Comment on Feistritzer and Riewald, page 3178
APC can trigger activation of the thrombin receptor PAR1 on endothelial cells. Is this important for the pharmacologic actions of APC? Endothelial barrier protection by PAR1 transactivation of S1P1 raises this possibility.
Activated protein C (APC) was recently reported to benefit patients with severe sepsis1 in accord with prior studies in nonhuman primates.2 The lack of apparent benefit of other anticoagulants in clinical trials as well as direct effects of APC on cells in vitro suggest that APC may have anti-inflammatory actions that are independent of its anticoagulant function.2 In 2002, Riewald et al3 showed that protease activated receptor-1 (PAR1) on endothelial cells can be activated by the endothelial protein C receptor (EPCR)/APC complex and suggested that this phenomenon might mediate APC's effects in sepsis. PAR1 activation does trigger potentially antiapoptotic and cytoprotective responses in endothelial cells,2 but the best characterized endothelial responses to PAR1 activation are proinflammatory. When might PAR1 activation be beneficial versus detrimental and how might activation by APC versus thrombin favor the former? In this issue of Blood, Feistritzer and Riewald propose a possible answer to this question.
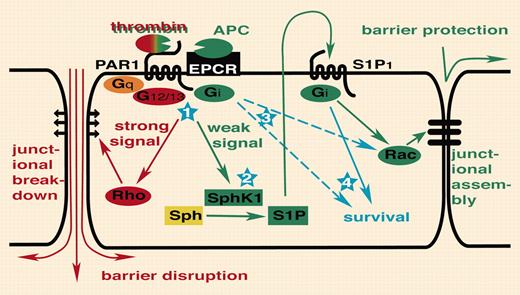
Breakdown of endothelial barrier function is recognized as a key event in the pathogenesis of sepsis. Feistritzer and Riewald found that pretreatment of endothelial monolayers in culture with APC attenuated permeability increases induced by thrombin or vascular endothelial growth factor (VEGF). The protective effects of APC and disruptive effects of thrombin were both blocked with a cleavagesite–specific antibody to PAR1, suggesting that the opposing responses to the 2 agonists were mediated by the same receptor. Low picomolar thrombin induced APC-like protective effects and supraphysiologic APC induced thrombin-like disruptive effects, arguing that the level of receptor activation determined the type of response. Because sphingosine 1–phosphate (S1P) enhances endothelial barrier function like APC and low thrombin,4 Feistritzer and Riewald sought and found evidence that the protective-effect of APC on endothelial barrier function required sphingosine kinase 1 (SphK1) and the S1P receptor S1P1, leading to the transactivation model in the figure.
How these intriguing in vitro observations relate to the pathophysiology of sepsis and the pharmacology of APC remains to be determined. Even in the presence of EPCR, APC is a poor activator of PAR1 compared with thrombin. While cleavage of endothelial PAR1 was not detected at APC concentrations in the range of those obtained in the plasma of septic patients infused with APC,5 the barrier protective effects of APC were detected at such low concentrations. It is worth noting that the extent to which cleavage of endothelial PAR1 by thrombin in the setting of disseminated intravascular coagulation (DIC) might obviate any opportunity for APC to participate is unknown and that alternative APC targets and anti-inflammatory mechanisms that do not involve PAR1 have been suggested.2 And while there are many caveats, the observation that Par1–/– mice die at the same rate as wild type in a model of endoxemia6 argues that activation of PAR1, at least by endogenous mediators, does not play either a necessary beneficial or harmful role in this model. Nonetheless, the current study suggests that low- versus high-level PAR1 activation can have distinct, even opposite, consequences and raises some answerable questions. Does PAR1 activation in endothelial cells stimulate SphK1 activity and S1P formation and release as the model predicts? Might this mechanism also play a role in the potentially beneficial effects of PAR1 activation described in other cell types or settings? Most interestingly, might S1P1 agonists protect endothelial barrier function in models of sepsis? We expect the study by Feistritzer and Riewald will prompt new studies of the potential roles of APC, PARs, and sphingosine phosphate receptors in endothelial biology and pathophysiology. ▪FIG1
PAR1 transactivation of S1P1 signaling. Thrombin activation of PAR1 disrupts endothelial barrier function via G12/13 and Rho (illustrated in red). In this issue ofBlood,Feistritzer and Riewald report that APC or picomolar thrombin can engage the same receptor with the opposite outcome, endothelial barrier protection, by transactivation of S1P1 (illustrated in green). Several questions remain to be answered (illustrated in blue). (1) Thrombin and APC can trigger both protective as well as disruptive responses depending on the rate of receptor activation. How is a difference in the level of receptor activation translated into activation of different signaling pathways? Do differences in coupling efficiency and/or intrinsic shutoff rates for different G proteins account for this? Or are different thresholds set by downstream pathways? (2) Does PAR1 indeed activate Sphk1 and S1P release? How? (3) PAR1 and S1P1 both couple to Gi. Why is transactivation required? Is it about amplification, tempo or subcellular localization of signaling, different effector pathways specified by receptor rather than G protein, or something else? (4) Does transactivation of S1P1 contribute to antiapoptotic or other effects of APC and thrombin?
PAR1 transactivation of S1P1 signaling. Thrombin activation of PAR1 disrupts endothelial barrier function via G12/13 and Rho (illustrated in red). In this issue ofBlood,Feistritzer and Riewald report that APC or picomolar thrombin can engage the same receptor with the opposite outcome, endothelial barrier protection, by transactivation of S1P1 (illustrated in green). Several questions remain to be answered (illustrated in blue). (1) Thrombin and APC can trigger both protective as well as disruptive responses depending on the rate of receptor activation. How is a difference in the level of receptor activation translated into activation of different signaling pathways? Do differences in coupling efficiency and/or intrinsic shutoff rates for different G proteins account for this? Or are different thresholds set by downstream pathways? (2) Does PAR1 indeed activate Sphk1 and S1P release? How? (3) PAR1 and S1P1 both couple to Gi. Why is transactivation required? Is it about amplification, tempo or subcellular localization of signaling, different effector pathways specified by receptor rather than G protein, or something else? (4) Does transactivation of S1P1 contribute to antiapoptotic or other effects of APC and thrombin?

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal