Abstract
Hematopoietic progenitors committed to the myeloid lineage, the common myeloid and granulocyte-monocyte progenitors (CMP/GMP), have been shown to protect against opportunistic pathogens following myeloablative radiation; however, the efficacy of this approach has not been studied in the setting of chemotherapy-induced neutropenia. In this mouse model, the infusion of CMP/GMP on the day after 5-fluorouracil (5-FU) administration (D+1) resulted in a significant increase in the number of splenic neutrophils by D+8 when compared with 5-FU–only controls (P = .02), the majority of which were CMP/GMP-derived (54%). Moreover, 19% and 28% of neutrophils in the blood and bone marrow, respectively, were CMP/GMP-derived. Survival following intranasal challenge with the fungus Aspergillus fumigatus was significantly higher in CMP/GMP-infused mice than the controls (56% and 33% respectively; P = .019). Thus, a single infusion of CMP/GMP enhances tissue neutrophil content and increases survival against a lethal challenge with A fumigatus in the setting of chemotherapy-induced neutropenia.
Introduction
The use of chemotherapy for treatment of hematologic diseases commonly results in neutropenia, rendering the patient more susceptible to bacterial and fungal infections.1 The increased morbidity and mortality along with the seemingly inexorable emergence of resistance to antimicrobials underscore the need for alternative therapies. Two strategies are currently employed to circumvent neutropenia. The first is the administration of growth factors such as granulocyte colony-stimulating factor (G-CSF), which has been shown to shorten the duration of neutropenia but has not consistently improved survival.2-4 The second is the infusion of mature granulocytes. This procedure is severely hampered by the need for repeated transfusions and the inability to store the large number of cells needed.5-7
The recently characterized common myeloid progenitors (CMPs) and granulocyte-monocyte progenitors (GMPs) are subpopulations of progenitor cells that are committed to the myeloid lineages.8 The biology of CMPs and GMPs predicts that infusion of these cells would be beneficial in the amelioration of neutropenia. In a preclinical model of hematopoietic cell transplantation following myeloablative radiation, we demonstrated that rapid engraftment of myeloid lineages was achieved following transplantation of a graft enriched with CMPs and GMPs. 9 Moreover, the progenitor-derived cells protected against lethal infections with the ubiquitous fungus Aspergillus fumigatus and the bacteria Pseudomonas aeruginosa.
Here, we report that, in a mouse model of neutropenia induced by the chemotherapeutic agent 5-fluorouracil (5-FU), a single infusion of CMPs and GMPs is sufficient to protect against a lethal challenge with A fumigatus.
Study design
Mice
The F1 generation of the congenic strains of C57BL/Ka-Thy1.1 (CD45.2) and C57BL/Ka-Thy1.1 (CD45.1) mice was used for host mice. Donor myeloid cell progenitors were purified from the C57BL/Ka-Thy1.2 (CD45.1) strain. All mice were bred and maintained at the animal care facility at Stanford University School of Medicine. Donor mice were used at 6 to 8 weeks and recipients at 12 to 16 weeks.
5-FU treatment
A single dose of 150 mg/kg 5-FU (American Pharmaceutical Partners, Schaumburg, IL) was administered intravenously 30 hours prior to myeloid progenitor cell transplantation.
Myeloid progenitor cell isolation and transplantation
Myeloid progenitor cells were purified from whole bone marrow as described previously.8 Briefly, CMPs and GMPs were identified and isolated by (1) excluding cells expressing interleukin-7 receptor α (IL-7Rα) chain, CD19, immunoglobulin M (IgM), Thy 1.1, Ter119, Gr-1, and CD45R/B220; and (2) the positive CMP/GMP markers c-kit, Sca-1, CD34, and CD16/32. Cells were sorted using a modified fluorescence-activated cell sorter (FACSVantage; BD Biosciences, San Jose, CA). Host mice were anesthetized (Isoflurane; Abbott Laboratories, North Chicago, IL) and cells were transplanted into the retro-orbital cavity. Mice were observed until fully recovered.
Preparation of A fumigatus conidia
A clinical isolate of A fumigatus that had caused fatal sinusitis in a patient following allogeneic bone marrow transplantation was used to generate a conidial suspension as described previously.9 Suspensions were maintained at 4°C.
Inoculation of A fumigatus conidia
The A fumigatus conidia stock was diluted with sterile saline to a concentration of 3 × 106 to 4 × 106 cfu/20 μL to 30 μL. Mice were anesthetized with Avertin administered intraperitoneally (375 mg/kg). A fumigatus conidia were instilled intranasally with a precision microliter pipette (Rainin, Emeryville, CA).
Fungal culture of target organs
Mice were killed when exhibiting clinical evidence of disease or on the specified study days. Lungs were harvested for histologic examination and/or cultured onto Sabouraud dextrose agar plates (BD Biosciences, Cockeysville, MD) for fungal colonies.
Statistics
The log-rank test was performed on the survival results and the rank sum test was used to assess the significance of the absolute leukocyte counts.
Results and discussion
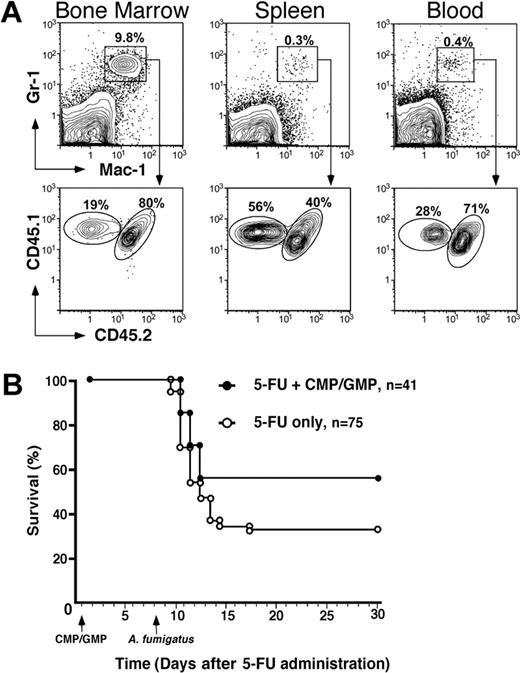
To assess the duration of the neutropenia resulting from the administration of 5-FU, peripheral blood leukocyte counts were measured at weekly intervals. Eight days following the administration of 5-FU (D+8), the absolute leukocyte counts in both experimental groups (5-FU only vs 5-FU+CMP/GMP) was only slightly lower than the lower limit of normal; however, mice in both groups demonstrated profound neutropenia (Table 1). By D+13, total leukocyte and absolute neutrophil counts had rebounded to above-normal levels in both groups. In order to determine the early trafficking of CMP and GMP progeny, immunophenotypic analyses of peripheral blood, spleen, and bone marrow cells were performed on D+8. By this time, mice that had received a single infusion of 1 × 104 CMPs and 2 × 104 GMPs had a significantly higher number of splenic Mac-1+Gr-1+ neutrophils than the 5-FU–only group (Table 1; P = .02). Moreover, 54% of these splenic Mac-1+Gr-1+ neutrophils were derived from the donor CMP/GMP population. Therefore, the contribution of the myeloid progenitor populations more than doubled the total neutrophil pool in the spleen compartment. Moreover, CMP/GMP-derived Mac-1+Gr-1+ neutrophils were also identified in blood and bone marrow (Figure 1A). These data demonstrate that CMPs and GMPs migrate and home to hematopoietic sites, thus suggesting a functional engraftment in this model of chemotherapy-induced neutropenia.
Absolute leukocyte counts following 5-FU treatment with or without myeloid progenitor infusion
. | 5-FU only . | . | 5-FU + CMP/GMP . | . | ||
|---|---|---|---|---|---|---|
. | D + 8* . | D + 13† . | D + 8* . | D + 13† . | ||
| Blood | ||||||
| Absolute WBC counts, × 103/μL; range: 5.5-9.3 | 4.1 ± 0.4 | 12.0 ± 1.6 | 5.4 ± 0.5 | 13.0 ± 0.6 | ||
| Absolute neutrophil counts, × 103/μL; range: 0.825-2.604 | 0.002 ± 0.007 | 3.6 ± 0.8 | 0.03 ± 0.01 | 2.6 ± 0.5 | ||
| Spleen | ||||||
| Absolute splenocytes, × 108 | 3.3 ± 0.4 | ND | 4.5 ± 1.1 | ND | ||
| Total Mac-1+ Gr-1+, × 106 | 0.6 ± 0.2 | ND | 1.2 ± 0.3‡ | ND | ||
| CMP/GMP-derived, × 105 | - | - | 6.5 ± 2.0 (54)§ | ND | ||
. | 5-FU only . | . | 5-FU + CMP/GMP . | . | ||
|---|---|---|---|---|---|---|
. | D + 8* . | D + 13† . | D + 8* . | D + 13† . | ||
| Blood | ||||||
| Absolute WBC counts, × 103/μL; range: 5.5-9.3 | 4.1 ± 0.4 | 12.0 ± 1.6 | 5.4 ± 0.5 | 13.0 ± 0.6 | ||
| Absolute neutrophil counts, × 103/μL; range: 0.825-2.604 | 0.002 ± 0.007 | 3.6 ± 0.8 | 0.03 ± 0.01 | 2.6 ± 0.5 | ||
| Spleen | ||||||
| Absolute splenocytes, × 108 | 3.3 ± 0.4 | ND | 4.5 ± 1.1 | ND | ||
| Total Mac-1+ Gr-1+, × 106 | 0.6 ± 0.2 | ND | 1.2 ± 0.3‡ | ND | ||
| CMP/GMP-derived, × 105 | - | - | 6.5 ± 2.0 (54)§ | ND | ||
Data are mean absolute cell counts plus or minus standard error of the mean (SEM).
ND indicates not determined; -, population not present; ND, not measured.
For D + 8/Blood, n = 15 for 5-FU only and n = 9 for 5-FU + CMP/GMP. For D + 8/Spleen, n = 14 for 5-FU only and n = 10 for 5-FU + CMP/GMP
For D + 13/Blood, n = 7 for 5-FU only and n = 5 for 5-FU + CMP/GMP
Significant difference from D + 8 counts (P = .02)
Value in parentheses indicates the percentage of total Mac-1+ Gr-1+ cells
Analysis of the tissue distribution of CMP/GMP progeny and survival following A fumigatus infection. (A) Flow cytometric analysis of tissue neutrophils (Mac-1+Gr-1+) on D+8 identified the presence of donor CMP/GMP-derived cells (CD45.1+CD45.2-). Although present in both bone marrow and blood, the donor cells constituted the majority of splenic neutrophils in comparison to host cells (CD45.1+CD45.2+). (B) Kaplan-Meier plot of mice infected with 3 × 106 to 4 × 106 cfu of A fumigatus following treatment with 5-FU only (○) or 5-FU plus 1 × 103 CMPs and 2 × 103 GMPs (⬡). The myeloid progenitors were infused 30 hours after 5-FU administration and the mice were infected 8 days after chemotherapy via intranasal instillation of A fumigatus conidia. The group that had received the CMP/GMP infusion (n = 41) had a significantly higher survival rate than the group treated with 5-FU only (n = 75) (56% and 33% respectively; P = .019). Animals that succumbed to infection at 2 to 4 days after instillation showed clinical evidence of disease, whereas those that survived appeared healthy throughout the observation period (30 to 60 days).
Analysis of the tissue distribution of CMP/GMP progeny and survival following A fumigatus infection. (A) Flow cytometric analysis of tissue neutrophils (Mac-1+Gr-1+) on D+8 identified the presence of donor CMP/GMP-derived cells (CD45.1+CD45.2-). Although present in both bone marrow and blood, the donor cells constituted the majority of splenic neutrophils in comparison to host cells (CD45.1+CD45.2+). (B) Kaplan-Meier plot of mice infected with 3 × 106 to 4 × 106 cfu of A fumigatus following treatment with 5-FU only (○) or 5-FU plus 1 × 103 CMPs and 2 × 103 GMPs (⬡). The myeloid progenitors were infused 30 hours after 5-FU administration and the mice were infected 8 days after chemotherapy via intranasal instillation of A fumigatus conidia. The group that had received the CMP/GMP infusion (n = 41) had a significantly higher survival rate than the group treated with 5-FU only (n = 75) (56% and 33% respectively; P = .019). Animals that succumbed to infection at 2 to 4 days after instillation showed clinical evidence of disease, whereas those that survived appeared healthy throughout the observation period (30 to 60 days).
As potentially important immune effectors, tissue was analyzed for its content of dendritic cells (DCs), macrophages, and monocytes. Multiparameter flow cytometry in blood, spleen, and bone marrow did not identify host-derived myeloid DCs (CD11c+Mac-+). However, host plasmacytoid DCs (CD11c+B220+) were detected in the bone marrow and spleen, but not blood. Moreover, there was no significant difference in the total DCs between the 2 experimental groups in bone marrow and spleen compartment DCs (P = .26 vs P = .89, respectively). Interestingly, there were no CMP/GMP-derived DCs in these tissues.
Similarly, no CMP/GMP-derived monocytes/macrophages (Mac-+Gr-1-) were identified in any compartment. Analysis of absolute splenic counts of host-derived Mac-1+Gr-1- cells confirmed that there was no significant difference between the 2 experimental groups of 5-FU only vs 5-FU+CMP/GMP (P = .16).
The significance of these quantitative differences in Mac-+Gr-1+ cells was confirmed by the ability of the infusion of CMPs and GMPs to protect against lethal challenge with A fumigatus. Following intranasal instillation of 3 × 106 to 4 × 106 conidia, only 33% of the animals that had received only 5-FU survived (n = 75) compared with 56% of the group infused with CMP/GMP (n = 41; P = .019; Figure 1B). The majority of mice died within 3 days after infection. Cultures of organs confirmed the presence of A fumigatus in the lungs. The absence of A fumigatus in other tissues may be explained by the rapid morbidity and mortality due to the infection. Of note, the CMP/GMP infusions were well tolerated and histologic evaluations did not reveal evidence of pulmonary injury such as a diffuse neutrophilic infiltration attributable to the cells. A neutrophilic infiltrate proximately associated with the presence of hyphae was observed in the lung tissue of about half of the mice that had received the single infusion of progenitors; in contrast, this infiltrate was not observed in the mice that had received 5-FU alone (data not shown).
These data confirm that a single infusion of myeloid progenitor cells reduces susceptibility to infection with A fumigatus in a preclinical model of chemotherapy-induced neutropenia. Moreover, the infusion of CMP/GMP resulted in the ability to contain a rapidly invasive infection when introduced in the biologically relevant route of inhalation. We previously reported that following transplantation, the tissue and not the peripheral blood content of myeloid effectors correlates with effective innate immunity, and the in vivo depletion of Mac-1+Gr-1+ cells abrogates this protection.9 Thus, it is reasonable to conclude that the containment of infection results from appropriate homing to the site of infection.
In contrast to the efficacy of the single infusion of GMP/GMP, the use of mature granulocytes in clinical settings requires repeated infusions of freshly apheresed cells, which is not only cumbersome but likely engenders the production of antileukocyte antibodies.10 Additionally, in studies where the cells were characterized, up to 20% of the apheresed cells were not granulocytes and may have contributed to the beneficial or detrimental effects observed.11-13 As was observed following myeloablative radiation, CMP/GMP infusion was well tolerated even in these mice with a less severe degree of immunosuppression. It is interesting to note that the use of G-CSF to mobilize leukocytes may result in a more rapid and durable elevation of the peripheral blood leukocyte counts when compared with counts following infusions of dexamethasone-mobilized cells.11,12 Whether or not G-CSF administration influences the mobilization, proliferation, or function of the infused cells remains an area of investigation. G-CSF administration following transplantation of CMP/GMP shortened the period of susceptibility to lethal fungal infection following myeloablative radiation.9 Whether or not a similar salutary effect of G-CSF will be seen following progenitor infusions in the setting of chemotherapy-induced neutropenia is under investigation.
As human myeloid progenitors have already been characterized in the bone marrow14 and G-CSF–mobilized peripheral blood (A.B., unpublished data, June 2004), these data support further investigation into the feasibility of cellular-based therapies to replenish depleted cell populations or serve as a bridge pending recovery of the hematopoietic system following chemotherapy or radiation.
Prepublished online as Blood First Edition Paper, December 2, 2004; DOI 10.1182/blood-2004-07-2676.
Supported by the National Institutes of Health grant 2P01CA49605, the Amy Strelzer Manasevit Scholars Program, the American Society for Blood and Marrow Transplantation (ASBMT)/Roche New Investigator Award, the Center for Clinical Immunology at Stanford University, and an unrestricted educational grant from Fugisawa Healthcare, Inc.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal