopoietic cell isolation
K-Ras-/- mice were obtained from Jackson Laboratories (Bar Harbor, ME) in a 129/SV background. Studies were conducted with a protocol approved by the Indiana University Animal Care and Use Committee. The K-Ras allele was genotyped by polymerase chain reaction as previously described.16 K-Ras+/- mice were mated to produce day-13.5 K-Ras-/- and wild-type (WT) embryos. Fetal livers were isolated as previously described.19 Single-cell suspensions were prepared by pushing hepatic tissues through a 23-gauge needle.
Fetal liver touch preparations
Fetal liver touch preparations were performed as previously described19 from day-13.5 WT and K-Ras-/- embryos and stained with Wright Giemsa (Dade Behring, Newark, DE). Photomicrographs of touch preparations were taken with an Olympus DP11 microscope (Melville, NY).
Colony assays
Recombinant murine KitL and granulocyte macrophage-colony stimulating factor (GM-CSF) were obtained from Peprotech (Rocky Hill, NJ), and EPO was obtained from Amgen (Thousand Oaks, CA). Erythroid burst-forming unit (BFU-E), erythroid colony-forming unit (CFU-E), and granulocyte-macrophage colony-forming unit (CFU-GM) assays were performed exactly as previously described.19
C-kit+ cell isolation
Fetal liver cells were incubated with 5 μg of a fluorescein isothiocyanate (FITC)–conjugated c-kit monoclonal antibody (Pharmingen, San Diego, CA) per 106 cells, placed on ice for 30 minutes, pelleted, washed, and resuspended in phosphate-buffered saline. C-kit+ cells were purified by immunomagnetic bead enrichment as previously described.19
Apoptosis, proliferation, and differentiation assays
Sorted c-kit+ fetal liver cells were stained with annexin V–FITC (Pharmingen) exactly per manufacturer's protocol followed by flow cytometric analysis (FACS) as previously described.19 For survival assays, 10 000 c-kit+ fetal liver cells were plated in 96-well plates with no serum in the presence of varying concentrations of either EPO or KitL alone or in combination. After 48 hours in culture, cells were stained with annexin V–FITC followed by FACS analysis. For thymidine incorporation assays, 10 000 c-kit+ fetal liver cells were plated in 96-well plates in serum-free medium (X-VIVO 10; CAMBREX, Walkersville, MD) in the presence of varying concentrations of either EPO or KitL alone or in combination. After 48 hours in culture, cells were pulsed with tritiated thymidine (New Life Sciences, Boston, MA) for 16 to 24 hours, harvested on glass-fiber filters, and β emission was measured. In some experiments, freshly isolated c-kit+ cells were cultured in the presence of both KitL and EPO for 24, 48, and 72 hours to differentiate the cells to later stages of erythroid cell maturation. After culture at each time point, survival and proliferation assays were performed exactly as described in this section.
For the differentiation assay, fetal liver cells were stained with anti-CD71–FITC (Pharmingen), anti–Ter-119–phycoerythrin (PE; Pharmingen), and anti–c-kit–allophycocyanin (APC; Pharmingen) antibodies followed by FACS analysis as previously established.18
Results and discussion
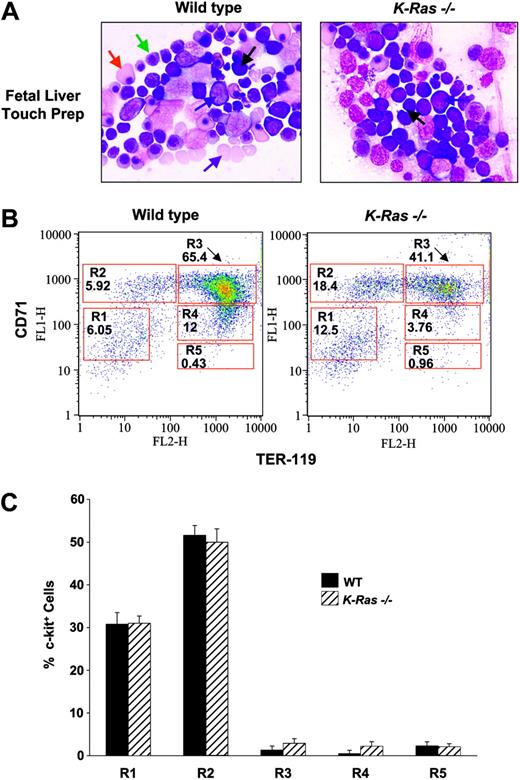
To determine the effect of K-Ras deficiency on fetal liver erythropoiesis, K-Ras+/- mice were intercrossed and day-13.5 embryos were harvested. As previously shown,16 K-Ras-/- embryos and fetal livers were small and pale compared with WT controls (data not shown). K-Ras-/- day-13.5 fetal liver touch preparations demonstrated an increase in immature proerythroblasts and early basophilic erythroblasts and a striking decrease in late basophilic, chromatophilic, and orthochromatophilic erythroblasts (Figure 1A). Further, there were very few mature enucleated erythrocytes in K-Ras-/- fetal livers (Figure 1A). In contrast, there was a continuum of erythropoiesis in WT fetal livers with mature enucleated erythrocytes identified throughout the fetal liver (Figure 1A).
Effect of K-Ras deficiency on fetal liver erythropoiesis. (A) Representative fetal liver touch preps from WT and K-Ras-/- day-13.5 embryos. Touch preparations were stained with Wright Giemsa. Numerous erythropoietic cells in all stages of differentiation are observed in WT fetal livers and are represented as follows: proerythroblasts by a blue arrow; basophilic erythroblasts by a black arrow; polychromatophilic erythroblasts by a green arrow; orthochromatophilic erythroblasts by a red arrow; and mature enucleated erythrocytes by a purple arrow. In contrast, a marked increase in proerythroblasts (blue arrow) and basophilic erythroblasts (black arrow) was observed in K-Ras-/- fetal livers with a concomitant decrease in more mature polychromatophilic and orthochromatophilic erythroblasts and a paucity of enucleated erythrocytes. Slides were visualized under an Olympus BX51 microscope (Olympus, Melville, NY) equipped with a UPlan FI 40 ×/0.75 objective lens. Photomicrographs were acquired with an Olympus DP11 camera using MicroSuite imaging software (Olympus). Five other experiments showed similar results. (B) Representative flow cytometric analysis of erythroid differentiation in vivo. Freshly isolated day-13.5 WT or K-Ras-/- fetal livers were doubly stained with anti-CD71–FITC and anti–Ter-119–PE. Regions R1 to R5 define distinct populations of erythroid progenitor cells at different stages of differentiation as described previously.18 Primitive progenitor cells (including mature BFU-Es and CFU-Es) are shown in R1, proerythroblasts and early basophilic erythroblasts in R2, early and late basophilic erythroblasts in R3, chromatophilic and orthochromatophilic erythroblasts in R4, and late orthochromatophilic and reticulocytes in R5, as previously described.18 Numbers represent the percentage of cells within that region. Day-13.5 wild-type fetal livers display normal distribution of erythroid cells at different stages of differentiation. K-Ras-/- fetal livers demonstrate a delay in differentiation at the basophilic erythroblast level: R2 to R3. Five other experiments showed similar results. (C) Percentage of freshly isolated K-Ras-/- or WT day-13.5 fetal liver cells expressing c-kit in regions R1 to R5, which define distinct populations of erythroid progenitor cells at different stages of differentiation. Freshly isolated day-13.5 WT or K-Ras-/- fetal livers were stained with anti-CD71–FITC, anti–Ter-119–PE, and anti–c-kit–APC antibodies as described in “Apoptosis, proliferation, and differentiation assays.” The majority of c-kit+ cells were identified in the more immature erythroid progenitor populations in R1 and R2 and no significant difference in the percentage of c-kit+ cells was observed in the different gated populations (R1-R5) between the 2 genotypes. Results represent the mean percentage of c-kit+ cells and error bars represent the standard error of the mean of 5 parallel independent experiments from embryos isolated from the same litter.
Effect of K-Ras deficiency on fetal liver erythropoiesis. (A) Representative fetal liver touch preps from WT and K-Ras-/- day-13.5 embryos. Touch preparations were stained with Wright Giemsa. Numerous erythropoietic cells in all stages of differentiation are observed in WT fetal livers and are represented as follows: proerythroblasts by a blue arrow; basophilic erythroblasts by a black arrow; polychromatophilic erythroblasts by a green arrow; orthochromatophilic erythroblasts by a red arrow; and mature enucleated erythrocytes by a purple arrow. In contrast, a marked increase in proerythroblasts (blue arrow) and basophilic erythroblasts (black arrow) was observed in K-Ras-/- fetal livers with a concomitant decrease in more mature polychromatophilic and orthochromatophilic erythroblasts and a paucity of enucleated erythrocytes. Slides were visualized under an Olympus BX51 microscope (Olympus, Melville, NY) equipped with a UPlan FI 40 ×/0.75 objective lens. Photomicrographs were acquired with an Olympus DP11 camera using MicroSuite imaging software (Olympus). Five other experiments showed similar results. (B) Representative flow cytometric analysis of erythroid differentiation in vivo. Freshly isolated day-13.5 WT or K-Ras-/- fetal livers were doubly stained with anti-CD71–FITC and anti–Ter-119–PE. Regions R1 to R5 define distinct populations of erythroid progenitor cells at different stages of differentiation as described previously.18 Primitive progenitor cells (including mature BFU-Es and CFU-Es) are shown in R1, proerythroblasts and early basophilic erythroblasts in R2, early and late basophilic erythroblasts in R3, chromatophilic and orthochromatophilic erythroblasts in R4, and late orthochromatophilic and reticulocytes in R5, as previously described.18 Numbers represent the percentage of cells within that region. Day-13.5 wild-type fetal livers display normal distribution of erythroid cells at different stages of differentiation. K-Ras-/- fetal livers demonstrate a delay in differentiation at the basophilic erythroblast level: R2 to R3. Five other experiments showed similar results. (C) Percentage of freshly isolated K-Ras-/- or WT day-13.5 fetal liver cells expressing c-kit in regions R1 to R5, which define distinct populations of erythroid progenitor cells at different stages of differentiation. Freshly isolated day-13.5 WT or K-Ras-/- fetal livers were stained with anti-CD71–FITC, anti–Ter-119–PE, and anti–c-kit–APC antibodies as described in “Apoptosis, proliferation, and differentiation assays.” The majority of c-kit+ cells were identified in the more immature erythroid progenitor populations in R1 and R2 and no significant difference in the percentage of c-kit+ cells was observed in the different gated populations (R1-R5) between the 2 genotypes. Results represent the mean percentage of c-kit+ cells and error bars represent the standard error of the mean of 5 parallel independent experiments from embryos isolated from the same litter.
Based on this observation, we next compared erythroid cell differentiation in vivo between K-Ras-/- and WT fetal livers. We used a recently developed flow cytometric assay, which readily distinguishes various stages of erythroid cell differentiation by analyzing the cell surface expression of CD71 and Ter-119.18 Consistent with our phenotypic observations (Figure 1A), we observed a delay in terminal erythroid differentiation in K-Ras-/- fetal livers compared with WT controls (Figure 1B). Specifically, K-Ras-/- fetal livers had a marked increase in early progenitor and proerythroblasts (region 1 [R1]; 12% vs 6%) and in early basophilic erythroblasts (R2; 18% vs 6%) with a decrease in more mature late basophilic erythroblasts (R3; 41% vs 65%) and chromatophilic and orthochromatophilic erythroblasts (R4; 4% vs 12%; Figure 1B). To further characterize the delay in erythroid cell maturation observed in K-Ras-/- fetal livers, we costained fetal liver cells with antibodies directed against CD71, Ter-119, and c-kit and analyzed the cells by FACS. Interestingly, c-kit+ cells were observed mostly in the R1 and R2 populations, which define early progenitors, proerythroblasts, and early basophilic erythroblasts, but there was no significant difference in the percentage of c-kit+ cells in the different gated populations (R1-R5) between the 2 genotypes (Figure 1C). However, since there is an increased percentage of cells in R1 and R2 in K-Ras-/- fetal livers (Figure 1B), these data indicate that there is an overall increase in the percentage of c-kit+ cells in K-Ras-/- fetal livers compared with WT controls, which is consistent with an increased percentage of early erythroid progenitors and a delay in erythroid maturation observed in K-Ras-/- day-13.5 embryos. Collectively, these results argue that K-Ras is important for normal fetal liver erythroid cell differentiation in vivo.
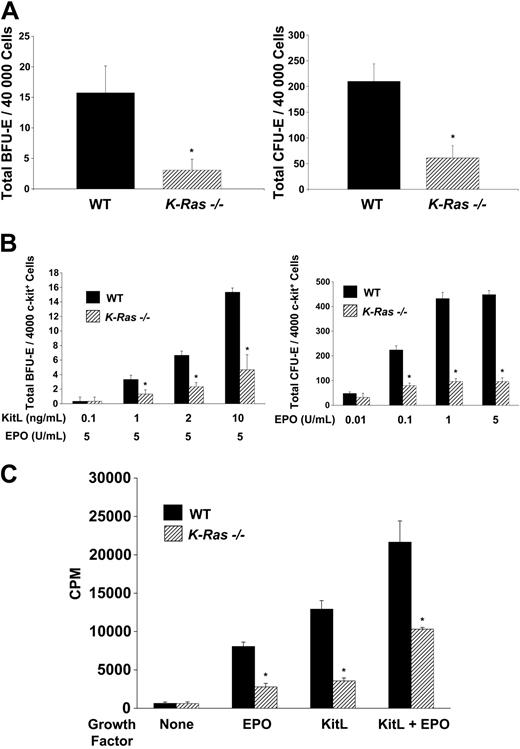
We next assayed for early (BFU-E) and late (CFU-E) erythroid progenitors in WT and K-Ras-/- fetal livers. The frequency of BFU-Es and CFU-Es in day-13.5 K-Ras-/- livers were reduced compared with WT controls (Figure 2A). To directly test the effect of K-Ras deficiency on erythroid colony formation independent of the effects of the microenvironment, we performed BFU-E and CFU-E assays with equal numbers of sorted WT and K-Ras-/- c-kit+ cells. To confirm that there were no differences in cell viability, an aliquot of sorted c-kit+ cells was stained with annexin V to identify apoptotic cells. K-Ras-/- and WT c-kit+ cells had a similar percentage of annexin V–positive cells (data not shown). A marked reduction in the frequency of both BFU-Es and CFU-Es was observed in K-Ras-/- c-kit+ cells compared with WT controls (Figure 2B). Since the number of both BFU-Es and CFU-Es generated from K-Ras-/- c-kit+ cells were reduced, these data argue that K-Ras activation is important for the proliferation and/or survival of both early and late erythroid progenitors in response to EPO and KitL. Interestingly, the number of myeloid colonies (CFU-GMs) generated in response to GM-CSF from K-Ras-/- c-kit+ fetal liver cells was reduced compared with WT controls (30.17 ± 2.29 vs 184.13 ± 13.08; n = 5; P < .001), indicating that K-Ras activation may also be essential for the development of other hematopoietic cell lineages. Nevertheless, these results argue that an intrinsic K-Ras-/- hematopoietic progenitor cell defect contributes at least in part to reduction of erythroid progenitors and mature erythrocytes in day-13.5 K-Ras-/- fetal livers.
Erythroid colony formation and proliferation of K-Ras-/- and WT c-kit+ day-13.5 fetal liver cells in response to EPO and KitL. (A) Frequency of erythroid progenitors in WT and K-Ras-/- nonfractionated day-13.5 fetal liver cells. Total nucleated fetal liver cells (40 000 cells/mL) were plated for growth of BFU-Es in methylcellulose medium supplemented with 10 ng/mL KitL and 5 U/mL EPO. For CFU-Es, total nucleated fetal liver cells (40 000 cells/mL) were plated in methylcellulose medium supplemented with 5 U/mL EPO. CFU-Es and BFU-Es were counted by indirect microscopy at days 2 and 7 of culture, respectively. Results represent the mean number of colonies and error bars represent the standard error of the mean of 5 parallel independent experiments, which were performed in triplicate from embryos isolated from the same litter. *P < .05 by Student paired t test. (B) Frequency of erythroid progenitors in c-kit+–sorted cells isolated from day-13.5 WT and K-Ras-/- fetal livers. C-kit+ cells were isolated by immunomagnetic bead enrichment and sorted as described in “C-kit+ cell isolation” to a purity of more than 85% as tested by fluorescence cytometry (data not shown). C-kit+ cells (4000 cells/mL) were plated for growth of BFU-Es in methylcellulose medium containing various concentrations of KitL as indicated combined with a single concentration of EPO (5 U/mL). C-kit+ cells (4000 cells/mL) were plated for growth of CFU-Es in methylcellulose medium containing various concentrations of EPO alone as indicated. CFU-Es and BFU-Es were counted by indirect microscopy at days 2 and 7 of culture, respectively. Results represent the mean number of colonies per 4000 c-kit+ ± SEM of 5 parallel independent experiments. Colony assays were performed in triplicate from c-kit+ cells isolated from embryos isolated from the same litter. *P < .05 by Student paired t test. (C) Proliferation of WT and K-Ras-/- c-kit+ day-13.5 fetal liver cells in response to no growth factors, EPO and KitL alone, or in combination. Freshly isolated c-kit+ fetal liver cells were plated in a 96-well plate at a concentration of 10 000 cells/well in serum-free medium with no additional growth factors or 10 ng/mL KitL and 5 U/mL EPO in combination or alone. After 48 hours of culture, cells were pulsed with tritiated thymidine and harvested 16 hours later for measurement of β emission. CPM indicates counts per minute. Results represent the mean thymidine incorporation ± SEM of 5 independent experiments. Thymidine incorporation assays were performed in triplicate from c-kit+ cells isolated from embryos harvested from the same litter. *P < .05 by Student paired t test.
Erythroid colony formation and proliferation of K-Ras-/- and WT c-kit+ day-13.5 fetal liver cells in response to EPO and KitL. (A) Frequency of erythroid progenitors in WT and K-Ras-/- nonfractionated day-13.5 fetal liver cells. Total nucleated fetal liver cells (40 000 cells/mL) were plated for growth of BFU-Es in methylcellulose medium supplemented with 10 ng/mL KitL and 5 U/mL EPO. For CFU-Es, total nucleated fetal liver cells (40 000 cells/mL) were plated in methylcellulose medium supplemented with 5 U/mL EPO. CFU-Es and BFU-Es were counted by indirect microscopy at days 2 and 7 of culture, respectively. Results represent the mean number of colonies and error bars represent the standard error of the mean of 5 parallel independent experiments, which were performed in triplicate from embryos isolated from the same litter. *P < .05 by Student paired t test. (B) Frequency of erythroid progenitors in c-kit+–sorted cells isolated from day-13.5 WT and K-Ras-/- fetal livers. C-kit+ cells were isolated by immunomagnetic bead enrichment and sorted as described in “C-kit+ cell isolation” to a purity of more than 85% as tested by fluorescence cytometry (data not shown). C-kit+ cells (4000 cells/mL) were plated for growth of BFU-Es in methylcellulose medium containing various concentrations of KitL as indicated combined with a single concentration of EPO (5 U/mL). C-kit+ cells (4000 cells/mL) were plated for growth of CFU-Es in methylcellulose medium containing various concentrations of EPO alone as indicated. CFU-Es and BFU-Es were counted by indirect microscopy at days 2 and 7 of culture, respectively. Results represent the mean number of colonies per 4000 c-kit+ ± SEM of 5 parallel independent experiments. Colony assays were performed in triplicate from c-kit+ cells isolated from embryos isolated from the same litter. *P < .05 by Student paired t test. (C) Proliferation of WT and K-Ras-/- c-kit+ day-13.5 fetal liver cells in response to no growth factors, EPO and KitL alone, or in combination. Freshly isolated c-kit+ fetal liver cells were plated in a 96-well plate at a concentration of 10 000 cells/well in serum-free medium with no additional growth factors or 10 ng/mL KitL and 5 U/mL EPO in combination or alone. After 48 hours of culture, cells were pulsed with tritiated thymidine and harvested 16 hours later for measurement of β emission. CPM indicates counts per minute. Results represent the mean thymidine incorporation ± SEM of 5 independent experiments. Thymidine incorporation assays were performed in triplicate from c-kit+ cells isolated from embryos harvested from the same litter. *P < .05 by Student paired t test.
Since these data establish that K-Ras is essential for erythropoiesis in vivo, we next tested whether the K-Ras-/- erythroid phenotype was also linked to either a decrease in proliferation and/or survival of hematopoietic progenitors in response to KitL or EPO. We performed thymidine incorporation assays using K-Ras-/- and WT c-kit+ cells. Remarkably, K-Ras-/- c-kit+ cells displayed a 50% to 60% decrease in proliferation in response to either EPO or KitL alone and EPO and KitL in combination (Figure 2C). Similar differences in proliferation between the 2 experimental genotypes were obtained when WT and K-Ras-/- c-kit+ cells were differentiated to later stages of erythroid cell maturation in vitro and thymidine incorporation assays were performed with the different populations of erythroid progenitors in response to either EPO or KitL alone or in combination (data not shown). One explanation for this result is that K-Ras-/- c-kit+ cells were undergoing an accelerated rate of apoptosis. To test this possibility, we compared the survival of K-Ras-/- and WT c-kit+ cells in response to either EPO and/or KitL in the absence of serum. Both K-Ras-/- and WT c-kit+ cells had a similar percentage of annexin V–positive cells at the initiation of the experiment (data not shown). After 24, 48, and 72 hours in culture, no differences were detected in the percentage of K-Ras-/- or WT c-kit+ apoptotic cells (annexin V–positive) in response to either EPO or KitL alone or in combination (data not shown). Thus, these data indicate that K-Ras activation is essential for the proliferation of erythroid progenitor cells in response to both EPO and KitL.
These studies establish a previously unrecognized role for K-Ras in regulating fetal erythropoiesis in vivo. Specifically, K-Ras is essential for the differentiation of erythroid progenitor cells to late basophilic erythroblasts and the proliferation of hematopoietic progenitors in response to EPO and KitL. Recent data demonstrate that Ras isoforms undergo differential posttranslational modification (reviewed in Ehrhardt et al,12 Bar-Sagi,20 and Hancock et al21 ) and compartmentalization within the cell (reviewed in Prior and Hancock,22 Hancock,23 and Chiu et al24 ) and that individual Ras isoforms can differentially activate discrete signaling pathways (reviewed in Ehrhardt et al12 ). Our data provide evidence that single Ras isoforms may also provide unique signals in hematopoietic cells in vivo.
Prepublished online as Blood First Edition Paper, January 11, 2005; DOI 10.1182/blood-2004-05-2021.
Supported by 1 KO8 CA096579-01 (D.A.I.). D.A.I. is a recipient of a Basil O'Connor Award from the March of Dimes (5-FY02-254).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal