Abstract
We previously demonstrated that autologous natural killer (NK)–cell therapy after hematopoietic cell transplantation (HCT) is safe but does not provide an antitumor effect. We hypothesize that this is due to a lack of NK-cell inhibitory receptor mismatching with autologous tumor cells, which may be overcome by allogeneic NK-cell infusions. Here, we test haploidentical, related-donor NK-cell infusions in a nontransplantation setting to determine safety and in vivo NK-cell expansion. Two lower intensity outpatient immune suppressive regimens were tested: (1) low-dose cyclophosphamide and methylprednisolone and (2) fludarabine. A higher intensity inpatient regimen of high-dose cyclophosphamide and fludarabine (Hi-Cy/Flu) was tested in patients with poor-prognosis acute myeloid leukemia (AML). All patients received subcutaneous interleukin 2 (IL-2) after infusions. Patients who received lower intensity regimens showed transient persistence but no in vivo expansion of donor cells. In contrast, infusions after the more intense Hi-Cy/Flu resulted in a marked rise in endogenous IL-15, expansion of donor NK cells, and induction of complete hematologic remission in 5 of 19 poor-prognosis patients with AML. These findings suggest that haploidentical NK cells can persist and expand in vivo and may have a role in the treatment of selected malignancies used alone or as an adjunct to HCT.
Introduction
Human natural killer (NK) cells are a subset of peripheral blood lymphocytes defined by the expression of CD56 or CD16 and the absence of the T-cell receptor (CD3).1 They recognize and kill transformed cell lines in a major histocompatibility complex (MHC)–unrestricted fashion and produce cytokines critical to the innate immune response. NK-cell function, distinct from the MHC-restricted cytolytic activity of T cells, may play a role in antitumor surveillance.2 The effects of NK-cell infusions have been studied in adoptive immunotherapy clinical trials. In these studies, autologous lymphokine-activated killer cells obtained from peripheral blood mononuclear cells (PBMCs) were administered to patients along with exogenous high-dose interleukin-2 (IL-2). Up to 20% of patients responded to these infusions of NK-cell–containing populations.3
In contrast to NK cells, T cells recognize targets through an antigen-specific T-cell receptor (TCR) and interact with targets only if human leukocyte antigen (HLA) MHC antigens are also recognized. Although NK-cell killing is MHC-unrestricted, NK cells display a number of activating and inhibitory receptors that ligate MHC molecules to modulate the immune response.4,5 NK-cell receptors that recognize antigens at the HLA-A, -B, or -C loci are members of the immunoglobulin superfamily and are termed killer immunoglobulin receptors (KIRs).6,7 Other receptor families (natural killer group 2 [NKG2]/CD94) that recognize antigens of the nonclassical HLA-E, -F, or -G loci and other ligand specificities have also been described.8-10 Engagement of these NK-cell receptors results in stimulation or inhibition of NK-cell effector function depending on intracellular signaling mediated through the cytoplasmic tail or adaptor molecules associated with each receptor.11-13 The NK-cell response to a target thus depends on the net effect of activating and inhibitory receptors.
Clinical trials have assessed the effects of low-dose IL-2 administration on activation of NK cells in patients with cancer. We have demonstrated the safety and feasibility of daily subcutaneous IL-2 injections following high-dose chemotherapy and autologous hematopoietic cell transplantation (HCT). Whereas IL-2 significantly expanded the number of circulating NK cells in vivo, these NK cells were not maximally cytotoxic as determined by in vitro assays.14 Subsequent studies tested infusion of IL-2–activated NK-cell–enriched populations or intravenous IL-2 infusions combined with subcutaneous IL-2. Although these approaches augmented in vivo NK-cell function, no consistent efficacy of autologous NK-cell therapy could be detected in cancer patients when compared with cohorts of matched controls.15
We hypothesized that autologous NK cells may be suppressed by the physiologic response resulting from NK-cell recognition of “self” MHC molecules. This notion is supported by recent data from haploidentical T-cell–depleted transplantation studies, which suggested that KIR mismatch with tumor MHC (ie, KIR ligand) may lead to greater tumor kill. In these studies, Ruggeri et al16 showed that stratifying patients by their KIR ligand mismatch would select for patients with alloreactive NK cells that protect against acute myeloid leukemia (AML) relapse. Although virtually untested in solid tumors, these clinical data strongly support a therapeutic role for allogeneic NK cells in myeloid leukemia.17 We present data on the biologic effects of haploidentical NK-cell infusions administered to cancer patients as cell-based immunotherapy with the goal of demonstrating a feasible and safe method that permits in vivo donor NK-cell expansion.
Patients and methods
Patient eligibility
There were 43 patients enrolled in this study, including 10 with metastatic melanoma, 13 with metastatic renal cell carcinoma, 1 with refractory Hodgkin disease after autologous HCT, and 19 with poor-prognosis AML. Patients with AML were eligible with primary refractory disease (no complete remission [CR] after 2 or more different induction regimens), relapsed disease not in CR after 1 or more cycles of standard reinduction therapy (not required if older than 60 years), secondary AML from myelodysplatic syndrome (MDS), or with relapsed disease at least 3 months after HCT. All patients except the one with AML/MDS and one with renal cell carcinoma had received prior therapy. All patients had adequate organ function. Non-AML patients were required to have a platelet count greater than 80 000/μL, a hemoglobin (Hgb) level greater than 9 gm/dL, and an absolute neutrophil count greater than 1000/μL. Corticosteroids or other immunosuppressive medications were not allowed for 3 days prior to study entry or while participating in the study.
Study design
Patients received one of 2 lower intensity outpatient regimens or 1 higher intensity inpatient regimen 48 hours prior to cell infusion (designated day 0). In the first regimen, 750 mg/m2 intravenous cyclophosphamide and 1000 mg/m2 methylprednisolone (Lo-Cy/mPred regimen) were administered to outpatients on day –2, followed by NK-cell infusion with increasing cell doses to determine the maximum number of NK cells that could be safely infused from a single lymphapheresis collection. A second course of treatment at a higher cell dose was given to 4 of the 17 patients. Once the maximum tolerated dose of NK cells was established, other preparative regimens were tested. Intravenous fludarabine (Flu; 25 mg/m2) was administered daily for 5 days (days –6 to –2) in an outpatient setting in a cohort of patients with metastatic renal cell carcinoma (n = 7). A high-dose regimen consisting of 60 mg/kg intravenous cyclophosphamide (9 patients received 1 dose and 10 patients received 2 doses) and 25 mg/m2 intravenous fludarabine for 5 consecutive days (Hi-Cy/Flu regimen) was administered in an inpatient setting in the cohort of patients with poor-prognosis AML.
In all cases, haploidentical related donors underwent lymphapheresis for 3 to 5 hours on the day prior to cell infusion (day –1). PBMCs were collected using a Fenwal CS-3000 Plus blood cell separator (no. 4R4538) with granulocyte separation chamber and small volume collection chamber (SVCC; Fenwal Division, Baxter Healthcare, Deerfield, IL). Up to 2 × 1010 PBMCs were incubated with magnetic activated cell sorting (MACS) colloidal super-paramagnetic CD3 MicroBeads (Miltenyi Biotec, Auburn, CA) that consist of monoclonal mouse anti-human CD3 antibodies conjugated to microspheres. T cells were depleted from the lymphapheresis product using the Miltenyi Biotec CliniMACS cell selection device under good manufacturing practice (GMP) conditions (AmCell, Sunnyvale, CA). A sample of the cell product was then evaluated by flow cytometry in order to identify the number of T cells, NK cells, B lymphocytes, and monocytes. The CD3-depleted product, containing less than 5 × 106 CD3+ T cells/kg recipient, was placed in VueLife Teflon (FEP) bags (American Fluoroseal, Gaithersburg, MD) and incubated overnight at 37°C under 5% CO2 in X-VIVO 15 medium (2 × 106 cells/mL) and 1000 U/mL IL-2 (Proleukin; Chiron, Emeryville, CA). On the morning of infusion (day 0), cells were washed twice with 0.9% sodium chloride for injection using a COBE 2991 cell processor (COBE BCT, Lakewood, CO). Cells were resuspended with 5% human serum albumin in 0.9% sodium chloride for injection. A sample of the product was evaluated for lot release testing. All cell products contained more than 70% viable cells for infusion.
Following premedication with acetaminophen (650 mg) and diphenhydramine (25 mg), cells were administered by intravenous infusion. Patients treated with the Lo-Cy/mPred preparative regimen subsequently were given 1 × 105, 1 × 106, 1 × 107, or 2 × 107 cells/kg of recipient body weight (at least 3 per cohort). Grade 3 or greater related nonhematologic toxicity, other than constitutional symptoms and fatigue, constituted dose-limiting toxicity (DLT). The maximum tolerated cell dose was defined as the dose at which less than 33% of patients experienced DLT. As there was no DLT in the dose escalation phase, all subsequent patients receiving Flu or Hi-Cy/Flu were administered the 2 × 107 cells/kg dose. On the evening of the cell infusion, patients began daily IL-2 (1.75 × 106 IU/m2) for 14 days by subcutaneous injection. The last 14 patients with AML were treated with a modified IL-2 regimen of 10 million units (MUs) 3 times weekly as indicated. Written informed consent was obtained from all patients and donors with approval from the University of Minnesota Institutional Review Board. The clinical protocol was covered by the Investigational New Drug (IND) application BB-IND 8847 (J. M., sponsor).
Laboratory methods
Mixed lymphocyte reaction (MLR). PBMCs were obtained by Ficoll-Hypaque separation of blood from patients before and after the Lo-Cy/mPred regimen. Patient cells were used as responders against donor cell stimulators obtained from the lymphapheresis product prior to IL-2 incubation. Irradiated donor stimulators (104 cells/well) were incubated with limiting dilutions of recipient PBMCs (starting at a 1:1 ratio) for 7 days at 37°C, under 5% CO2. Tritiated thymidine (3.7 × 104 [1 μCi/well]) was added during the last 16 hours of culture prior to transferring cells onto filters using a multiwell harvester. Filters were counted on a Perkin-Elmer Matrix 9600 and proliferation was measured as counts per minute (Perkin-Elmer, Shelton, CT).
Chimerism assay. Donor and recipient PBMC samples, previously typed for HLA class I, were compared to identify unique alleles that discriminate donor cells among a recipient background. Using the IMGT HLA database (the international ImMunoGeneTics information system, http://imgt.cines.fr), the informative donor allele was aligned with the recipient alleles and subtypes to locate unique regions of the informative donor sequences. Primers were designed with a minimum of 2 or more base pair differences. The success of primers in discriminating between donor and recipient alleles was verified by reverse transcriptase–polymerase chain reaction (RT-PCR) of a titration curve using preinfusion patient samples serially diluted with known concentrations of donor cells. Once successful primers were established, postinfusion PBMC samples were compared with the standard curve to assess the level of donor cDNA. Identical conditions were used in separate β-actin control reactions. PCR products were run on an ethidium bromide–stained 2% agarose gel and visualized under ultraviolet light.
Phenotyping, cytotoxicity, and ELISA. Fluorescein isothiocyanate (FITC), phycoerythrin (PE), peridinin chlorophyll-alpha protein (PerCP), and allophycocyanin (APC)–coupled control immunoglobulins or specific antibodies directed at CD56, CD3, CD19, CD14, and KIR (BD PharMingen, San Diego, CA) were used to evaluate NK and other cells of treated patients. Cytotoxicity assays were performed against K562 targets as previously reported.18 IL-15 concentrations were determined on frozen plasma by commercial enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN) with an assay sensitivity of 2 pg/mL.
Statistics. Results of experimental points obtained from multiple experiments were reported as mean plus or minus 1 standard error of the mean (SEM). Comparisons were performed by 2-sided Student t test. The complete remission rates in the AML cohort were compared using the Fisher exact test. Simple linear regression was used to study the relationship between the absolute lymphocyte count and the IL-15 concentration.
Results
Characteristics of the NK-cell product
Forty-three patients with advanced cancer were treated with 48 haploidentical NK-cell infusions. Three preparative regimens of different intensity (Lo-Cy/mPred; Flu; Hi-Cy/Flu) were used to prevent immunologic rejection of donor cells by recipient. The goals of this study were to assure safety of the infusion, promote persistence of donor cells for at least 2 weeks after infusion, and to expand haploidentical NK cells in vivo. Processing of the infusion product under GMP conditions was the same for all regimens. Characteristics of the last 36 products after the cell dose escalation was complete (all subjects received ∼2 × 107 total nucleated cells/kg) showed that the apheresis product prior to manipulation contained 11% ± 1% [5%-19%] CD56+/CD3– NK cells, and NK cells were enriched to 40% ± 2% [18%-68%] after processing. The final IL-2–activated product contained an NK-cell dose of 8.5 ± 0.5 [2.2-15] × 106 cells/kg. Cell processing resulted in a significant reduction of T cells in all products starting with 64% ± 1.3% in the apheresis product to 0.9% ± 0.1% after CD3 depletion, yielding a final T-cell dose of 1.75 ± 0.3 × 105 cells/kg. There was an average of 40-fold less T cells than the NK cells. Other components of the final product included monocytes (25% ± 1.6%) and B lymphocytes (19% ± 2.0%). Clinical apheresis products were found to have a significant increase in cytotoxic function against K562 targets after CD3 depletion, which increased further after overnight incubation with 1000 U/mL IL-2 (data not shown). All products tested below the accepted limit for endotoxin and Gram stain was negative on the day of infusion. Subsequent microbiologic cultures of all infusions were negative.
Establishing a safe haploidentical NK-cell dose with Lo-Cy/mPred preparation
The low-intensity outpatient preparative regimen (Lo-Cy/mPred) was administered to the first 17 patients (10 with melanoma, 6 with renal cell carcinoma, and 1 with refractory Hodgkin disease) followed by infusion of escalating doses of haploidentical NK cells. All patients except 1 with renal cell carcinoma received prior therapy. Three patients with melanoma and 1 with renal cell carcinoma demonstrated stable disease on restaging CT scan performed approximately 6 weeks following NK infusion and subsequently received a second course of treatment. Three patients received 1 × 105 cells/kg, 3 received 1 × 106 cells/kg, 6 received 1 × 107 cells/kg, and 9 received 2 × 107 cells/kg, which was the highest CD3-depleted cell dose that could be routinely obtained from a single unmobilized lymphapheresis collection.
Nonhematologic toxicity was minimal following Lo-Cy/mPred and haploidentical NK-cell infusions at all cell doses (Table 1). There were no adverse reactions during or after the cell infusion and graft-versus-host disease was not observed. The majority of patient symptoms were due to the low-dose IL-2 injections, although these were tolerable, and 88% (260/294) of the planned IL-2 doses were administered. Constitutional symptoms consisting of low-grade fever, chills, and myalgias were common as previously seen using this regimen without NK-cell infusions.14
Toxicity associated with Lo-Cy/mPred regimen
Symptom . | Events, out of 21 courses (%) . | Grade or duration . |
|---|---|---|
| Nausea | 18 (86%) | Grade 1-2 |
| Insomnia | 6 (28%) | 2-3 days |
| Injection site reaction | 15 (71%) | Mild |
| Vomiting | 11 (52%) | Grade 1-2 |
| Dyspnea | 2 (10%) | Grade 2 |
| Stroke* | 1 (5%) | Grade 4 |
Symptom . | Events, out of 21 courses (%) . | Grade or duration . |
|---|---|---|
| Nausea | 18 (86%) | Grade 1-2 |
| Insomnia | 6 (28%) | 2-3 days |
| Injection site reaction | 15 (71%) | Mild |
| Vomiting | 11 (52%) | Grade 1-2 |
| Dyspnea | 2 (10%) | Grade 2 |
| Stroke* | 1 (5%) | Grade 4 |
Documented carotid artery plaque likely related to radiation therapy.
Grade 2 and 3 anemia was observed in 7 and 6 treatment courses, respectively, although 7 of these patients had grade 2 anemia at study entry. Two patients experienced grade 4 neutropenia and 2 others developed grade 3 neutropenia following cyclophosphamide. There were no episodes of neutropenic fever and thrombocytopenia was not observed. Hematologic toxicity was transient and there was no occurrence of prolonged cytopenia or marrow aplasia. A maximum tolerated dose was not reached and the largest cell dose was that achievable during a single lymphapheresis collection.
Restaging evaluation by CT scan was performed 4 to 6 weeks following NK infusion and every 3 months thereafter. Two patients with renal cell carcinoma demonstrated stable disease at 20 and 21 months after NK-cell infusion. Four patients with melanoma demonstrated stable disease after cell infusion and received a second course of therapy 4 to 9 months after the initial NK-cell administration. However, these patients demonstrated disease progression 4 to 6 weeks after the second infusion.
Determining whether haploidentical NK cells expanded in vivo after Lo-Cy/mPred
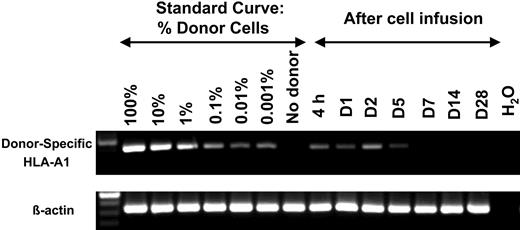
In order to detect the presence of donor cells in serial PBMC samples, unique RT-PCR primers for donor-specific MHC class I alleles were generated for 6 patients that did not cross-react with recipient MHC allele sequences. In 5 of 6 patients, donor cells persisted for 5 days but were not detected at later time points (Figure 1). However, one patient demonstrated persistence of donor cells for as long as 138 days after haploidentical infusion. Additionally, donor cells comprised less than 1% of circulating PBMCs in all patients treated with the Lo-Cy/mPred regimen, and there was no evidence of in vivo NK-cell expansion in any patient. The reason for this lack of expansion was explored further.
Transient persistence of donor cells following haploidentical NK-cell infusions without in vivo expansion after Lo-Cy/mPred. Each donor/recipient pair was evaluated for a unique class I MHC allele present in the donor but not the recipient. After reverse transcription, PCR primers were chosen to amplify donor-derived informative transcripts without cross reactivity with recipient alleles. A standard curve was established using donor cell dose titration, while showing little or no product in the control sample that does not contain donors cells. Patient PBMC samples were analyzed in a similar manner at various time points after infusion. Shown is a representative donor/recipient pair where HLA-A1 was informative (β-actin control for equal sample loading). There was persistence of donor cells through day 5 (D5) after NK-cell infusion but none at later time points. There was no consistent increase in band density after infusion, suggesting that in vivo expansion did not occur. A similar pattern demonstrating persistence of donor cells through day 5 was seen in 5 of 6 donor/recipient pairs tested.
Transient persistence of donor cells following haploidentical NK-cell infusions without in vivo expansion after Lo-Cy/mPred. Each donor/recipient pair was evaluated for a unique class I MHC allele present in the donor but not the recipient. After reverse transcription, PCR primers were chosen to amplify donor-derived informative transcripts without cross reactivity with recipient alleles. A standard curve was established using donor cell dose titration, while showing little or no product in the control sample that does not contain donors cells. Patient PBMC samples were analyzed in a similar manner at various time points after infusion. Shown is a representative donor/recipient pair where HLA-A1 was informative (β-actin control for equal sample loading). There was persistence of donor cells through day 5 (D5) after NK-cell infusion but none at later time points. There was no consistent increase in band density after infusion, suggesting that in vivo expansion did not occur. A similar pattern demonstrating persistence of donor cells through day 5 was seen in 5 of 6 donor/recipient pairs tested.
The intent of the preparative regimen was to prevent immediate rejection of donor cells. However, there was no significant change in absolute lymphocyte counts following Lo-Cy/mPred just prior to the haploidentical cell infusions (data not shown). The persistence of patient lymphocytes after this low-dose regimen permitted functional testing of patient effector cells against donor cells as a surrogate assay to determine whether recipient lymphocytes were immediately rejecting donor cells. PBMCs obtained from patients before and after Lo-Cy/mPred treatment were incubated with irradiated donor PBMCs, and allogeneic reactivity was assessed in a proliferation assay measuring [3H]-thymidine uptake by responder cells in a standard mixed lymphocyte reaction (MLR). The MLRs using patient cells before and 48 hours after Lo-Cy/mPred were not different (data not shown). These findings suggest that the inability to expand haploidentical NK cells in vivo may be due to inadequate clearing of recipient cells and inadequate immunosuppression.
In vivo expansion of haploidentical NK cells with alternative low- and high-intensity immune-suppressive regimens
After showing that NK cells were rejected with the low-intensity Lo-Cy/mPred regimen, in vivo NK-cell expansion was tested with 2 different immune suppressive regimens. Seven patients with renal cell carcinoma were treated with a lower intensity regimen (Flu) as the preparative therapy, and 19 patients with poor-prognosis AML were treated with a higher intensity regimen (Hi-Cy/Flu). Both regimens induced absolute lymphopenia by the time of the cell infusions although the impact on overall hematopoiesis differed. Flu alone induced lymphopenia without neutropenia, whereas those receiving Hi-Cy/Flu had marked pancytopenia at the time of cell infusion (data not shown). In addition to a major difference in hematologic toxicity between the regimens, patients receiving Hi-Cy/Flu had more significant toxicity associated with IL-2 injections. The complete course (14 days) of daily subcutaneous IL-2 was administered to 6 of 7 patients with renal cell carcinoma receiving Flu alone with only one patient stopping IL-2 after 6 doses due to constitutional symptoms. In contrast, only 2 of the first 5 patients with AML receiving Hi-Cy/Flu could complete the entire planned course due to persistent fever and associated constitutional symptoms. Given the clinical need to differentiate neutropenic fevers from infection, as opposed to IL-2–related toxicity, in the next 3 patients with AML the IL-2 dose and schedule was changed from 1.75 MU/m2 per day to 10 MU 3 times per week for 9 doses (n = 3). This was further reduced to 6 doses in the following 11 patients because of concern for neutropenia. We and others have used this alternative regimen to expand autologous NK cells in vivo with similar results to the lower dose daily regimen initially used, with fewer constitutional side effects.19
There was no unexpected clinical toxicity in patients with renal cell carcinoma receiving the Flu regimen and none of the patients required hospitalization. Patients with AML were expected to have a clinical course similar to that for patients receiving induction therapy for AML and were hospitalized throughout treatment. In one patient who had poor pulmonary function and unexplained pleural effusions prior to starting therapy, IL-2 administration was interrupted because of increasing pleural effusions and hypoxemia. The patient recovered from the acute event (grade 3), and was discharged from the hospital with persistent leukemia that did not respond to therapy. Subsequent to this event, pleural effusions were considered an exclusion criteria for enrollment. The only other pulmonary event was grade 2 shortness of breath and mild hypoxemia requiring transient low-flow oxygen toward the end of IL-2 therapy (on the 3-times-per-week regimen). This same patient also developed a diffuse skin rash that on biopsy showed dermatitis suggestive of a drug rash and no evidence of graft-versus-host disease. These symptoms resolved spontaneously several days after IL-2 therapy was completed. The lack of graft-versus-host disease in this cohort is intriguing given that 2.1 ± 0.3 [0.5-6.5] × 105 T cells/kg were infused with the NK-cell product. Although graft-versus-host disease may be expected when these T-cell doses are infused in a haploidentical transplantation setting, the T cells infused here did not contribute significantly to engraftment, which likely explains the lack of graft-versus-host disease observed.
In vivo expansion of haploidentical NK cells in patients with AML receiving the Hi-Cy/Flu intensity preparative regimen
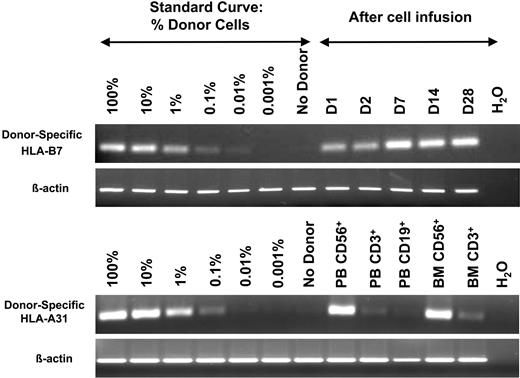
Using the RT-PCR–based chimerism assay, we defined engraftment as a densitometry-determined band density of at least 1% compared with the standard curve. Using this criteria, none of the patients with renal cell carcinoma (of the 5 tested) receiving fludarabine alone engrafted with haploidentical donor NK cells. Engraftment after infusions was less than 0.1% at all postinfusion time points. In marked contrast, 8 of 15 evaluable patients with AML receiving Hi-Cy/mPred showed at least 1% engraftment of donor cells at day 7 or beyond after haploidentical infusion (Figure 2 and 3). In one patient lymphocytes were sorted to purity. Circulating blood and marrow NK cells, but not T or B cells, were of donor origin (Figure 2).
In vivo expansion of haploidentical NK cells. After establishing a standard curve between donor and recipient using the informative HLA-B7 allele, postinfusion samples were analyzed in a patient receiving Hi-Cy/Flu. In contrast to patients receiving Lo-Cy/mPred, Hi-Cy/Flu not only increased the persistence of donor cells but also facilitated in vivo expansion as demonstrated by the increased band density beyond day 2 (D2) (β-actin control for equal sample loading). In another patient, after establishing HLA-B31 as an informative allele, NK cells, T cells, and B cells were sorted from blood (PB) and marrow (BM). From this patient, essentially 100% of NK cells, but not T or B cells, were of donor origin.
In vivo expansion of haploidentical NK cells. After establishing a standard curve between donor and recipient using the informative HLA-B7 allele, postinfusion samples were analyzed in a patient receiving Hi-Cy/Flu. In contrast to patients receiving Lo-Cy/mPred, Hi-Cy/Flu not only increased the persistence of donor cells but also facilitated in vivo expansion as demonstrated by the increased band density beyond day 2 (D2) (β-actin control for equal sample loading). In another patient, after establishing HLA-B31 as an informative allele, NK cells, T cells, and B cells were sorted from blood (PB) and marrow (BM). From this patient, essentially 100% of NK cells, but not T or B cells, were of donor origin.
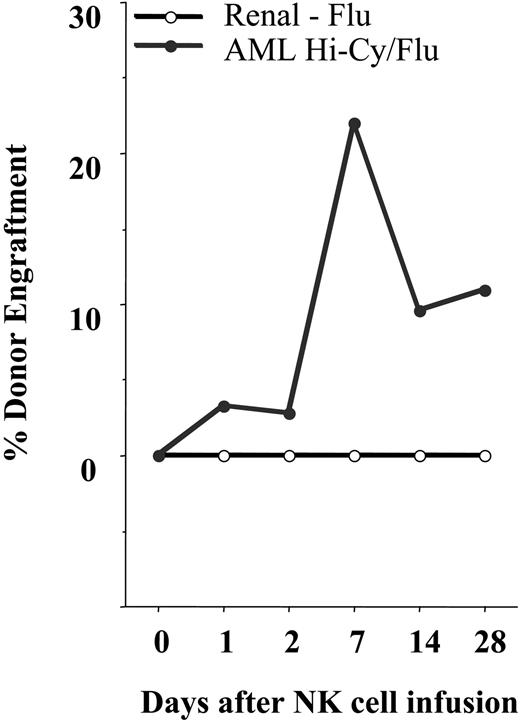
Hi-Cy/Flu induces in vivo expansion of NK cells. All patients with AML and renal cell carcinoma were analyzed using an RT-PCR–based chimerism assay when an informative donor allele lacking cross reactivity with the recipient could be established. Shown is the average percent donor PBMC engraftment for each cohort. There was in vivo expansion of donor cells by day 7 in the AML cohort receiving Hi-Cy/Flu (•) but not in the renal cell carcinoma cohort receiving Flu alone (○). Donor cells persisted for 28 days and beyond in some patients.
Hi-Cy/Flu induces in vivo expansion of NK cells. All patients with AML and renal cell carcinoma were analyzed using an RT-PCR–based chimerism assay when an informative donor allele lacking cross reactivity with the recipient could be established. Shown is the average percent donor PBMC engraftment for each cohort. There was in vivo expansion of donor cells by day 7 in the AML cohort receiving Hi-Cy/Flu (•) but not in the renal cell carcinoma cohort receiving Flu alone (○). Donor cells persisted for 28 days and beyond in some patients.
Five patients achieved a morphologic CR on this protocol, and characteristics associated with response were evaluated. Patients were stratified into those with predicted graft-versus-host alloreactivity using the KIR ligand mismatch strategy. Four patients were KIR ligand mismatched in the graft-versus-host direction and 3 of 4 (75%) achieved a complete remission. This was in marked contrast to those without alloreactivity in the graft-versus-host direction where only 2 of 15 (13%) achieved remission (P = .04). Of those patients who achieved complete remission, the number of circulating NK cells was significantly greater than the nonremission group (51% ± 13% vs 8% ± 4% of circulating lymphocytes, P = .027) corresponding to higher NK-cell cytotoxicity against K562 targets 14 days after NK-cell infusion (50 ± 13 vs 12 ± 6, E:T 20:1, P = .04). Lastly, 4 of 5 patients in CR missing at least one KIR ligand allowed for evaluation of the frequency of alloreactive NK cells in the donor product and those circulating in the patient 14 to 28 days after NK-cell infusions (Table 2).
Frequency of alloreactive NK cells
Missing KIR ligand* . | % Alloreactive in donor product† . | % Alloreactive in patient† . |
|---|---|---|
| Cw3 | 21 | 95 |
| Bw4 | 16 | 23 |
| Cw4 | 34 | 25 |
| Bw4 and Cw4 | 37 and 22 | 11 and 2 |
Missing KIR ligand* . | % Alloreactive in donor product† . | % Alloreactive in patient† . |
|---|---|---|
| Cw3 | 21 | 95 |
| Bw4 | 16 | 23 |
| Cw4 | 34 | 25 |
| Bw4 and Cw4 | 37 and 22 | 11 and 2 |
Based on high-resolution HLA-C typing.
Determined as the percent of NK cells expressing KIR corresponding to the recipient missing ligand.
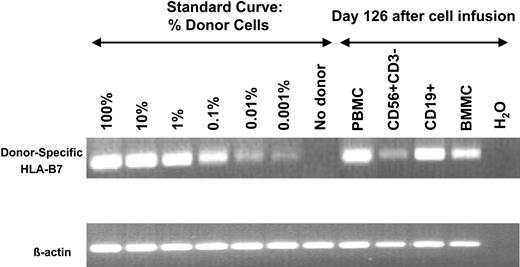
A patient in CR who had 100% donor NK-cell engraftment provided definitive proof of principle that donor cells expanded in vivo. This patient, a 50-year-old man with AML, M1, underwent a nonmyeloablative (Hi-Cy/Flu, total body irradiation 200 cGy) double umbilical cord blood transplantation (one male and one female donor) in second clinical remission following successful therapy for marrow and central nervous system relapse. He achieved 100% donor chimerism from one of the cord blood units (male) by day 38 after transplantation. Four weeks later, chimerism studies showed reappearance of recipient DNA followed by morphologic bone marrow relapse. He received Hi-Cy/Flu haploidentical NK cells from his sister about 100 days after cord blood transplantation. Full and sustained haploidentical NK-cell engraftment was shown at day 14 by RT-PCR for HLA-B7, which was unique to the donor, but not recipient cells or the umbilical cord blood graft (Figure 2). This engraftment was verified at day 14 by clinical engraftment methods using variable nucleotide tandem repeats showing 97% engraftment of haploidentical donor cells (an informative pattern unique from the engrafted cord and recipient) and by G-banding showing 60% female metaphases. Also at day 14, 92% of circulating PBMCs were functional CD56+/CD3– NK cells (Figure 4A-B). After 3 weeks of neutropenia granulocyte–colony-stimulating factor was started. The neutrophil count eventually recovered (absolute neutrophil count > 500) 3 weeks later (6 weeks after NK-cell infusion) at which time a bone marrow was found to be entirely of umbilical cord blood origin with normal male metaphases in all cells, whereas there was no evidence of cells from the haploidentical donor. The patient died without evidence of leukemia 126 days after haploidentical NK-cell infusion as a result of Epstein-Barr virus (EBV) reactivation (162 300 copies of EBV DNA/mL blood) with B-cell lymphoproliferation and hemophagocytosis. There were 8% haploidentical donor cells in the marrow but a large number of circulating CD19+ B cells of haploidentical, but not umbilical cord blood or recipient origin (Figure 5).
In vivo expanded NK cells were functional after Hi-Cy/Flu. PBMCs were collected 14 days after haploidentical cell infusions from the patient shown in Figure 2. (A) 92% of all circulating cells were CD56+/CD3– NK cells corresponding to an absolute circulating NK-cell count of 650/μL. (B) Fresh day-14 samples were tested without further activation in a standard 4-hour chromium release assay demonstrating cytotoxicity against K562. • indicates K562; ○, Raji.
In vivo expanded NK cells were functional after Hi-Cy/Flu. PBMCs were collected 14 days after haploidentical cell infusions from the patient shown in Figure 2. (A) 92% of all circulating cells were CD56+/CD3– NK cells corresponding to an absolute circulating NK-cell count of 650/μL. (B) Fresh day-14 samples were tested without further activation in a standard 4-hour chromium release assay demonstrating cytotoxicity against K562. • indicates K562; ○, Raji.
EBV reactivation induced in vivo expansion of haploidentical B cells. Using the informative HLA-B7 allele, blood and bone marrow mononuclear cells (BMs) were evaluated in a patient who developed fevers, hemophagocytosis, and EBV reactivation 126 days after haploidentical NK-cell infusions and about 226 days following umbilical cord blood transplantation. PB containing 5% CD56+/CD3– NK cells and 68% CD19+ B cells were sorted to purity (documented by postsort analysis) and their origin was evaluated. Haploidentical NK cells were minimally present in the 120-day sample, unlike that observed at earlier time points (14 days) after infusion. However, most of the EBV-driven B cells were from the HLA-B7 haploidentical NK-cell donor.
EBV reactivation induced in vivo expansion of haploidentical B cells. Using the informative HLA-B7 allele, blood and bone marrow mononuclear cells (BMs) were evaluated in a patient who developed fevers, hemophagocytosis, and EBV reactivation 126 days after haploidentical NK-cell infusions and about 226 days following umbilical cord blood transplantation. PB containing 5% CD56+/CD3– NK cells and 68% CD19+ B cells were sorted to purity (documented by postsort analysis) and their origin was evaluated. Haploidentical NK cells were minimally present in the 120-day sample, unlike that observed at earlier time points (14 days) after infusion. However, most of the EBV-driven B cells were from the HLA-B7 haploidentical NK-cell donor.
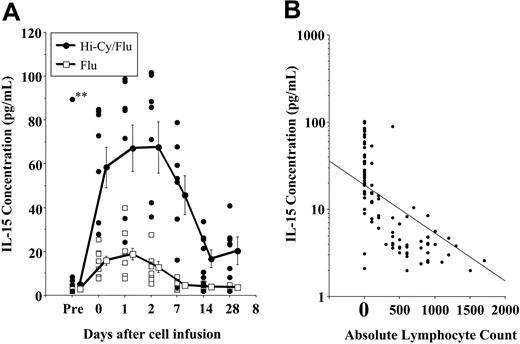
The persistence of adoptively transferred haploidentical NK cells in patients with AML treated with Hi-Cy/Flu was striking compared with the same cell infusion given to patients treated with Lo-Cy/mPred or Flu regimens. We tested whether endogenous cytokines play a role in this process. IL-15 was an ideal candidate to drive homeostatic NK-cell expansion based on recent mouse studies20,21 and because of the established role of IL-15 in NK-cell differentiation.22-24 There was no increase in plasma IL-15 concentration in those patients treated with Lo-Cy/mPred (data not shown). IL-15 was only slightly increased several days after Flu when compared with samples obtained prior to treament (Figure 6A). In marked contrast, patients with AML had significant increases in plasma IL-15, starting before haploidentical cell infusions and sustained for several weeks after completion of Hi-Cy/Flu therapy. There was an inverse correlation between the absolute lymphocyte count and the IL-15 concentration (r =–.62, P < .0001; Figure 6B).
Hi-Cy/Flu induces endogenous IL-15, which correlates with in vivo haploidentical NK-cell expansion. Plasma was collected from patients before and after the indicated preparative regimens. The day-0 sample was collected prior to haploidentical NK-cell infusion. A marked increase in IL-15 concentrations was detected in patients receiving Hi-Cy/Flu (•) compared with those receiving Flu alone (□). (A) The individual symbols represent patient samples obtained at the indicated time points. The plotted lines represent the mean plus or minus SEM of samples from each preparative regimen. Endogenous IL-15 was significantly higher with the Hi-Cy/Flu preparative regimen. A single pediatric patient (designated **) on oral etoposide prior to enrollment was the only patient who had high IL-15 levels prior to treatment. (B) At all postchemotherapy time points, an inverse correlation between the IL-15 concentration and the absolute lymphocyte count was detected among all patients receiving Flu alone or Hi-Cy/Flu.
Hi-Cy/Flu induces endogenous IL-15, which correlates with in vivo haploidentical NK-cell expansion. Plasma was collected from patients before and after the indicated preparative regimens. The day-0 sample was collected prior to haploidentical NK-cell infusion. A marked increase in IL-15 concentrations was detected in patients receiving Hi-Cy/Flu (•) compared with those receiving Flu alone (□). (A) The individual symbols represent patient samples obtained at the indicated time points. The plotted lines represent the mean plus or minus SEM of samples from each preparative regimen. Endogenous IL-15 was significantly higher with the Hi-Cy/Flu preparative regimen. A single pediatric patient (designated **) on oral etoposide prior to enrollment was the only patient who had high IL-15 levels prior to treatment. (B) At all postchemotherapy time points, an inverse correlation between the IL-15 concentration and the absolute lymphocyte count was detected among all patients receiving Flu alone or Hi-Cy/Flu.
Discussion
In this study, we demonstrate that adoptively transferred human NK cells derived from haploidentical related donors can be expanded in vivo. Of interest, in vivo NK-cell expansion occurs after preparation with a high dose (Hi-Cy/Flu) but not lower doses of immunosuppression (Lo-Cy/mPred or Flu). Successful lymphocyte adoptive transfer following intensive immunosuppresion is not surprising. Lymphopenia may change the competitive balance between transferred lymphocytes and endogenous lymphocytes. Alternatively, lymphopenia may induce survival factors or deplete cellular or soluble inhibitory factors.25,26 In murine studies, preparative regimens sufficient to induce lymphopenia allowed homeostatic T-cell expansion in vivo that potentiated effective antitumor immunity.27 This concept has been tested in human T-cell clinical trials by Rosenberg's group.28 T-cell lymphopenia was induced by Hi-Cy/Flu, similar to what was used here. Successful adoptive transfer and expansion of NK cells may also require intense immunosuppression. Prlic et al20 showed that mature NK cells proliferated only in an NK-cell–deficient host where the endogenous NK-cell pool was absent.
We also demonstrate that NK-cell adoptive therapy is associated with a striking rise in endogenous IL-15 levels, reminiscent of the role IL-7 plays in CD4+ T-cell homeostasis.29 IL-15 is required for the final steps of in vitro NK-cell differentiation from CD34+ progenitors.22-24 Cooper et al21 was the first to show that IL-15 was absolutely required for in vivo expansion and survival of NK cells, in mice, in part through bcl-2 expression. Transfer of NK cells into IL-15–/– hosts resulted in loss of NK cells by 4 days after transfer. IL-15 receptor alpha knockout mice generate IL-15 but do not have NK cells and are unable to undergo successful adoptive transfer. This implies that IL-15 responsiveness by cells other than NK cells may be important in driving this response. IL-15 transgenic mice markedly expand their NK cells and CD8+ T cells, ultimately resulting in an NK/T-lymphocytic leukemia.30 The endogenous origin of IL-15 in our patients was unclear. Our data support the notion that IL-15 levels increased only after an intensive lymphocyte-depleting preparative regimen as demonstrated by the inverse correlation between IL-15 concentrations and the absolute lymphocyte count. This does not exclude the possibility that IL-15 may be produced following chemotherapy-induced damage to gastrointestinal mucosa or other cells of epithelial origin.31-34 The effects of exogenous IL-2 administration in these patients needs to be explored as it does add toxicity to the regimen. Further clinical testing may demonstrate that expansion will occur in the presence of IL-15 alone.
Donor NK-cell infusions were feasible and tolerated without unexpected toxicity except for the umbilical cord blood transplantation patient who developed EBV reactivation after treatment. The risk of posttransplantation lymphoproliferative disease approached 10% when HCT is performed using a T-cell–depleted and mismatched graft.35 Although a single event, this finding is important to understand the possible consequences of allogeneic NK-cell therapy in heavily pretreated immunosuppressed patients. It also emphasizes that the CD3-depleted final product, enriched for NK cells but containing B cells, may need further purification to lessen the possibility of this complication. Clinical ex vivo selection methods to address this issue using CD3 depletion followed by CD56 selection are now in place36 and will be tested. We have previously shown that monocytes serve as accessory cells for NK-cell expansion in vitro18 but the role of accessory cells in vivo, if any, is unknown. We need to verify that removal of monocytes and B cells does not change the in vivo expansion potential of NK cells seen here before recommending a purified NK-cell product in all future studies.
In summary, this is the first study to demonstrate that adoptively transferred human NK cells can be expanded in vivo. Expansion was dependent on the more intense Hi-Cy/Flu preparative regimen, which induced lymphopenia, and the more potent immunosuppression that was associated with high endogenous concentrations of IL-15, none of which was observed following Lo-Cy/mPred and Flu alone. It is intriguing that this same regimen is the basis for many transplantation regimens and may help explain the robust NK-cell reconstitution seen in that setting. In this study, NK-enriched cells were obtained from related haploidentical donors by efficient depletion of CD3 from PBMCs, although contaminating B cells and monocytes remained in the final product. A maximum tolerated dose was not reached and the largest cell dose administered was that obtained during a single lymphapheresis collection. Although tumor response was not a primary goal of this study, 5 of 19 poor-prognosis patients with AML achieved complete remission after haploidentical NK-cell therapy, with a significantly higher complete remission rate when KIR ligand mismatched donors were used, a strategy that predicts NK-cell alloreactivity.16,37 The precise role of the cells versus the high-intensity chemotherapy regimen in responding patients cannot be definitively determined in this current study. However, the benefit of alloreactivity and the preferential expansion of functional NK cells in responding patients is consistent with at least a partial effect from the NK cells. Our data suggest that prospective selection of KIR ligand–mismatched donors is warranted when possible, which will be assessed in subsequent larger clinical trails.
Prepublished online as Blood First Edition Paper, January 4, 2005; DOI 10.1182/blood-2004-07-2974.
Supported by National Institutes of Health grants PO1-CA-65493 (J.S.M., J.E.W., P.B.M.), R01-HL-55417 (J.S.M.), and R01-CA-72669 (B.R.B.), and supported in part by grant M01-RR00400 from the National Cancer Institute for Research Resources.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We would like to thank our dedicated research nurses, Dixie Lewis and Roby Nicklow, for their outstanding research support and contribution to patient education, and Melinda Berthold, Diane Kadidlo, and Darin Sumstad for their excellent technical assistance.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal