Abstract
Previously, we suggested that imatinib incorporation into conventional chemotherapy as an alternative (imatinib interim therapy) might be a useful strategy for bridging the time to allogeneic stem cell transplantation (SCT) for newly diagnosed Philadelphia chromosome–positive acute lymphoblastic leukemia (Ph+ ALL). Here, we provide an updated report on this strategy in 29 patients. At the time of enrollment, 23 patients (79.3%) achieved complete remission (CR). After the first imatinib cycle, the median breakpoint cluster region–Abelson oncogene locus (BCR-ABL)/ABL ratios decreased by 0.77 log in 25 (86.2%) responders, and their BCR-ABL/ABL ratios decreased further by 0.34 log after the second imatinib cycle, which included 7 molecular CR. One patient (4.3%) relapsed during the imatinib therapy. The remaining 3 patients were primarily refractory to both imatinib and chemotherapy. Twenty-five (86.2%) of the 29 patients received transplants in first CR. With a median follow-up duration of 25 months after SCT, the 3-year estimated probabilities of relapse, nonrelapse mortality, disease-free survival, and overall survival were 3.8%, 18.7%, 78.1%, and 78.1%, respectively. In comparison to our historical control data, first-line imatinib interim therapy appears to provide a good quality of CR and a survival advantage for patients with Ph+ ALL. Further long-term follow-up is needed to validate the results of this study.
Introduction
The Philadelphia chromosome (Ph) is the most frequent cytogenetic abnormality in adult acute lymphoblastic leukemia (ALL), with an overall incidence of 20% to 40%.1-4 Most patients with Ph+ ALL have an extremely poor prognosis when treated by chemotherapy alone.2,3,5-9 Current chemotherapy regimens induce complete remissions (CRs) in more than 70%, but most patients relapse within 6 to 11 months of treatment and die of the disease. The 5-year overall survival rates for those treated with intensive chemotherapy alone is less than 10%. To date, only allogeneic stem cell transplantation (SCT) performed early during remission has permitted long-term survival (35%-65%).10-15 Outcome for allogeneic SCT in an actively relapsed or a refractory status is poor. Because the results of allogeneic SCT correlate with the pretransplantation leukemia burden, improved treatment strategies are clearly needed to ensure CR at the time of SCT for patients with Ph+ ALL.
Imatinib (Glivec, STI571), a selective breakpoint cluster region–Abelson oncogene locus (BCR-ABL) protein tyrosine kinase inhibitor, has been demonstrated to induce overall responses (CR + marrow-CR + partial marrow response) in 60% to 70% of patients with relapsed or refractory Ph+ ALL, including patients that have previously undergone transplantation, with limited toxicity.16-18 Unfortunately, the median time to progression is only 2 to 3 months, which reflects the time required to develop resistance to imatinib. Considering the frequency and kinetics of resistance, it is felt likely that imatinib monotherapy is not sufficient as a first-line treatment for Ph+ ALL. Reports of additive or synergistic effects in vitro of imatinib in combination with cytotoxic agents (eg, anthracyclines, cytarabine, or vincristine) support the use of clinical trials to test the feasibility of combination therapies.19-21 In this regard, the initiation of imatinib earlier in the disease course with cytotoxic agents either as alternative or concurrent schedules may reduce the likelihood of resistance and improve treatment outcome.
From this point of view, we undertook a prospective phase 2 study to evaluate the effect of imatinib incorporation into conventional chemotherapy as an alternative before allogeneic SCT (imatinib interim therapy) for newly diagnosed Ph+ ALL. Our previously published preliminary data suggested that first-line imatinib interim therapy might be a useful strategy for bridging the time to allogeneic SCT for newly diagnosed Ph+ ALL.22 Here, we report on the updated results of this strategy, which better define the role of imatinib interim therapy. In addition, we evaluated whether first-line imatinib interim therapy would improve the outcome of allogeneic SCT.
Patients, materials, and methods
Eligibility
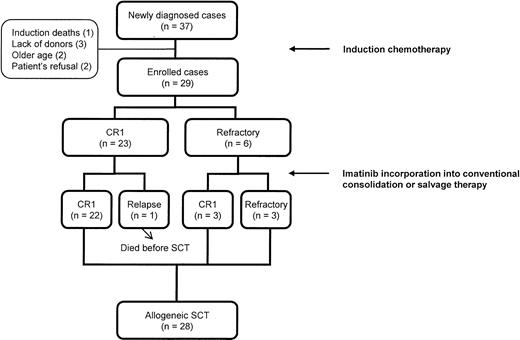
Between September 2000 and August 2003, 37 (28.2%) of the 131 adults (aged 15 years or older) with ALL were Ph+ at the time of diagnosis. Of these, 29 patients with newly diagnosed Ph+ ALL who completed induction chemotherapy and intended to undergo human leukocyte antigen (HLA)–matched allogeneic (sibling or unrelated) SCT were enrolled in this study. Eight patients were excluded because of death during induction (n = 1), lack of donors (n = 3), old age (n = 2), and refusal (n = 2) (Figure 1). Eligibility criteria consisted of (1) karyotypic and/or molecular evidence of t(9;22) in leukemic cells, (2) an age younger than 60 years, (3) Eastern Cooperative Oncology Group performance status of 0 to 2, (4) adequate renal and hepatic functions (serum creatinine < 152.5 μmol/L [< 2 mg/dL] and bilirubin < 51.3 μmol/L [< 3 mg/dL]), (5) adequate cardiac status (New York Heart Association class ≤ II), and (6) a negative pregnancy test. All patients in the study provided written informed consent, and the study protocol was approved by the institutional review board of The Catholic University of Korea.
Overall scheme of disease status until the time of allogeneic stem cell transplantation in patients treated with imatinib interim therapy.
Overall scheme of disease status until the time of allogeneic stem cell transplantation in patients treated with imatinib interim therapy.
Therapy
Induction therapy was started with hyperfractionated cyclophosphamide (300 mg/m2, every 12 hours, days 1 to 3), vincristine (1.4 mg/m2, days 4 and 11), idarubicin (12 mg/m2, days 4 and 11), and dexamethasone (40 mg, days 1 to 4 and days 11 to 14), which was mainly based on the hyper-CVAD (cyclophosphamide, vincristine, Adriamycin, and dexamethasone) regimen.6 After induction treatment with the recovery of white blood cell (WBC; ≥ 3 × 109/L) and platelet (≥ 60 × 109/L) counts, the first imatinib cycle (400-600 mg/d for 4 weeks) was started. Patients who achieved CR after induction therapy were randomly assigned to receive 400 mg or 600 mg imatinib daily. Patients not in CR received the imatinib therapy at a daily dose of 600 mg. Subsequently, patients in CR received consolidation chemotherapy consisting of high-dose cytarabine (2 g/m2, every 12 hours, days 1 to 5) and mitoxantrone (12 mg/m2, days 1 to 2) followed by a second imatinib cycle bridging the time to SCT. Patients not in CR received salvage chemotherapy, which was cytarabine (2 g/m2, every 12 hours, days 1 to 4), mitoxantrone (12 mg/m2, days 1 to 4), and etoposide (100 mg/m2, days 5 to 7). Central nervous system (CNS) prophylaxis was performed by intrathecally administering triple agents (methotrexate, cytarabine, and methylprednisolone) during the induction and consolidation courses (6 times in total). At the time of diagnosis, CNS leukemia was considered present when there was neurologic involvement or when cerebrospinal fluid (CSF) studies showed 5 or more leukemic blasts/μL CSF fluid, ensuring no sample contamination by peripheral blood (PB). Patients with CNS disease received intrathecal therapy twice weekly until the CSF study findings were negative during the induction course, and then cranial irradiation (2340 cGy) was added.
The preparative regimen consisted of total body irradiation (TBI; 1320 cGy) and cyclophosphamide (120 mg/kg) for patients in first CR and TBI (1200 cGy), cytarabine (12 g/m2), and melphalan (140 mg/m2) for patients in more advanced pretransplantation disease status. Some older patients (older than 50 years) were given a reduced intensity regimen consisting of fludarabine (180 mg/m2) and melphalan (140 mg/m2). Graft-versus-host disease (GVHD) prophylaxis was attempted by administering cyclosporine plus methotrexate. Cyclosporine was administered intravenously at a dosage of 3 mg/kg as a continuous infusion from day -1. Subsequently, when patients were able to tolerate oral administration, they received cyclosporine orally at 6 mg/kg/d in 2 divided doses until day 90. The dose of cyclosporine was then gradually tapered and in the absence of GVHD discontinued 6 months after SCT. If residual leukemia was detected during the follow-up period after SCT, cyclosporine was rapidly discontinued. All patients also received a short course of methotrexate, 10 mg/m2 on days 1, 3, 6, and 11. Acute GVHD was treated with high-dose steroids, and extensive chronic GVHD was treated with cyclosporine and steroids.
Minimal residual disease monitoring
For minimal residual disease (MRD) monitoring, 272 bone marrow (BM) samples were available from all patients receiving imatinib interim therapy and were analyzed by real-time quantitative polymerase chain reaction (RQ-PCR). Samples were collected at diagnosis, before and after imatinib therapy, and then at 3, 6, 9, 12, 18, 24, and 36 months after SCT. Mononuclear cells were isolated by Histopaque density gradient centrifugation (Sigma, St Louis, MO; density 1.077 g/mL). Total RNA was extracted using an RNAqueous kit (Ambion, Austin, TX), and reverse transcription was performed using 1 μg RNA. Plasmid standard titrations with the defined copy numbers for BCR-ABL and for reference ABL were analyzed simultaneously with the patient's samples. As previously described,15 we designed 1 set of primers for each type of BCR-ABL transcript, ABL, and TaqMan probes. RQ-PCR was performed in triplicate using iCycler software 2.1 (Bio-Rad, Hercules, CA) with the standard conditions (95°C for 10 minutes, 50 cycles at 95°C for 15 seconds, and 60°C for 1 minute). The 50-μL PCR reaction mix contained 5 μL 1 × PCR buffer (4.5 mM MgCl2, 0.2 mM dNTP (deoxyribonucleoside triphosphate), 0.2 μM primer, 140 nM TaqMan probe, 1.25 U AmpliTaq gold DNA polymerase, and 4 μL target cDNA). The quantity of BCR-ABL transcript was normalized for ABL expression (sensitivity, 10-5). Negative results were confirmed by nested PCR.15
Definitions and evaluation of response
CR was defined as the reconstitution of normal BM cellularity with less than 5% leukemic blasts, together with an absolute neutrophil count of greater than 1.5 × 109/L and a platelet count greater than 100 × 109/L. Molecular CR was defined by a negative PCR in BM aspirates. Relapse was defined by the reappearance of more than 5% leukemic cells in BM aspirates or extramedullary leukemia in patients with previously documented CR. Patients were considered refractory if PB blasts or extramedullary disease had not been eliminated, BM blasts had not been reduced below 5%, or both.
Acute GVHD was classified according to previous published criteria.23 The clinical and laboratory parameters collected to assess the grading of acute GVHD included the percentage of body surface area covered by a skin rash, volume of diarrhea, and total bilirubin level. Tissue biopsy samples were obtained to confirm the diagnosis of acute GVHD whenever clinically feasible. The diagnosis and grading of chronic GVHD were established with the use of the clinical and pathologic criteria proposed by Sullivan et al.24 Studies included hematopoietic and chemical parameters, skin and lip biopsies, Schirmer test, pulmonary function tests, and other examinations as indicated. Patients with sustained donor engraftment who survived more than 14 days and more than 3 months after SCT were assessed with respect to the occurrence and severity of acute and chronic GVHD, which were graded by a single investigator at our institution.
Statistical analysis
The clinical characteristics and treatment outcomes of patients receiving imatinib interim therapy (imatinib group) were compared with the historical control group of 33 consecutive patients with Ph+ ALL who completed induction chemotherapy and proceeded to HLA-matched sibling or unrelated SCT without imatinib interim therapy from 1996 to 2000 (immediately preceded the imatinib interim therapy trial). No autologous or HLA-mismatched SCTs were performed in this group. Except for induction chemotherapy (idarubicin, vincristine, prednisone, and l-asparaginase) and CNS prophylaxis (methotrexate plus cranial irradiation), these patients were treated with the same protocols (postinduction chemotherapy, preparative regimen, and GVHD prophylaxis or treatment) that were given for patients receiving imatinib interim therapy. The outcomes of 23 of these 33 historical patients in first or second CR treated with SCT from HLA-matched sibling donors has been previously reported.15 Here, we analyzed the results of 33 historical patients, which included 5 patients that previously underwent SCT from HLA-matched unrelated donors, and 5 with a refractory pretransplantation disease status. The proportions of patients with a given characteristic were compared by using the chi-square or Fisher exact tests. Differences in the means of continuous variables were checked using the Mann-Whitney U test. Survival duration was calculated from the date of transplantation until death or the date when last known alive. Nonrelapse mortality (NRM) was defined as death occurring in relapse-free patients. Death resulting from any cause after relapse was considered to have been caused by relapse. When calculating disease-free survival (DFS), both relapses and deaths in CR were counted as adverse events. The cumulative relapse rate was calculated by using the same type of analysis used for DFS, except for those who died in CR, who were censored at the time of death. Survival curves were plotted using the Kaplan-Meier method and compared by the log-rank test. Statistical analyses were performed using SPSS 11.5 software (SPSS, Chicago, IL).
Results
Patient characteristics
In the imatinib group, there were 14 men and 15 women with a median age of 36 years (range, 18-55 years). Karyotype analysis revealed additional chromosomal changes in 16 (55.2%) of the 29 patients. As to the breakpoint within the BCR gene, 16 patients (55.2%) had the p190BCR-ABL transcript (e1a2), and the other 13 (44.8%) had p210BCR-ABL (b2a2 or b3a2). Twenty-three (79.3%) of the 29 patients achieved CR after induction chemotherapy, while the other 6 patients (20.7%) were refractory. Their main clinical features were compared with 33 historical patients with Ph+ ALL. Presenting characteristics and CR rates after induction chemotherapy were not significantly different between the 2 groups (Table 1).
Clinical and biologic characteristics of patients
. | No. . | . | . | |
|---|---|---|---|---|
. | Imatinib group (n = 29) . | Historical group (n = 33) . | P . | |
| Median age, y (range) | 36 (18-55) | 35 (15-48) | .160 | |
| Sex | .622 | |||
| Male (%) | 14 (48.3) | 18 (54.5) | ||
| Female (%) | 15 (51.7) | 15 (45.5) | ||
| Median hemoglobin level, g/dL (range) | 9.9 (4.9-13.5) | 8.5 (3.8-13.7) | .205 | |
| Median WBC count, × 109/L (range) | 32.3 (1.8-239.0) | 50.3 (1.6-481.0) | .658 | |
| Median platelet count, × 109/L (range) | 42.0 (12.0-313.0) | 33.0 (9.0-131.0) | .402 | |
| Median LDH level, IU/L (range) | 1173 (442-6366) | 1767 (250-4188) | .494 | |
| Extramedullary involvement | .987 | |||
| Yes (%) | 14 (48.3) | 16 (48.5) | ||
| No (%) | 15 (51.7) | 17 (51.5) | ||
| CNS leukemia | .926 | |||
| Yes (%) | 1 (3.6) | 1 (3.0) | ||
| No (%) | 28 (96.4) | 32 (97.0) | ||
| Immunophenotype | .814 | |||
| B-lineage (%) | 21 (72.4) | 23 (69.7) | ||
| B + myeloid antigen (%) | 8 (27.6) | 10 (30.3) | ||
| Karyotype | .599 | |||
| Ph alone (%) | 13 (44.8) | 17 (51.5) | ||
| Additional changes (%) | 16 (55.2) | 16 (48.5) | ||
| BCR-ABL subtype | .773 | |||
| p210BCR-ABL (%) | 13 (44.8) | 16 (48.5) | ||
| p190BCR-ABL (%) | 16 (55.2) | 17 (51.5) | ||
| Response to induction therapy | .803 | |||
| CR (%) | 23 (79.3) | 27 (81.8) | ||
| Refractory (%) | 6 (20.7) | 6 (18.2) | ||
| Median time to SCT*, d (range) | 138 (56-217) | 150 (130-234) | .137 | |
. | No. . | . | . | |
|---|---|---|---|---|
. | Imatinib group (n = 29) . | Historical group (n = 33) . | P . | |
| Median age, y (range) | 36 (18-55) | 35 (15-48) | .160 | |
| Sex | .622 | |||
| Male (%) | 14 (48.3) | 18 (54.5) | ||
| Female (%) | 15 (51.7) | 15 (45.5) | ||
| Median hemoglobin level, g/dL (range) | 9.9 (4.9-13.5) | 8.5 (3.8-13.7) | .205 | |
| Median WBC count, × 109/L (range) | 32.3 (1.8-239.0) | 50.3 (1.6-481.0) | .658 | |
| Median platelet count, × 109/L (range) | 42.0 (12.0-313.0) | 33.0 (9.0-131.0) | .402 | |
| Median LDH level, IU/L (range) | 1173 (442-6366) | 1767 (250-4188) | .494 | |
| Extramedullary involvement | .987 | |||
| Yes (%) | 14 (48.3) | 16 (48.5) | ||
| No (%) | 15 (51.7) | 17 (51.5) | ||
| CNS leukemia | .926 | |||
| Yes (%) | 1 (3.6) | 1 (3.0) | ||
| No (%) | 28 (96.4) | 32 (97.0) | ||
| Immunophenotype | .814 | |||
| B-lineage (%) | 21 (72.4) | 23 (69.7) | ||
| B + myeloid antigen (%) | 8 (27.6) | 10 (30.3) | ||
| Karyotype | .599 | |||
| Ph alone (%) | 13 (44.8) | 17 (51.5) | ||
| Additional changes (%) | 16 (55.2) | 16 (48.5) | ||
| BCR-ABL subtype | .773 | |||
| p210BCR-ABL (%) | 13 (44.8) | 16 (48.5) | ||
| p190BCR-ABL (%) | 16 (55.2) | 17 (51.5) | ||
| Response to induction therapy | .803 | |||
| CR (%) | 23 (79.3) | 27 (81.8) | ||
| Refractory (%) | 6 (20.7) | 6 (18.2) | ||
| Median time to SCT*, d (range) | 138 (56-217) | 150 (130-234) | .137 | |
WBC indicates white blood cell; LDH, lactate dehydrogenase; CNS, central nervous system; Ph, Philadelphia chromosome; CR, complete remission; SCT, allogeneic stem cell transplantation.
Interval from the start of induction chemotherapy to the date of transplantation
Disease status until the time of SCT
As shown in Figure 1, 22 of the 23 patients who achieved CR after induction chemotherapy remained in sustained first CR until the time of transplantation in the imatinib group. Only 1 patient relapsed after the first imatinib cycle. Furthermore, 3 of the 6 patients who were refractory to chemotherapy achieved CR after imatinib interim therapy. The clinical and biologic characteristics of patients who had primary refractoriness to imatinib, chemotherapy, or both are shown in Table 2. Of these, 4 patients who had the p190BCR-ABL transcript were primarily refractory to imatinib, and 3 of them had additional chromosomal changes at the time of diagnosis. The relapse rate in the imatinib group was lower than that of the historical group before SCT (1 [4.3%] of 23 versus 11 [40.7%] of 27, P = .003). In addition, the imatinib group had a higher rate of proceeding to SCT in sustained or newly achieved first CR than the historical group (25 [86.2%] of 29 versus 17 [51.5%] of 33, P = .004; Table 3).
Presenting characteristics of patients who had primary refractoriness to imatinib and/or chemotherapy
No. . | Age, y/sex . | Hb level, g/dL . | WBC count, × 109/L . | Platelet count, × 109/L . | LDH level, IU/L . | EMI . | Immunophenotype . | Karyotype . | BCR-ABL isoform . |
|---|---|---|---|---|---|---|---|---|---|
| 10* | 27/M | 11.6 | 223.6 | 22.0 | 6366 | No | B-lineage | 46,XY,del(9)(p22),t(9;22)(q34;q11.2),der(19) t(9;19)(q12;q13)[14]/48,idem, + 8, - der (19)t(9;19), + der(22)t(9;22)[10]/46,XY[1] | p190 |
| 12† | 39/M | 8.0 | 179.2 | 15.0 | 1880 | Yes | B-lineage | 46,XY,t(9;22)(q34;q11.2)[16]/46,XY[4] | p210 |
| 14‡ | 44/M | 12.0 | 122.7 | 58.0 | 2050 | No | B + My | 45,XY, - 7,t(9;22)(q34;q11.2)[25] | p190 |
| 16† | 31/M | 10.2 | 4.7 | 23.0 | 1159 | Yes | B + My | 46,XY,t(9;22)(q34;q11.2)[20] | p190 |
| 19† | 28/F | 7.9 | 13.4 | 12.0 | 4470 | No | B + My | 46,XX,t(9;22)(q34;q11.2),del(9)(p22)[3]/47, idem, + der(22)t(9;22)[17] | p190 |
| 24‡ | 44/F | 10.2 | 1.8 | 33.0 | 574 | Yes | B-lineage | 46,XX,t(9;22)(q34;q11.2)[25] | p190 |
| 26‡ | 20/M | 10.5 | 102.5 | 32.0 | 1082 | Yes | B + My | 45,XY, - 7,t(9;22)(q34;q11.2)[12]/45,idem, der(16)t(1;16)(q12;q11.2)[2]/46,XY[11] | p190 |
No. . | Age, y/sex . | Hb level, g/dL . | WBC count, × 109/L . | Platelet count, × 109/L . | LDH level, IU/L . | EMI . | Immunophenotype . | Karyotype . | BCR-ABL isoform . |
|---|---|---|---|---|---|---|---|---|---|
| 10* | 27/M | 11.6 | 223.6 | 22.0 | 6366 | No | B-lineage | 46,XY,del(9)(p22),t(9;22)(q34;q11.2),der(19) t(9;19)(q12;q13)[14]/48,idem, + 8, - der (19)t(9;19), + der(22)t(9;22)[10]/46,XY[1] | p190 |
| 12† | 39/M | 8.0 | 179.2 | 15.0 | 1880 | Yes | B-lineage | 46,XY,t(9;22)(q34;q11.2)[16]/46,XY[4] | p210 |
| 14‡ | 44/M | 12.0 | 122.7 | 58.0 | 2050 | No | B + My | 45,XY, - 7,t(9;22)(q34;q11.2)[25] | p190 |
| 16† | 31/M | 10.2 | 4.7 | 23.0 | 1159 | Yes | B + My | 46,XY,t(9;22)(q34;q11.2)[20] | p190 |
| 19† | 28/F | 7.9 | 13.4 | 12.0 | 4470 | No | B + My | 46,XX,t(9;22)(q34;q11.2),del(9)(p22)[3]/47, idem, + der(22)t(9;22)[17] | p190 |
| 24‡ | 44/F | 10.2 | 1.8 | 33.0 | 574 | Yes | B-lineage | 46,XX,t(9;22)(q34;q11.2)[25] | p190 |
| 26‡ | 20/M | 10.5 | 102.5 | 32.0 | 1082 | Yes | B + My | 45,XY, - 7,t(9;22)(q34;q11.2)[12]/45,idem, der(16)t(1;16)(q12;q11.2)[2]/46,XY[11] | p190 |
M indicates male; F, female; Hb, hemoglobin; EMI, extramedullary involvement; My, myeloid antigen.
Patient with refractoriness to imatinib therapy
Patients with refractoriness to chemotherapy
Patients with refractoriness to both imatinib therapy and chemotherapy
Disease status until the time of allogeneic stem cell transplantation
. | Imatinib group (n = 29) . | Historical group (n = 33) . | P . |
|---|---|---|---|
| After induction therapy | |||
| CR1 (%) | 23 (79.3) | 27 (81.8) | .803 |
| Refractory (%) | 6 (20.7) | 6 (18.2) | |
| After consolidation therapy | |||
| Sustained CR1 (%) | 22/23 (95.7) | 16/27 (59.3) | .003 |
| Relapse (%) | 1/23 (4.3) | 11/27 (40.7) | |
| After salvage therapy | |||
| CR1 (%) | 3/6 (50.0) | 1/6 (16.7) | .545 |
| Refractory (%) | 3/6 (50.0) | 5/6 (83.3) | |
| Pretransplantation disease status | |||
| CR1 (%)* | 25 (86.2)† | 17 (51.5) | .004 |
| CR2 (%) | 0 | 9 (27.3) | |
| Refractory (%) | 4 (13.8) | 7 (21.2) |
. | Imatinib group (n = 29) . | Historical group (n = 33) . | P . |
|---|---|---|---|
| After induction therapy | |||
| CR1 (%) | 23 (79.3) | 27 (81.8) | .803 |
| Refractory (%) | 6 (20.7) | 6 (18.2) | |
| After consolidation therapy | |||
| Sustained CR1 (%) | 22/23 (95.7) | 16/27 (59.3) | .003 |
| Relapse (%) | 1/23 (4.3) | 11/27 (40.7) | |
| After salvage therapy | |||
| CR1 (%) | 3/6 (50.0) | 1/6 (16.7) | .545 |
| Refractory (%) | 3/6 (50.0) | 5/6 (83.3) | |
| Pretransplantation disease status | |||
| CR1 (%)* | 25 (86.2)† | 17 (51.5) | .004 |
| CR2 (%) | 0 | 9 (27.3) | |
| Refractory (%) | 4 (13.8) | 7 (21.2) |
CR1 indicates first complete remission; and CR2, second complete remission.
Sustained CR1 during the consolidation phase plus newly achieved CR1 after the imatinib or salvage chemotherapy
Includes 7 patients with molecular CR
MRD-based role of imatinib interim therapy before SCT
The kinetics of the BCR-ABL transcript correlated well with the clinical courses of patients receiving imatinib interim therapy (Figure 2). At the time of enrollment, the median BCR-ABL/ABL ratios were 2.96 (0.14-7.90) × 10-3 in 23 patients in CR. In the 6 refractory patients, the median BCR-ABL/ABL ratios were more than 10-2. After the first imatinib cycle, 22 of the 23 patients in CR remained in sustained CR. In paired samples (before and after the first imatinib cycle) from these patients, their median BCR-ABL/ABL ratios showed a decrease of 0.83 (0.20-1.85) log (Figure 2A-B). Meanwhile, 1 patient (patient no. 10) who had achieved CR before the first imatinib cycle relapsed; the BCR-ABL/ABL ratio also increased from 2.96 × 10-3 to 2.55 × 10-2 (Figure 2C). In the remaining 6 patients refractory to induction chemotherapy, 3 (patient nos. 12, 16, 19) achieved CR with a decrease in MRD (-0.89 log, -0.76 log, and -0.60 log, respectively) after the first imatinib cycle (Figure 2D), while the other 3 patients (patient nos. 14, 24, 26) showed no hematologic or molecular responses (Figure 2C). Overall, the BCR-ABL/ABL ratios were reduced by 0.77 (0.20-1.85) log in 25 (86.2%) and increased by 0.81 (0.54-1.10) log in 4 (13.8%) patients after the first imatinib cycle.
Kinetics of the BCR-ABL transcript and its clinical correlation in 29 patients with Ph+ ALL treated with imatinib interim therapy. (A) Plot showing quantitative MRD levels for 15 patients with hematologic CR after imatinib interim therapy. (B) Plot showing quantitative MRD levels for 7 patients with molecular CR after imatinib interim therapy. Their median BCR-ABL/ABL ratios decreased by 0.83 log after the first imatinib cycle and 0.34 log after the second imatinib cycle, respectively. (C) Plot showing quantitative MRD levels for 4 patients with a primary refractoriness to imatinib therapy. One patient (patient no. 10) died of septicemia before transplantation. (D) Plot showing quantitative MRD levels for 3 refractory patients after induction chemotherapy. These patients achieved CR with a decrease in MRD after imatinib therapy. Twenty-five patients underwent allogeneic SCT with a first CR (A + B + D), while the remaining 3 patients had resistant leukemia at the time of SCT (C). Of these, 7 patients (patient nos. 3, 6, 12, 16, 19, 24, 26) still had residual leukemia at 3 or 6 months after SCT. However, with the exception of 2 cases (patient nos. 16, 19), their BCR-ABL/ABL ratios rapidly decreased to an undetectable level after the development of acute (patient no. 24) or chronic (patient nos. 3, 6, 12, 26) GVHD induced by the rapid withdrawal of cyclosporine. Of 2 patients without GVHD, 1 (patient no. 16) showed an increase in MRD at 6 months (1.35 × 10-5 → 6.78 × 10-5) and subsequently died of overt hematologic relapse following a further increase in MRD (8.12 × 10-2) at 12 months after SCT. The overall treatment outcomes of the patients receiving imatinib interim therapy are listed in Table 4. Dx indicates diagnosis; p, post (ie, after); Ind, induction; C, consolidation; IM, imatinib; S, salvage chemotherapy; M, months; aGVHD, acute GVHD; cGVHD, chronic GVHD.
Kinetics of the BCR-ABL transcript and its clinical correlation in 29 patients with Ph+ ALL treated with imatinib interim therapy. (A) Plot showing quantitative MRD levels for 15 patients with hematologic CR after imatinib interim therapy. (B) Plot showing quantitative MRD levels for 7 patients with molecular CR after imatinib interim therapy. Their median BCR-ABL/ABL ratios decreased by 0.83 log after the first imatinib cycle and 0.34 log after the second imatinib cycle, respectively. (C) Plot showing quantitative MRD levels for 4 patients with a primary refractoriness to imatinib therapy. One patient (patient no. 10) died of septicemia before transplantation. (D) Plot showing quantitative MRD levels for 3 refractory patients after induction chemotherapy. These patients achieved CR with a decrease in MRD after imatinib therapy. Twenty-five patients underwent allogeneic SCT with a first CR (A + B + D), while the remaining 3 patients had resistant leukemia at the time of SCT (C). Of these, 7 patients (patient nos. 3, 6, 12, 16, 19, 24, 26) still had residual leukemia at 3 or 6 months after SCT. However, with the exception of 2 cases (patient nos. 16, 19), their BCR-ABL/ABL ratios rapidly decreased to an undetectable level after the development of acute (patient no. 24) or chronic (patient nos. 3, 6, 12, 26) GVHD induced by the rapid withdrawal of cyclosporine. Of 2 patients without GVHD, 1 (patient no. 16) showed an increase in MRD at 6 months (1.35 × 10-5 → 6.78 × 10-5) and subsequently died of overt hematologic relapse following a further increase in MRD (8.12 × 10-2) at 12 months after SCT. The overall treatment outcomes of the patients receiving imatinib interim therapy are listed in Table 4. Dx indicates diagnosis; p, post (ie, after); Ind, induction; C, consolidation; IM, imatinib; S, salvage chemotherapy; M, months; aGVHD, acute GVHD; cGVHD, chronic GVHD.
Twenty-three of the 25 patients in CR received a second imatinib cycle following consolidation chemotherapy. Median BCR-ABL/ABL ratios were observed to further reduce by 0.60 (0.06-1.58) log after consolidation chemotherapy, but no patient achieved a molecular CR. After the second imatinib cycle, the median BCR-ABL/ABL ratios decreased by 0.34 (0.07-1.3)1 log, compared with levels after consolidation. In addition, the BCR-ABL transcript was not detected in 7 patients (patient nos. 1, 4, 15, 17, 22, 27, 29) (Figure 2B). Meanwhile, 2 resistant patients (patient nos. 10, 24) after the first imatinib cycle were also resistant to the second imatinib cycle with an increase in MRD. The remaining 2 patients in CR (patient nos. 12, 13) and 2 resistant patients (patient nos. 14, 26) proceeded directly to SCT after the first imatinib cycle. All patients tolerated the imatinib therapy well.
Transplantation outcome
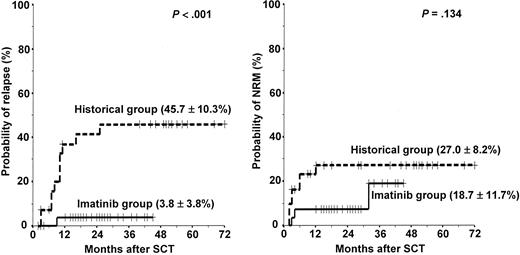
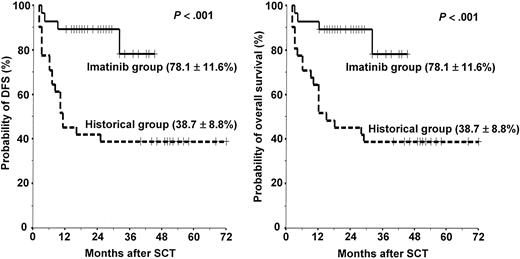
The overall treatment outcomes of the 29 patients in the imatinib group are listed in Table 4. Twenty-eight (25 first CR, 3 refractory) of the 29 patients underwent HLA-matched sibling (n = 22) and unrelated (n = 6) SCT after the completion of first (n = 4) or second (n = 24) imatinib cycles at a median time of 138 days (range, 56-217 days) from the start of induction therapy. Some patients received additional imatinib therapy or chemotherapy until an unrelated donor became available. However, 1 patient (patient no. 10) who showed resistance against imatinib died of septicemia before SCT. A conditioning regimen was started 7 days after the last day of the imatinib cycle. All patients achieved a successful engraftment. Acute GVHD was observed in 10 patients (35.7%; 7 grade II, 2 grade III, 1 grade IV). Twelve (46.2%) of the 26 evaluable patients had chronic GVHD (9 limited, 3 extensive). With a median follow-up duration of 25 months (range, 12+ to 45+ months) for surviving patients after SCT, 24 patients are alive with a leukemia-free status, and 4 patients have died (early or late transplant-related complications in 3 [1 hemorrhagic cystitis, 1 grade IV acute GVHD, and 1 extensive chronic GVHD] and relapse mortality in 1). The 3-year estimated probabilities of relapse, NRM, DFS, and overall survival were 3.8% ± 3.8%, 18.7% ± 11.7%, 78.1% ± 11.6%, and 78.1% ± 11.6%, respectively (Figures 3, 4).
Overall treatment outcome of patients treated with first-line imatinib interim therapy (n = 29)
. | . | . | . | . | . | Response to imatinib* . | . | . | . | . | GVHD (grade) . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age, y/sex . | BCR-ABL isoform . | Karyotype . | Status before imatinib . | Imatinib dose, mg/d . | After first imatinib . | After second imatinib . | Pre-SCT status . | Graft source . | Time to SCT, d . | Acute . | Chronic . | Current DFS status; cause of death . | ||
| 1 | 39/F | p190 | Additional | CR | 600 | CR (-0.20) | Mol CR (UD) | Mol CR | MSD/BM | 128 | + (II) | + (E) | Died 32 mo; NRM | ||
| 2 | 29/M | p210 | Additional | CR | 400 | CR (-0.89) | CR (-0.27) | CR | MSD/BM | 136 | - | - | Alive 45 + mo | ||
| 3 | 40/M | p210 | Additional | CR | 600 | CR (-0.40) | CR (-0.79) | CR | MSD/BM | 132 | - | + (L) | Alive 43 + mo | ||
| 4 | 39/F | p190 | Additional | CR | 400 | CR (-0.35) | Mol CR (UD) | Mol CR | MSD/BM | 127 | - | - | Alive 42 + mo | ||
| 5 | 26/M | p190 | Ph alone | CR | 400 | CR (-1.22) | CR (-0.31) | CR | MSD/BM | 134 | - | - | Alive 39 + mo | ||
| 6 | 20/M | p210 | Additional | CR | 600 | CR (-0.97) | CR (-0.08) | CR | MSD/BM | 158 | - | + (L) | Alive 37 + mo | ||
| 7 | 43/F | p210 | Additional | CR | 600 | CR (-1.85) | CR (-0.09) | CR | MSD/BM | 139 | + (III) | NA | Died 3 mo; NRM | ||
| 8 | 40/F | p190 | Ph alone | CR | 600 | CR (-0.50) | CR (-1.09) | CR | MSD/BM | 126 | - | + (L) | Alive 34 + mo | ||
| 9 | 31/M | p210 | Ph alone | CR | 400 | CR (-0.93) | CR (-0.11) | CR | MSD/BM | 120 | - | - | Alive 32 + mo | ||
| 10 | 27/M | p190 | Additional | CR | 600 | Relapse (+0.93) | Relapse (+1.63) | Relapse | NA | NA | NA | NA | Died before SCT | ||
| 11 | 38/F | p190 | Additional | CR | 400 | CR (-0.20) | CR (-0.07) | CR | MSD/BM | 141 | + (II) | + (E) | Alive 29 + mo | ||
| 12 | 39/M | p210 | Ph alone | Refractory | 600 | CR (-0.89) | NA | CR | MSD/BM | 126 | - | + (L) | Alive 28 + mo | ||
| 13 | 30/M | p190 | Additional | CR | 400 | CR (-1.48) | NA | CR | MSD/BM | 56 | - | - | Alive 27 + mo | ||
| 14 | 44/M | p190 | Additional | Refractory | 600 | Refractory (+0.54) | NA | Refractory | MSD/PB | 137 | + (IV) | NA | Died 4 mo; NRM | ||
| 15 | 18/M | p190 | Additional | CR | 400 | CR (-0.48) | Mol CR (UD) | Mol CR | MSD/BM | 151 | - | + (E) | Alive 26 + mo | ||
| 16 | 31/M | p190 | Ph alone | Refractory | 600 | CR (-0.76) | CR (-1.31) | CR | MUD/BM | 191 | - | - | Died 9 mo; relapse | ||
| 17 | 55/F | p210 | Ph alone | CR | 600 | CR (-0.77) | Mol CR (UD) | Mol CR | MSD/BM | 126 | - | + (L) | Alive 25 + mo | ||
| 18 | 21/F | p210 | Ph alone | CR | 600 | CR (-0.48) | CR (-0.81) | CR | MSD/BM | 141 | + (II) | - | Alive 24 + mo | ||
| 19 | 28/F | p190 | Additional | Refractory | 600 | CR (-0.60) | CR (-1.02) | CR | MSD/BM | 151 | - | - | Alive 22 + mo | ||
| 20 | 36/F | p210 | Ph alone | CR | 400 | CR (-1.13) | CR (-0.56) | CR | MUD/BM | 217 | + (III) | + (L) | Alive 20 + mo | ||
| 21 | 23/F | p190 | Additional | CR | 400 | CR (-0.44) | CR (-0.16) | CR | MUD/BM | 176 | - | - | Alive 19 + mo | ||
| 22 | 40/F | p190 | Additional | CR | 400 | CR (-0.39) | Mol CR (UD) | Mol CR | MSD/BM | 162 | - | - | Alive 19 + mo | ||
| 23 | 48/F | p210 | Ph alone | CR | 600 | CR (-0.89) | CR (-0.31) | CR | MSD/BM | 215 | + (II) | - | Alive 18 + mo | ||
| 24 | 44/F | p190 | Ph alone | Refractory | 600 | Refractory (+1.10) | Refractory (+0.90) | Refractory | MUD/PB | 171 | + (II) | + (L) | Alive 18 + mo | ||
| 25 | 35/F | p190 | Ph alone | CR | 400 | CR (-0.89) | CR (-0.37) | CR | MSD/BM | 135 | - | - | Alive 17 + mo | ||
| 26 | 20/M | p190 | Additional | Refractory | 600 | Refractory (+0.67) | NA | Refractory | MSD/PB | 101 | + (II) | + (L) | Alive 16 + mo | ||
| 27 | 28/M | p210 | Ph alone | CR | 600 | CR (-1.23) | Mol CR (UD) | Mol CR | MUD/BM | 176 | - | + (L) | Alive 15 + mo | ||
| 28 | 37/M | p210 | Additional | CR | 400 | CR (-0.70) | CR (-0.62) | CR | MUD/BM | 181 | + (II) | - | Alive 14 + mo | ||
| 29 | 52/F | p210 | Ph alone | CR | 400 | CR (-1.26) | Mol CR (UD) | Mol CR | MSD/PB | 129 | - | - | Alive 12 + mo | ||
. | . | . | . | . | . | Response to imatinib* . | . | . | . | . | GVHD (grade) . | . | . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. . | Age, y/sex . | BCR-ABL isoform . | Karyotype . | Status before imatinib . | Imatinib dose, mg/d . | After first imatinib . | After second imatinib . | Pre-SCT status . | Graft source . | Time to SCT, d . | Acute . | Chronic . | Current DFS status; cause of death . | ||
| 1 | 39/F | p190 | Additional | CR | 600 | CR (-0.20) | Mol CR (UD) | Mol CR | MSD/BM | 128 | + (II) | + (E) | Died 32 mo; NRM | ||
| 2 | 29/M | p210 | Additional | CR | 400 | CR (-0.89) | CR (-0.27) | CR | MSD/BM | 136 | - | - | Alive 45 + mo | ||
| 3 | 40/M | p210 | Additional | CR | 600 | CR (-0.40) | CR (-0.79) | CR | MSD/BM | 132 | - | + (L) | Alive 43 + mo | ||
| 4 | 39/F | p190 | Additional | CR | 400 | CR (-0.35) | Mol CR (UD) | Mol CR | MSD/BM | 127 | - | - | Alive 42 + mo | ||
| 5 | 26/M | p190 | Ph alone | CR | 400 | CR (-1.22) | CR (-0.31) | CR | MSD/BM | 134 | - | - | Alive 39 + mo | ||
| 6 | 20/M | p210 | Additional | CR | 600 | CR (-0.97) | CR (-0.08) | CR | MSD/BM | 158 | - | + (L) | Alive 37 + mo | ||
| 7 | 43/F | p210 | Additional | CR | 600 | CR (-1.85) | CR (-0.09) | CR | MSD/BM | 139 | + (III) | NA | Died 3 mo; NRM | ||
| 8 | 40/F | p190 | Ph alone | CR | 600 | CR (-0.50) | CR (-1.09) | CR | MSD/BM | 126 | - | + (L) | Alive 34 + mo | ||
| 9 | 31/M | p210 | Ph alone | CR | 400 | CR (-0.93) | CR (-0.11) | CR | MSD/BM | 120 | - | - | Alive 32 + mo | ||
| 10 | 27/M | p190 | Additional | CR | 600 | Relapse (+0.93) | Relapse (+1.63) | Relapse | NA | NA | NA | NA | Died before SCT | ||
| 11 | 38/F | p190 | Additional | CR | 400 | CR (-0.20) | CR (-0.07) | CR | MSD/BM | 141 | + (II) | + (E) | Alive 29 + mo | ||
| 12 | 39/M | p210 | Ph alone | Refractory | 600 | CR (-0.89) | NA | CR | MSD/BM | 126 | - | + (L) | Alive 28 + mo | ||
| 13 | 30/M | p190 | Additional | CR | 400 | CR (-1.48) | NA | CR | MSD/BM | 56 | - | - | Alive 27 + mo | ||
| 14 | 44/M | p190 | Additional | Refractory | 600 | Refractory (+0.54) | NA | Refractory | MSD/PB | 137 | + (IV) | NA | Died 4 mo; NRM | ||
| 15 | 18/M | p190 | Additional | CR | 400 | CR (-0.48) | Mol CR (UD) | Mol CR | MSD/BM | 151 | - | + (E) | Alive 26 + mo | ||
| 16 | 31/M | p190 | Ph alone | Refractory | 600 | CR (-0.76) | CR (-1.31) | CR | MUD/BM | 191 | - | - | Died 9 mo; relapse | ||
| 17 | 55/F | p210 | Ph alone | CR | 600 | CR (-0.77) | Mol CR (UD) | Mol CR | MSD/BM | 126 | - | + (L) | Alive 25 + mo | ||
| 18 | 21/F | p210 | Ph alone | CR | 600 | CR (-0.48) | CR (-0.81) | CR | MSD/BM | 141 | + (II) | - | Alive 24 + mo | ||
| 19 | 28/F | p190 | Additional | Refractory | 600 | CR (-0.60) | CR (-1.02) | CR | MSD/BM | 151 | - | - | Alive 22 + mo | ||
| 20 | 36/F | p210 | Ph alone | CR | 400 | CR (-1.13) | CR (-0.56) | CR | MUD/BM | 217 | + (III) | + (L) | Alive 20 + mo | ||
| 21 | 23/F | p190 | Additional | CR | 400 | CR (-0.44) | CR (-0.16) | CR | MUD/BM | 176 | - | - | Alive 19 + mo | ||
| 22 | 40/F | p190 | Additional | CR | 400 | CR (-0.39) | Mol CR (UD) | Mol CR | MSD/BM | 162 | - | - | Alive 19 + mo | ||
| 23 | 48/F | p210 | Ph alone | CR | 600 | CR (-0.89) | CR (-0.31) | CR | MSD/BM | 215 | + (II) | - | Alive 18 + mo | ||
| 24 | 44/F | p190 | Ph alone | Refractory | 600 | Refractory (+1.10) | Refractory (+0.90) | Refractory | MUD/PB | 171 | + (II) | + (L) | Alive 18 + mo | ||
| 25 | 35/F | p190 | Ph alone | CR | 400 | CR (-0.89) | CR (-0.37) | CR | MSD/BM | 135 | - | - | Alive 17 + mo | ||
| 26 | 20/M | p190 | Additional | Refractory | 600 | Refractory (+0.67) | NA | Refractory | MSD/PB | 101 | + (II) | + (L) | Alive 16 + mo | ||
| 27 | 28/M | p210 | Ph alone | CR | 600 | CR (-1.23) | Mol CR (UD) | Mol CR | MUD/BM | 176 | - | + (L) | Alive 15 + mo | ||
| 28 | 37/M | p210 | Additional | CR | 400 | CR (-0.70) | CR (-0.62) | CR | MUD/BM | 181 | + (II) | - | Alive 14 + mo | ||
| 29 | 52/F | p210 | Ph alone | CR | 400 | CR (-1.26) | Mol CR (UD) | Mol CR | MSD/PB | 129 | - | - | Alive 12 + mo | ||
GVHD indicates graft-versus-host disease; DFS, disease-free survival; Mol CR, molecular complete remission; UD, undetectable; NA, not available; MSD, matched sibling donor; MUD, matched unrelated donor; BM, bone marrow; PB, peripheral blood; E, extensive; L, limited; mo, months; and NRM, nonrelapse mortality.
Values in parentheses indicate the changes of BCR-ABL/ABL ratio between each paired sample (before and after imatinib level)
Probabilities of relapse and nonrelapse mortality in the imatinib group versus the historical group. Solid line indicates imatinib group; dotted line, historical group.
Probabilities of relapse and nonrelapse mortality in the imatinib group versus the historical group. Solid line indicates imatinib group; dotted line, historical group.
Probabilities of disease-free survival and overall survival in the imatinib group versus the historical group. Solid line indicates imatinib group; dotted line, historical group.
Probabilities of disease-free survival and overall survival in the imatinib group versus the historical group. Solid line indicates imatinib group; dotted line, historical group.
In the historical group, 31 (17 first CR, 9 second CR, 5 refractory) of the 33 patients underwent HLA-matched sibling (n = 26) and unrelated (n = 5) SCT. Median time to SCT was 150 days (range, 130-234 days). Two patients died of disease progression before SCT. Because of the higher incidence of advanced (> first CR) pretransplantation disease status, patients in the historical group received a more intensive preparative regimen (TBI + cytarabine + melphalan). Acute GVHD was observed in 17 (54.8%) patients (7 grade II, 8 grade III, 2 grade IV). Sixteen (66.7%) of the 24 evaluable patients had chronic GVHD (7 limited, 9 extensive). The median follow-up of surviving patients in the historical group was 51 months (range, 40+ to 72+ months) after SCT. To date, 19 of the 31 patients that received SCT have died, 11 because of the relapse and progression of leukemia and 8 because of the transplant-related complications. The 5-year estimated probabilities of relapse, NRM, DFS, and overall survival were 45.7% ± 10.3%, 27.0% ± 8.2%, 38.7% ± 8.8%, and 38.7% ± 8.8%, respectively. The imatinib group showed better transplantation outcomes in terms of the probabilities of relapse (P < .001), DFS (P < .001), and overall survival (P < .001) than the historical group (Figures 3, 4).
MRD monitoring after SCT in the imatinib group
All but 2 (patient nos. 7, 14) of the patients who received a transplant in the imatinib group were monitored for MRD level after SCT as scheduled (Figure 2). Nineteen patients showed a sustained molecular CR, while the remaining 7 (patient nos. 3, 6, 12, 16, 19, 24, 26) had residual leukemia 3 or 6 months after SCT. However, with the exception of 2 patients (patient nos. 16, 19), their BCR-ABL/ABL ratios rapidly reduced to an undetectable level after the development of acute (patient no. 24) or chronic (patient nos. 3, 6, 12, 26) GVHD induced by the rapid withdrawal of cyclosporine. Of 2 patients without evidence of GVHD, 1 (patient no. 16) showed an increase in MRD at 6 months (1.35 × 10-5 → 6.78 × 10-5) and subsequently died of overt hematologic relapse following a further increase in MRD (8.12 × 10-2) at 12 months after SCT.
Discussion
Despite considerable improvements in the management of Ph+ ALL, a substantial proportion of patients continue to die as a result of disease progression. Consequently, patients at highest risk of relapse or resistance are likely to benefit from the identification of new criteria that enable patient outcome to be predicted. The quantification of BCR-ABL transcripts in Ph+ ALL has been used to assess treatment response and to detect residual leukemia prior to overt hematologic relapse.15,25-28 Radich et al25 studied 36 adults with Ph+ ALL and found that patients who were PCR positive after SCT have a significantly higher probability of relapse than PCR-negative patients. Recently, Scheuring et al29 reported on the usefulness of MRD analysis, using RQ-PCR in patients with relapsed or refractory Ph+ ALL treated with imatinib monotherapy as a salvage treatment. They found that 40 (71%) of the 56 patients achieved a good response with a significant decrease in MRD (1.37 logs in BM, 2.64 logs in PB), and that the BCR-ABL levels in BM and PB after 2 weeks of imatinib treatment and in BM after 4 weeks had a predictive relevance to response and progression-free survival.
On the basis of these results, we investigated the MRD-based role of imatinib as a first-line interim therapy in newly diagnosed Ph+ ALL. Using RQ-PCR, we found that median BCR-ABL/ABL ratios reduced by 0.77 log in 25 responders (86.2%) after the first imatinib cycle, compared with levels after induction. After the second imatinib cycle, median BCR-ABL/ABL ratios decreased by 0.34 log, compared with levels after consolidation. Furthermore, imatinib interim therapy possibly induced molecular CR in 7 patients. Indeed, 28 of the 29 enrolled patients underwent allogeneic SCT as scheduled after the completion of imatinib interim therapy. Of these, which included 7 patients with molecular CR, 25 were in first CR at the time of SCT. Patients receiving imatinib interim therapy showed a lower incidence of relapse during the consolidation phase and a higher rate of proceeding to SCT in first CR than the historical patients. A possibility of effect of different induction regimen or CNS prophylaxis on the pretransplantation disease status may be excluded in this study because CR rates after induction chemotherapy and incidence of CNS leukemia were similar between the 2 groups (Table 1). Several potential factors could account for the increased success of performing allogeneic SCT in first CR, including increased availability of HLA-matched unrelated donors and development of supportive care. Nevertheless, considering the similar presenting features and the same postinduction chemotherapy protocol, it is unlikely that these results were caused merely by selection biases. Rather, these results likely reflect an effect of imatinib on improving the quality of response and further reducing leukemia burden before SCT. Shimoni et al30 also reported on the substantial activity of pretransplantation imatinib monotherapy for patients with chronic myeloid leukemia in blastic crisis (n = 10) and Ph+ ALL (n = 5). In their study, 11 of the 15 patients received transplants in the chronic phase or in CR. Therefore, first-line imatinib interim therapy allowed SCT to be conducted in a more favorable status in association with a low MRD level in patients with Ph+ ALL.
A variety of potential mechanisms of resistance against imatinib have already been reported, including point mutations in the adenosine triphosphate binding domain or activation loop of the BCR-ABL oncoprotein, genomic amplification of the BCR-ABL fusion gene, up-regulation of BCR-ABL transcription, enhanced imatinib efflux mediated by the up-regulation of multidrug resistance proteins, or the decreased cellular bioavailability of imatinib.31-35 In our study, 4 (13.8%) of the 29 patients were primarily refractory to imatinib. These 4 patients had the p190BCR-ABL transcript, and 3 of them had additional chromosomal changes at the time of diagnosis. However, we did not identify any parameters predictive of response to imatinib. Hofmann et al36 suggested that gene expression profiling of Ph+ ALL blasts by microarray analysis could discriminate between imatinib-sensitive and imatinibrefractory leukemias, and that genes related to apoptosis pathways and cell cycle control were differently expressed in sensitive and resistant cells. Recently, Wassmann et al37 suggested that day 14 BM with a cutoff at 5% blasts and some presenting variables, including BCR-ABL amplification, a high WBC count, and circulating PB blasts, were important clinical parameters for predicting response to imatinib in 68 patients with Ph+ ALL receiving imatinib salvage therapy. If this analysis has the potential to identify patients at risk of relapse or resistance, tailored therapeutic interventions should be undertaken.
No data are available regarding the influence of imatinib on the outcome of SCT, and the optimal timing of imatinib discontinuation has not been defined. In the present study, the conditioning regimen was started 7 days from the last day of the imatinib cycle. We did not observe any detrimental effects of imatinib on engraftment, GVHD, or transplant-related organ toxicity, compared with the historical data. With a median follow-up of 25 months after SCT, 24 of the 28 patients who received transplants in the imatinib group are currently alive without evidence of leukemia and have yet to reach median survival. Outcome in the imatinib group was better than in the historical group with respect to the probabilities of relapse, DFS, and overall survival. However, this observation must be interpreted with caution because there were some limitations to the study design. The controls were a historical cohort of patients, making this a retrospective comparison. Although all patients who received transplants in the 2 groups were treated with the same protocols for conditioning and GVHD prophylaxis or treatment, it is difficult to compare treatment outcomes of historical approaches initiated many years ago with the therapeutic approaches of today. In addition, it is important to note that the follow-up time of the imatinib group was relatively short, so patients in the imatinib group remain at risk of developing further late events, such as disease relapse. Despite the limitations of our study, our results are supported by 2 other reports. Recently, Thomas et al38 reported an encouraging result of concurrent imatinib plus chemotherapy for 20 patients with Ph+ ALL. In their study, 15 (75.0%) of their 20 patients remained alive without evidence of leukemia after a median follow-up of 20 months from the start of induction therapy. Of their 20 patients, 10 (50.0%) received allogeneic SCT in first CR, and, at a median follow-up of 12 months after SCT, all but 1 of those patients remained alive without disease. Of the 10 patients who had not undergone allogeneic SCT, 5 patients remained alive in sustained CR (4+ to 24+ months). The investigators suggested that outcome for concurrent imatinib plus chemotherapy was better than with prior regimes (historical data) in terms of CR rates (P < .01), DFS (P < .001), and overall survival rates (P = .001). According to data from the International Bone Marrow Transplant Registry (n = 6991),39 patients with acute leukemia who were free of their leukemia 24 months after allogeneic SCT showed the high probability of living for 5 or more years (95% confidence interval, 88%-90%). Taken together, our results suggest that the imatinib interim therapy might provide a survival advantage for patients with Ph+ ALL.
The role of allogeneic SCT remains to be determined, as durable remissions were observed in some patients with Ph+ ALL who received concurrent imatinib plus chemotherapy alone.38 We also observed a phenomenon maintaining durable CR in the absence of allogeneic SCT. In the present study, 3 patients who had sustained CR (including 1 molecular CR) until the completion of 3 courses of imatinib interim therapy were excluded because of a lack of HLA-matched donors. For those patients, maintenance therapy was planned with 6-mercaptopurine (50 mg/m2/d), methotrexate (15 mg/m2/wk), and imatinib (400 mg/d) for 2 years. Of them, 1 patient who achieved molecular CR remains alive with a leukemia-free status after a follow-up of 26 months from the date of initiation of therapy. Conversely, the remaining 2 patients died of their disease progression at 11 and 15 months, respectively (data not shown).
Previously, we reported a phenomenon involving increased MRD followed by a rapid return to a PCR-negative status after the development of chronic GVHD in some historical patients.15 The current follow-up of the historical group showed that the probability of relapse was significantly lower in patients with chronic GVHD than that in patients without chronic GVHD (22.1% ± 11.3% versus 75.0% ± 15.3%, P = .009). In the present study, we were able to repeatedly observe the relationship between GVHD and antileukemic activity by serially monitoring MRD level in the imatinib group. Seven of the 28 patients who received transplants had residual leukemia at 3 or 6 months after SCT. Of them, 5 patients showed a decrease in MRD to an undetectable level after the development of acute (n = 1) or chronic GVHD (n = 4), induced by the rapid withdrawal of cyclosporine. However, the remaining 2 patients showed no evidence of GVHD after cyclosporine withdrawal, and 1 of them showed a pattern of MRD increase at 6 months after SCT. At this time, we recommended preemptive imatinib therapy and donor lymphocyte infusion (DLI), but the patient refused. Unfortunately, the patient died of overt hematologic relapse with a high MRD level 12 months after SCT. Regarding the role of GVHD in ALL, the Seattle group first described an inverse relationship between relapse and GVHD, especially chronic GVHD.40 According to data from the International Bone Marrow Transplant Registry,41 acute GVHD had a stronger antileukemic activity in patients with ALL, whereas chronic GVHD was more important at reducing the probability of relapse in patients with early-phase myeloid leukemia. Conversely, other investigators have confirmed the importance of chronic GVHD in reducing relapse in ALL.42-44 These conflicting results may be due to either the different prophylactic methods used for GVHD or to heterogeneities of patient populations. However, the clinical significance of GVHD in Ph+ ALL has not been widely discussed in the literature. Cornelissen et al14 suggested that Ph+ ALL does illustrate the graft-versus-leukemia effect, because they found significantly fewer relapses and better DFS in adults with Ph+ ALL undergoing HLA-matched unrelated transplantation as compared with ALL patients with other karyotypes. According to data from the Seattle group,28 patients with chronic GVHD showed a significantly lower risk of relapse (relative risk = .33, P = .038) in Ph+ ALL. Regarding the treatment options for relapsed patients with acute and chronic leukemias, DLI has been widely used. However, the efficacy of DLI was uniformly dismal in patients with ALL who experience overt hematologic relapse, probably because of the rapid pace of the disease and the delayed antileukemic effect of DLI.45 Furthermore, results with imatinib monotherapy were disappointing for most patients with Ph+ ALL with hematologic relapse after allogeneic SCT.18 Therefore, we suggest that MRD-based postgrafting immunomodulation (eg, immunosuppressant discontinuation, dose-escalating DLI, etc) in combination with imatinib at the earliest level of disease recurrence may have a potential role in the long-term control of residual leukemia in Ph+ ALL.
In summary, this study suggests that first-line imatinib interim therapy may be a feasible treatment strategy in terms of providing a good quality of CR and securing a survival advantage for patients with Ph+ ALL. In addition, MRD monitoring makes evaluating the role of imatinib and postgrafting immunomodulation in individual patients with Ph+ ALL possible. Further long-term follow-up is needed to validate the results of this study.
Prepublished online as Blood First Edition Paper, January 18, 2005; DOI 10.1182/blood-2004-09-3785.
Supported by a Korea Research Foundation Grant (KRF-2004-013-E00011).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the dedicated nurses of our stem cell transplantation program, our fellows and house-staff, and the medical technicians for their excellent assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal