Abstract
Chronic lymphocytic leukemia (CLL) B cells become sensitive to Fas (CD95)–mediated apoptosis 3 to 5 days after CD40 ligation. However, CD4+ cytotoxic T lymphocytes (CTLs) can kill CLL B cells via a Fas-ligand (CD178)–dependent process within 24 hours after CD40 cross-linking, when ligation of CD95 alone is insufficient to induce apoptosis. In addition to CD95, CD40-activated CLL cells also express DR5, a receptor for tumor-necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) that is expressed by CD4+ CTL. In addition, CD40 ligation in vitro and in vivo induces CLL cells to express the proapoptotic protein, BH3 interacting domain death agonist (Bid), which can facilitate crosstalk between mitochondrial-dependent, apoptosis-inducing pathways and death receptors, such as death receptor 5 (DR5). To evaluate whether ligation of CD95 and/or DR5 can induce apoptosis of CD40-activated CLL cells, we generated artificial cytotoxic effector cells that express both human TRAIL and CD178 (Chinese hamster ovary [CHO]–CD178/TRAIL) or only TRAIL (CHO-TRAIL) or CD178 (CHO-CD178). CHO-CD178/TRAIL cells were significantly more effective in killing CD40-activated CLL cells than either CHO-TRAIL or CHO-CD178 and, unlike the latter, could kill CLL cells 24 hours after CD40 ligation. We conclude that CD40 ligation induces CLL cells to express the proapoptotic molecule Bid and the death receptors CD95 and DR5, the latter of which can act synergistically to induce caspase-dependent apoptosis of CD40-activated CLL B cells.
Introduction
Patients with chronic lymphocytic leukemia (CLL) treated with intravenous infusions of autologous leukemia cells transfected with an adenovirus vector encoding the CD40 ligand (Ad-CD154) experienced acute reductions in leukemia cell counts and lymph node size.1 Such changes were associated with induction of the death receptor Fas (CD95) on bystander, nontransfected CLL cells in vivo.1 Conceivably, the induced expression of CD95 on the entire leukemia cell population rendered it susceptible to CD95-mediated apoptosis by cells bearing the CD95 ligand (CD178), such as activated T cells or natural killer (NK) cells.2,3 Consistent with this hypothesis, we found that CLL cells acquire latent sensitivity to CD95-mediated apoptosis 3 to 5 days following CD40 ligation.4 Although sole ligation of CD95 was not sufficient to induce apoptosis of newly activated CLL cells during the first few days after CD40 cross-linking, we observed that CD4+ cytotoxic T lymphocytes (CTLs) could kill such newly activated CLL cells via a CD95-dependent pathway(s).4
Cells sensitive to CD95-mediated apoptosis can be divided into 2 types, type I and type II.5,6 Whereas the apoptotic pathway in type I cells involves CD95-induced activation of procaspases 8 and 3 and downstream caspase substrates, the pathway in type II cells depends upon crosstalk between mitochondrial-death–dependent pathways and extrinsic death receptors, such as CD95. This crosstalk is mediated by cleavage of the proapoptotic protein BH3 interacting domain death agonist (Bid) by activated caspase 8, which in turn results in the release of proapoptotic factors such as cytochrome c and Smac from the mitochondria to the cytosol.7-9 Here, cytochrome c in conjunction with adenosine triphosphate (ATP) activates caspase 9 in the so-called apoptosome, while Smac binds to inhibitors of apoptosis proteins (IAPs), thereby mitigating their inhibitory effect on effector caspases, such as caspase 3 or 7.
Proximal events in apoptosis mediated by death receptors of the tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL) are very similar to CD95, having an absolute requirement for Fas-associated death domain (FADD) and caspase 8 in the TRAIL-induced formation of death-inducing signaling complex (DISC) required for initiation of apoptosis via the extrinsic pathway.10,11 Events downstream of TRAIL-induced cleavage of caspase 8 follow a pattern of cell death that is independent or dependent on mitochondria factors, depending on whether the cell is type I or type II, respectively.12,13 In addition, ligation of TRAIL receptors also can induce activation of caspases other than caspase 8, which in turn can induce cleavage of Bid in type II cells for initiation of apoptosis via the intrinsic pathway.14 Certain differences between CD95- and TRAIL receptor–mediated apoptosis signaling are suggested by the finding that TRAIL, in contrast to CD178, is cytotoxic against many tumor cells but not against most normal cells15-17 and that certain CD95-resistant cell lines still exhibit sensitivity to TRAIL-mediated apoptosis.18 The results from such studies show that, despite having many similar features, CD95 and TRAIL receptors can trigger apoptosis via distinctive signaling pathways, which conceivably could work synergistically to induce apoptosis of cells expressing both CD95 and TRAIL death receptors.
To test these hypotheses, we examined CLL B cells for expression of TRAIL death receptors and Bid before and after CD40 ligation and examined activated CD4+ CTLs for concomitant expression of CD178 and TRAIL. In addition, we generated artificial CTLs using Chinese hamster ovary (CHO) cells transduced to express human CD178 (CHO-CD178), TRAIL (CHO-TRAIL), or both CD178 and TRAIL (CHO-CD178/TRAIL). Using these artificial cytotoxic effector cells, we could address whether physiologic co-ligation of CD95 and TRAIL death receptors could act synergistically to induce apoptosis of CLL cells following CD40 activation.
Materials and methods
Cells
Blood was obtained after written informed consent from patients with CLL. Approval was obtained from the University of California, San Diego's institutional review board for these studies. Informed consent was provided according to the Declaration of Helsinki. The mononuclear cells were isolated by density centrifugation over Ficoll-Paque (Amersham Biosciences, Piscataway, NJ) and viably frozen in fetal bovine serum (FBS) (Omega Scientific, Tarzana, CA) containing 10% dimethyl sulfoxide (DMSO). CHO cells, HeLa cells, and the JURKAT T-cell line were obtained from the American Type Culture Collection (ATCC, Manassas, VA). CHO cells that express CD178 (CHO-CD178 cells) and HeLa cells that express human CD154 (HeLa-CD154 cells) were as described.4 CHO-TRAIL cells or CHO-CD178/TRAIL cells were generated by respectively infecting CHO cells or CHO-CD178 cells with a replication-defective adenovirus type 5 vector encoding human TRAIL (Ad-TRAIL) supplied by Dr Mark Cantwell (Tragen, San Diego, CA). CHO cells were monitored via flow cytometry for expression of TRAIL using a phycoerythrin-conjugated monoclonal antibody (mAb) (BD-PharMingen, San Diego, CA).
CD40 activation
CLL cells were cocultured for 24 hours with HeLa cells or HeLa-CD154 in RPMI culture medium (Irvine Scientific, Santa Ana, CA) supplemented with 10% fetal bovine serum (Gibco/Invitrogen, Carlsbad, CA) in 5% CO2 at 37°C. All time points of CD40 activation refer to days after completion of the 24 hours' coculture of the CLL cells with HeLa or HeLa-CD154 cells. CD40 activation was monitored on CD19+ CLL cells by flow cytometry of cells stained with fluorochrome-conjugated mAb (CD95; BD-PharMingen) or biotinylated mAb (DR4 and DR5; Alexis, Carlsbad, CA) developed with second-step staining with streptavidin-allophycocyanin (PharMingen). In each case, isotype control mAb of irrelevant specificity was used to monitor for nonspecific cytophillic antibody binding. To compare expression levels of surface antigens, we calculated the mean fluorescence intensity ratio (MFIR) for each antigen. This is the ratio of mean fluorescence of the cells stained with the fluorochrome-conjugated, or biotinylated, specific mAb divided by the mean fluorescence intensity of the cells stained with a fluorochrome-conjugated, or biotinylated, isotype-control mAb of irrelevant specificity.
T-cell activation
CD4 T cells were isolated from the peripheral blood mononuclear cells (PBMCs) of patients with CLL using the magnetic beads coated with anti-CD4 mAbs (DYNAL, Oslo, Norway). The T cells were cultured at a density of 106 cells/mL with anti-CD3/CD28 beads (DYNAL) in XVIVO-15 medium (Cambrex, East Rutherford, NJ) supplemented with 5% human AB serum and 100 U/mL of recombinant human interleukin-2 (IL-2) (BD-PharMingen). Anti-CD3/CD28 beads were added on days 0 and 19. After 21 days, the T cells were harvested and examined for expression of TRAIL and CD178 by flow cytometry after staining with fluorochrome-conjugated anti-TRAIL and biotinylated anti-CD178 mAb and second-step staining with streptavidin allophycocyanin (APC) (BD-PharMingen).
Death receptor–mediated apoptosis
Apoptosis of CLL cells was monitored by a flow cytometric assay using 3,3′ dihexyloxacarbocyanine iodide (DiOC6) (Molecular Probes, Eugene, OR) and propidium iodide (PI) (Molecular Probes), as previously described.19 CLL-target cells were cocultured with CHO effector cells for 8 hours at a 1:10 ratio. To distinguish CHO cells from CLL cells, the CHO cells were labeled with PKH26 (Sigma, Saint Louis, MO) immediately prior to coculture. Control samples were incubated with the pan-caspase inhibitor N-carbobenzoxy-Val-Ala-Asp fluoromethyl ketone (zVAD-fmk) (Calbiochem, San Diego, CA) or with blocking anti-CD178 mAb NOK-2 or anti-TRAIL mAb RIK-2 (BD-PharMingen). The zVAD-fmk was dissolved as a 50 mM stock solution in DMSO and stored at –20°C. After 8 hours, the cells were stained with 40 nM DiOC6 for 15 minutes at 37°C and analyzed using a FACS-Calibur (Becton Dickinson, San Jose, CA). The percent specific killing of JURKAT target cells was determined by subtracting the level of apoptosis observed in the target cell population cultured with CHO cells from the level of apoptosis observed in the target cell population cultured with cells plus CD178 and/or TRAIL.
Immunoblot analysis
CLL cells were cocultured with HeLa cells or CD154-expressing HeLa cells for 24 hours, separated, and then cultured alone for 1, 2, or 4 days prior to harvesting to prepare a whole-cell lysate. Alternatively, mononuclear cells were isolated from patients with CLL before or 1 or 14 days after infusion with Ad-CD154–transduced autologous CLL cells, as described.1 For preparation of whole-cell lysates, the cells were collected by centrifugation at 250g for 10 minutes at 4°C, washed once in ice-cold phosphate-buffered saline (PBS), and then lysed with sonification in radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [tris(hydroxymethyl)aminomethane]/HCl, pH 7.4, 1% Nonidet P-40, 0.25% Na-Deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 150 mM NaCl, 5 mM EDTA [ethylenediaminetetraacetic acid], 1 mM phenylmethylsulfonyl fluoride (PMSF), 10 μg/mL aprotinin, 10 μg/mL leupeptin, and 10 μg/mL pepstatin) for 15 minutes on ice. The lysate was cleared from insoluble debris by centrifugation at 21 000g for 10 minutes at 4°C, and the supernatant was stored at –80°C. Forty micrograms of protein lysate was loaded onto each lane of a 5% to 15% gradient sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gel (Bio-Rad, Hercules, CA) and transferred to a polyvinylidene fluoride microporous membrane (Millipore, Billerica, MA). Filters were probed with anti-Bid antibody (Cell Signaling Technology, Beverly, MA) and goat anti–rabbit immunoglobulin (IgG) horseradish peroxidase (HRP) (Santa Cruz, Santa Cruz, CA). Stripped filters were probed with anti–β-actin (Sigma) and then goat anti–mouse IgG-HRP (Santa Cruz) to assess the level of β-actin present in each sample lane.
Results
Expression of death receptors on CLL cells following CD40 ligation
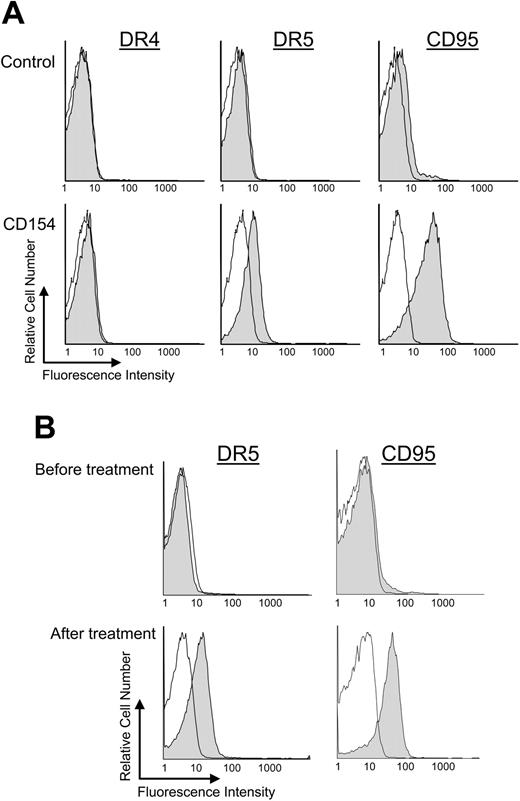
We examined CLL cells for expression of death receptor 4 (DR4), DR5, and CD95 before and at various times after CD40-ligation. The expression levels of each of these receptors were negligible on each of the samples (n = 6) prior to CD40 activation (Figure 1A-B). Furthermore, expression of DR4 did not change following coculture with CD154-expressing cells (Figure 1A, data not shown). However, within 24 hours of coculture with CD154-expressing cells, the CD19+ CLL cells expressed both DR5 and CD95 (Figure 1A).4 The MFIR of DR5, for example, increased from a pretreatment value of 1.3 ± 0.2 (average MFIR ± SD, n = 6) to 2.3 ± 0.2 (average MFIR ± SD, n = 6) within 24 hours after CD40 activation. CLL cells cocultured with nontransduced cells that did not express CD154, however, were not induced to express higher levels of DR5 or CD95 (data not shown).
Expression of death receptors after CD40 ligation on CLL B cells in vitro and in vivo. Surface expression of death receptors on CD19+ CLL cells was monitored by flow cytometry. (A) CLL B cells were cocultured for 24 hours with HeLa cells (control) or HeLa cells expressing CD154 (CD40 ligation). Representative shaded histograms show the expression of DR4 (left column), DR5 (middle column), or CD95 (right column) on CLL cells 24 hours after coculture with HeLa cells (top row) or HeLa cells that express CD154 (bottom row). The open histograms represent the fluorescence of the cells stained with a fluorochrome-conjugated isotype control mAbs of irrelevant specificity. (B) CLL B cells were isolated from gene therapy patients before treatment and 24 hours after infusion of Ad-CD154–transduced CLL cells (after treatment). Representative shaded histograms show the expression of DR5 (left column) or CD95 (right column). The open histograms represent the fluorescence of the cells stained with isotype-matched control mAbs of irrelevant specificity.
Expression of death receptors after CD40 ligation on CLL B cells in vitro and in vivo. Surface expression of death receptors on CD19+ CLL cells was monitored by flow cytometry. (A) CLL B cells were cocultured for 24 hours with HeLa cells (control) or HeLa cells expressing CD154 (CD40 ligation). Representative shaded histograms show the expression of DR4 (left column), DR5 (middle column), or CD95 (right column) on CLL cells 24 hours after coculture with HeLa cells (top row) or HeLa cells that express CD154 (bottom row). The open histograms represent the fluorescence of the cells stained with a fluorochrome-conjugated isotype control mAbs of irrelevant specificity. (B) CLL B cells were isolated from gene therapy patients before treatment and 24 hours after infusion of Ad-CD154–transduced CLL cells (after treatment). Representative shaded histograms show the expression of DR5 (left column) or CD95 (right column). The open histograms represent the fluorescence of the cells stained with isotype-matched control mAbs of irrelevant specificity.
We also examined the viably frozen leukemia cell samples isolated from patients before and after Ad-CD154 gene therapy (n = 3).1 Again, negligible expression of DR5 or CD95 was observed on the CLL cells prior to Ad-CD154 gene therapy (Figure 1B). However, bystander CLL cells obtained 1 or more days after infusion of autologous Ad-CD154–transduced leukemia cells expressed high levels of both death receptors for at least 14 days after the infusion1 (Figure 1B, data not shown).
CLL-cell expression of Bid before and after CD40 ligation
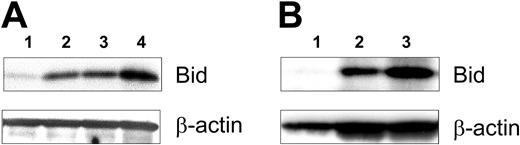
We examined whether CLL cells were type I or type II cells before and after CD40 activation, as assessed via the expression of the proapoptotic protein Bid. CLL cells (n = 8) prior to CD40 activation expressed negligible levels of Bid, as assessed via immunoblot analysis, consistent with the notion that resting CLL cells are type I cells (Figure 2A). However, following coculture with CD154-expressing cells, the CLL cells expressed increasingly greater amounts of Bid, as early as one day after CD40 activation (Figure 2A, lane 2). In contrast, CLL cells cocultured with HeLa cells lacking expression of CD154 were not induced to express Bid at any time point (Figure 2A, lane 1, data not shown). Similarly, we found that CLL cells isolated from patients (n = 2) 1 or 14 days after Ad-CD154 gene therapy also expressed high levels of Bid (Figure 2B, lanes 2 and 3). In contrast, CLL cells isolated prior to the infusion of Ad-CD154–transduced, autologous CLL cells did not have detectable expression of this proapoptotic protein (Figure 2B, lane 1).
Time course of bid expression after CD40 ligation on CLL B cells. In panel A the CLL cells were cocultured 1 day with HeLa cells or HeLa-CD154 that expressed the CD40 ligand. The CLL cells were separated from the adherent HeLa cells, cultured separately, and then used to prepare whole-cell lysates. Forty micrograms of cell lysate was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-Bid (top row) or anti–β-actin (bottom row), as indicated on the right side of the figure. The different lanes are of lysates prepared from CLL cells cultured for 1 day after coculture with HeLa cells (lane 1) or for 1 day (lane 2), 2 days (lane 3), or 4 days (lane 4) after coculture with HeLa-CD154 cells. In panel B, CLL cells were isolated from a patient before (lane 1), 1 day after (lane 2), or 14 days after (lane 3) infusion of 1 × 109 Ad-CD154–transduced autologous leukemia cells, as described.1 Forty micorgrams of cell lysate was applied to each lane for immunoblot analysis, as described in panel A.
Time course of bid expression after CD40 ligation on CLL B cells. In panel A the CLL cells were cocultured 1 day with HeLa cells or HeLa-CD154 that expressed the CD40 ligand. The CLL cells were separated from the adherent HeLa cells, cultured separately, and then used to prepare whole-cell lysates. Forty micrograms of cell lysate was applied to each lane of a polyacrylamide gel for immunoblot analyses with anti-Bid (top row) or anti–β-actin (bottom row), as indicated on the right side of the figure. The different lanes are of lysates prepared from CLL cells cultured for 1 day after coculture with HeLa cells (lane 1) or for 1 day (lane 2), 2 days (lane 3), or 4 days (lane 4) after coculture with HeLa-CD154 cells. In panel B, CLL cells were isolated from a patient before (lane 1), 1 day after (lane 2), or 14 days after (lane 3) infusion of 1 × 109 Ad-CD154–transduced autologous leukemia cells, as described.1 Forty micorgrams of cell lysate was applied to each lane for immunoblot analysis, as described in panel A.
Expression of Fas-ligand (CD178) and TRAIL on activated CLL T cells
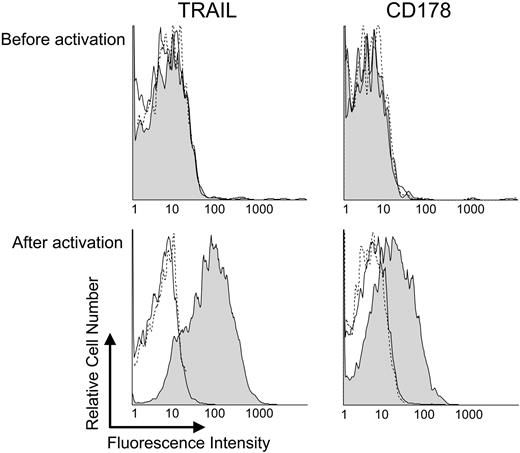
Prior studies indicated that activated cytotoxic CD4+ T cells could express CD1782 and possibly also TRAIL.20 To examine whether the blood CD4+ T cells of patients with CLL also could be induced to express such death-receptor ligands, we isolated blood CD4+ T cells from the mononuclear cells of patients with CLL (n = 8) and activated them via CD3/CD28 co-ligation, which in prior studies was found capable of inducing T-cell proliferation in vitro.21,22 Cultured CD3/CD28-activated T cells were assessed for expression of CD178 and TRAIL by flow cytometry. In contrast to resting T cells, T cells activated via CD3/CD28 expressed both CD178 and TRAIL (Figure 3).
Activated T cells from patients with CLL express CD178 and TRAIL. CD4+ T cells from patients with CLL were isolated and activated as described in “Materials and methods.” Surface expression of TRAIL or CD178 on CD4+ T cells was analyzed before (top row) or after (bottom row) CD3/CD28 activation. (C) The shaded histograms represent TRAIL (left column) or CD178 (right column) expression. Isotype-matched controls mAb staining are given as solid lines, whereas autofluorescence is provided as dashed lines.
Activated T cells from patients with CLL express CD178 and TRAIL. CD4+ T cells from patients with CLL were isolated and activated as described in “Materials and methods.” Surface expression of TRAIL or CD178 on CD4+ T cells was analyzed before (top row) or after (bottom row) CD3/CD28 activation. (C) The shaded histograms represent TRAIL (left column) or CD178 (right column) expression. Isotype-matched controls mAb staining are given as solid lines, whereas autofluorescence is provided as dashed lines.
Sensitivity to CD178 and/or TRAIL-induced apoptosis following CD40 activation
To address whether physiologic ligation of CD95 and/or DR5 on CLL cells could induce apoptosis, we generated artificial cytotoxic effector cells from CHO cells that expressed CD178 (CHO-CD178), TRAIL (CHO-TRAIL), or both CD178 and TRAIL (CHO-CD178/TRAIL). Expression of CD178 and/or TRAIL on the transduced CHO cells was assessed via flow cytometry and found to be similar to that of activated CD4+ T cells of patients with CLL (data not shown). In addition, the transduced effector cells were examined for their capacity to induce apoptosis of JURKAT T cells that expressed both CD95 and DR5. We found that CHO-CD178, CHO-TRAIL, or CHO-CD178/TRAIL induced apoptosis of 51% (± 16% SE, n = 5), 64% (± 14% SE), or 65% (± 9% SE) of the JURKAT cells within 8 hours of coculture, respectively. In contrast, nontransduced CHO cells did not induce apoptosis of JURKAT cells, even at later time points (data not shown). The capacity of CHO-CD178, CHO-TRAIL, or CHO-CD178/TRAIL cells to induce apoptosis of JURKAT cells could be blocked by addition to the cocultures of the nonspecific caspase inhibitor zVAD-fmk (at 50 μM) or the relevant blocking mAb for CD178 and/or TRAIL (data not shown).
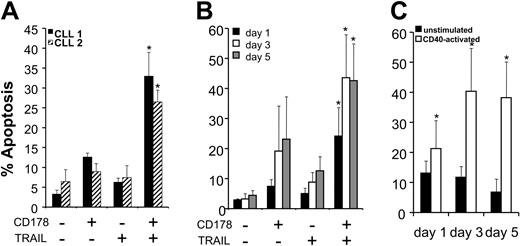
We cocultured each of CHO-effector cell populations with CLL cells that had been activated by prior coculture with CD154-expressing cells. Before and one day after CD40 activation, the CLL cells were resistant to apoptosis induced by either CHO-CD178 or CHO-TRAIL cytotoxic effector cells (Figure 4A-B). In contrast, CHO-CD178/TRAIL effector cells could induce apoptosis of CLL cells as early as 1 day after CD40 activation (Figure 4A-B).
CD178- and/or TRAIL-mediated apoptosis of CLL B cells after CD40-ligation. (A) CLL cells from representative patients 1 (▪) or 2 (▨) were cocultured with HeLa-CD154 cells to effect CD40 activation and then tested 24 hours later for their susceptibility to apoptosis induced by 8 hours' coculture with CHO cells (–/–), CHO-CD178 cells (+/–), CHO-TRAIL cells (–/+), or CHO-CD178/TRAIL cells (+/+), as indicated at the bottom of the figure. The bars depict the mean levels of apoptosis observed for 3 independent experiments, as indicated on the left axis. The error bars indicate the standard errors about the mean. For both samples, the mean levels of apoptosis observed upon coculture with CHO-CD178/TRAIL cells were significantly greater than those observed following coculture with CHO, CHO-CD178, or CHO-TRAIL cells (indicated by the bars with the asterisk) (Bonferroni t test ≥ 0.05). (B) Leukemia cells from 9 different patients were cocultured for 8 hours with each of the various CHO effector cells on days 1 (▪), 3 (□), or 5 (▦) after coculture with HeLa-CD154. The bars depict the mean levels of apoptosis that were observed for the 9 different CLL patient samples at the various times when cultured with CHO effector cells as indicated at the bottom of the figure. The error bars depict the standard deviation of the mean. The asterisk highlights the bars representing the mean levels of apoptosis observed in the CLL cell samples when cultured with CHO-CD178/TRAIL cells, each of which were significantly greater than the mean levels of apoptosis observed in the respective CLL cell samples cocultured with CHO cells, CHO-CD178 cells, or CHO-TRAIL cells (P ≥ .05, Bonferroni t test). (C) Unstimulated (▪) or CD40-activated (□) leukemic cells from 9 different patients were cocultured for 8 hours with CHO-CD178/TRAIL effector cells on day 1, day 3, and day 5 after HeLa coculture. The bars depict the mean levels of apoptosis and the error bars the standard deviation of the mean. The asterisks indicate significantly greater apoptosis levels of the CD40 activated versus the unstimulated samples on the respective days (P ≥ .05, Student t test).
CD178- and/or TRAIL-mediated apoptosis of CLL B cells after CD40-ligation. (A) CLL cells from representative patients 1 (▪) or 2 (▨) were cocultured with HeLa-CD154 cells to effect CD40 activation and then tested 24 hours later for their susceptibility to apoptosis induced by 8 hours' coculture with CHO cells (–/–), CHO-CD178 cells (+/–), CHO-TRAIL cells (–/+), or CHO-CD178/TRAIL cells (+/+), as indicated at the bottom of the figure. The bars depict the mean levels of apoptosis observed for 3 independent experiments, as indicated on the left axis. The error bars indicate the standard errors about the mean. For both samples, the mean levels of apoptosis observed upon coculture with CHO-CD178/TRAIL cells were significantly greater than those observed following coculture with CHO, CHO-CD178, or CHO-TRAIL cells (indicated by the bars with the asterisk) (Bonferroni t test ≥ 0.05). (B) Leukemia cells from 9 different patients were cocultured for 8 hours with each of the various CHO effector cells on days 1 (▪), 3 (□), or 5 (▦) after coculture with HeLa-CD154. The bars depict the mean levels of apoptosis that were observed for the 9 different CLL patient samples at the various times when cultured with CHO effector cells as indicated at the bottom of the figure. The error bars depict the standard deviation of the mean. The asterisk highlights the bars representing the mean levels of apoptosis observed in the CLL cell samples when cultured with CHO-CD178/TRAIL cells, each of which were significantly greater than the mean levels of apoptosis observed in the respective CLL cell samples cocultured with CHO cells, CHO-CD178 cells, or CHO-TRAIL cells (P ≥ .05, Bonferroni t test). (C) Unstimulated (▪) or CD40-activated (□) leukemic cells from 9 different patients were cocultured for 8 hours with CHO-CD178/TRAIL effector cells on day 1, day 3, and day 5 after HeLa coculture. The bars depict the mean levels of apoptosis and the error bars the standard deviation of the mean. The asterisks indicate significantly greater apoptosis levels of the CD40 activated versus the unstimulated samples on the respective days (P ≥ .05, Student t test).
Three to five days after CD40 activation the CLL cells became sensitive to apoptosis induced by CHO-CD178 or CHO-TRAIL effector cells (Figure 4B). CHO-CD178 effector cells could effect significant levels of CLL cell killing at day 3 and day 5 after CD40 activation, as noted in previous studies4 (Figure 4B). Moreover, CHO-TRAIL effector cells also induced killing of CLL cells 3 or 5 days after CD40 ligation at levels that were significantly higher than that observed with nontransduced CHO cells (P < .005, Student t test). CHO-CD178/TRAIL effector cells, on the other hand, induced significantly higher levels of apoptosis of CD40-activated CLL cells than either CHO-CD178 or CHO-TRAIL effector cells at any time point tested (Figure 4B) (P < .05, Bonferroni t test). In contrast, resting CLL cells, or CLL cells cocultured with nontransduced HeLa cells, were not sensitive to the cytotoxic effects of CHO-CD178/TRAIL effector cells at any time point tested (Figure 4C, data not shown).
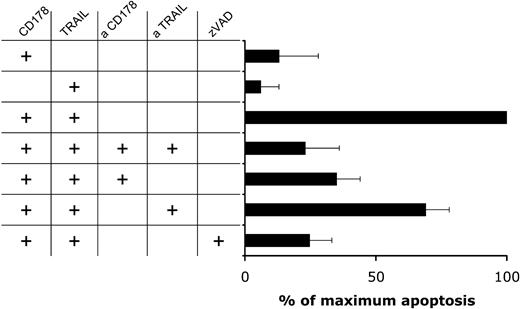
Addition of either anti-CD178 or anti-TRAIL mAbs to the cultures at 10 μg/mL prior to the cytotoxicity assay significantly inhibited the killing of CD40-activated CLL cells by CHO-CD178/TRAIL effector cells (Figure 5). This was similar to the effect of anti-CD178 mAb on apoptosis of CD40-activated CLL cells by activated CD4+ T cells.4 Moreover, addition of zVAD-fmk (at 50 μM) significantly inhibited the killing of CD40-activated CLL cells by CHO-CD178/TRAIL effector cells to background levels in control cultures or in cultures of CD40-activated CLL cells with CHO-CD178/TRAIL effectors with blocking mAb specific for CD178 and TRAIL (Figure 5) or of CD40-activated CLL cells cocultured with nontransduced CHO cells (data not shown). These results indicate that CD178 and TRAIL act synergistically to induce apoptosis of CD40-activated CLL cells via a caspase-dependent pathway.
CD178/TRAIL-induced apoptosis is dependent upon caspase activation and CD95 triggering. CLL B cells were cocultured for 24 hours with HeLa cells expressing CD154 as described in “Materials and methods.” After CD40 activation the CLL cells (n = 3) were cocultured with CHO effector cells expressing CD178, TRAIL, or both CD178 and TRAIL, as indicated on the left side. Thirty minutes before the initiation of each coculture, the various CHO–effector-cell populations were incubated with blocking mAbs specific for CD178 or TRAIL, or both blocking mAbs for CD178 and TRAIL, as indicated. In some cultures, we added zVAD to the CLL cells 30 minutes before the initiation of the coculture, as indicated on the left. A 100% value to the level of apoptosis achieved using CHO-CD178/TRAIL effector cells was used to compare the levels of CLL cell killing with that observed using other CHO effector cells and/or other culture conditions. Apoptosis induced by the other treatment was calculated relative as (percent apoptosis of treatment divided by percent apoptosis of CHO-CD178/TRAIL × 100). The error bars indicate the standard deviation of the mean.
CD178/TRAIL-induced apoptosis is dependent upon caspase activation and CD95 triggering. CLL B cells were cocultured for 24 hours with HeLa cells expressing CD154 as described in “Materials and methods.” After CD40 activation the CLL cells (n = 3) were cocultured with CHO effector cells expressing CD178, TRAIL, or both CD178 and TRAIL, as indicated on the left side. Thirty minutes before the initiation of each coculture, the various CHO–effector-cell populations were incubated with blocking mAbs specific for CD178 or TRAIL, or both blocking mAbs for CD178 and TRAIL, as indicated. In some cultures, we added zVAD to the CLL cells 30 minutes before the initiation of the coculture, as indicated on the left. A 100% value to the level of apoptosis achieved using CHO-CD178/TRAIL effector cells was used to compare the levels of CLL cell killing with that observed using other CHO effector cells and/or other culture conditions. Apoptosis induced by the other treatment was calculated relative as (percent apoptosis of treatment divided by percent apoptosis of CHO-CD178/TRAIL × 100). The error bars indicate the standard deviation of the mean.
Discussion
Encouraging results were reported for a trial in gene therapy for patients with CLL.1 Patients were given a single intravenous bolus infusion of autologous leukemia cells transduced with Ad-CD154, a recombinant adenovirus encoding CD154. This and prior studies indicated that transduction with Ad-CD154 induced infected and bystander noninfected leukemia cells to express CD95 along with immune co-stimulatory molecules required for effective presentation of antigen to T cells.23 Treated patients experienced significant reductions in leukemia cell counts and lymph node size that were associated with increases in the numbers of blood T cells that could recognize leukemia cells in vitro.1 In addition, bystander leukemia cells were induced to express CD95 in vivo for up to 2 weeks following the infusion of CD154-transfected cells. The expression of CD95 coincided with the reduction in leukemia cell counts and lymph node size.
In this study we found that CD40 ligation also could induce expression of another death receptor, namely DR5. The kinetics of CD40-induced expression of DR5 appeared similar to that of CD95 on CLL cells cocultured with CD154-bearing cells in vitro. Moreover, analyses of viably frozen leukemia cells from patients treated in this phase-1 gene therapy trial revealed de novo expression of DR5 on bystander CLL cells 1 day after treatment that persisted for at least 14 days thereafter. As such, the induced expression of DR5 on bystander CLL cells in vivo appears similar to that of CD95 following Ad-CD154 gene therapy.1
CD40 activation also induced expression of Bid, which was not expressed by resting CLL cells. The low-level expression of Bid in unstimulated CLL cells implies that these cells ordinarily lack Bid-dependent crosstalk from active caspase 8 to mitochondrial-dependent death pathways and therefore resemble type I cells. On the other hand, CD40 activation in vitro or in vivo induces expression of Bid that is paralleled by up-regulation of CD95 and DR5. Thus, while CD40 ligation induces expression of anti-apoptotic factors, such as both isoforms of flice-inhibitory protein (FLIP),4,23-25 myeloid cell leukemia factor 1 (Mcl-1),26 and B-cell leukemia protein xL (BCL-xL),24-26 it also paradoxically induces conversion of CLL cells into type II cells that potentially could be more sensitive to apoptosis via mitochondrial-dependent death pathways. Since the expression of Bid has been associated with sensitivity to anticancer drugs,27,28 the temporal events induced by CD40 activation could result in initial resistance and then increased sensitivity of CLL cells to antileukemia drugs. This could account for the conflicting reports of the resistance or increased sensitivity of CLL cells to fludarabine following CD40 ligation.29,30
Activation of caspase 8 in response to ligation of CD95 in type II cells can result in cleavage of Bid to truncated Bid (tBid), which then associates with B-cell lymphoma-2–associated X protein (Bax) to induce release from mitochondria of proapoptotic factors such as cytochrome c and Smac.31 In the cytosol, cytochrome c in conjunction with ATP activates caspase 9 in the so-called apoptosome, while Smac binds to cellular inhibitors of apoptosis proteins (cIAPs), releasing an inhibitory effect of IAPs on effector caspases, such as caspase 3 or 7. Bid also can be cleaved in response to TRAIL treatment and effects similar downstream targets, namely cytochrome c and second mitochondria-derived activator of caspases (SMAC).17 Recent studies also indicate that activated caspase 2 can participate in Bid cleavage in response to TRAIL.14,32 Furthermore, other reports indicate that events downstream from Bid cleavage, which lead to cytochrome c release, might be differentially regulated under conditions of CD95 versus DR5 triggering.18 As such, although similar, the signaling events induced by ligation of CD95 versus DR5 in type II cells have some noted differences. This could be relevant for the mechanism of apoptosis of CD40-activated CLL cells mediated by activated CD4+ T cells. Prior studies found that such activated cytotoxic CD4+ T cells could induce apoptosis of CD40-activated, but not resting, CLL cells via a CD178-dependent pathway.4 Moreover, CLL cells appeared susceptible to killing by such cells 1 day after CD40 ligation, when they still were resistant to apoptosis induced by anti-CD95 mAbs. In this study, we found that the activated blood CD4+ T cells of patients with CLL expressed both CD178 and TRAIL simultaneously, in agreement with prior studies on T cells from healthy donors.2,20 Co-expression of both ligands should allow such activated T cells to effect coligation of both CD95 and DR5 on cells bearing both death receptors. Moreover, we postulated that such co-ligation could function together to induce apoptosis of CLL cells previously activated via CD40 ligation.
To investigate for such interactions independent of other surface molecules, we generated artificial cytotoxic effector CHO cells that expressed one or both of these 2 ligands. CHO cells expressing both CD178 and TRAIL were significantly more effective than CHO cells expressing CD178 or TRAIL in killing CD40-activated CLL cells. Moreover, such double-ligand–expressing effector cells could kill CLL cells at time points after CD40 activation in which they still were resistant to killing by either single-ligand–expressing effector cell population alone. This synergy was dependent upon CD40 ligation of CLL cells in that either effector population failed to induce apoptosis of resting, nonactivated CLL cells. Similar to the apoptosis induced by activated CD4+ T cells,4 the apoptosis induced by CHO-CD178/TRAIL effector cells was dependent upon CD178-CD95 interactions. When CD178 was blocked by anti-CD178 mAb, the ability of CHO-CD178/TRAIL effector cells to induce apoptosis of CD40-activated CLL cells was significantly inhibited.
The synergy noted between CD178 and TRAIL might provide for added specificity in the clearance of cells by activated CD4+ CTL. Such effector cells might induce cytolysis of cells that express 2 or more death receptors more effectively than cells that have only one of these death receptors. In some respects this is similar to the requirement for coligation of multiple accessory molecules during cognate intercellular interactions to achieve optimal immune co-stimulation during the immune response to antigen.33 Similarly, a requirement for coligation of multiple death receptors via CD178 and TRAIL could be an important mechanism for enhancing the specificity of killing by innate immune effector mechanisms. Strategies that induce expression of more than one death receptor on a tumor cell population or that induce coligation of more than one type of death receptor expressed by a tumor cell population might prove more effective in effecting tumor clearance than those that target only one death receptor on the target tumor population.
Supported by a grant from the Dana Foundation, grant R37 CA49870 from the National Institutes of Health, and by a personal Dutch Cancer foundation and René Vogels foundation grant (A.P.K.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
Prepublished online as Blood First Edition Paper, August 31, 2004; DOI 10.1182/blood-2003-10-3684.
We are grateful to Dr Laura Rassenti, Monica Cook, Lang Huynh, and Traci Toy for their technical assistance, and to Dr Eric Eldering of the Academic Medical Center of The Netherlands for his helpful advice and discussions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal