Comment on Nencioni et al, page 3255
Proteasome inhibition can promote death by apoptosis in various tumor cells, yet the molecular basis of these apoptotic effects are not well understood, particularly since proteasome inhibition can also increase levels of the antiapoptotic protein myeloid cell leukemia-1 (Mcl-1).
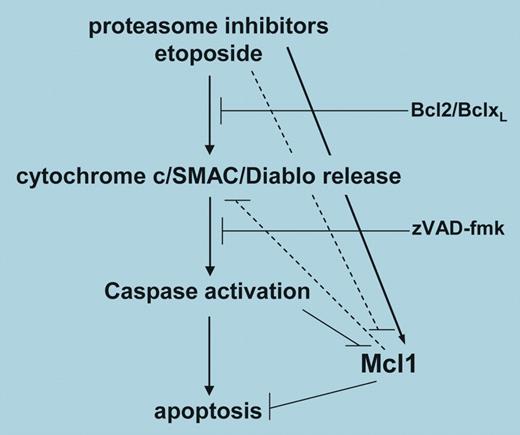
The cytotoxic activity of proteasome inhibitors is likely to involve modification of multiple components of the apoptotic signaling pathway. Initially, the ability of proteasome inhibitors to block the activation of nuclear factor kappa-B (NF-κB) was thought to be crucial for promoting apoptosis. However, a number of subsequent studies have concluded that inhibition of NF-κB alone cannot account for all apoptosis that occurs following proteasome inhibition. Nencioni and colleagues have investigated the effects of proteasome inhibition on members of the B-cell lymphoma/leukemia 2 (Bcl-2) family of proteins that play a central role in regulating apoptosis. Interestingly, the levels of most Bcl-2 family members do not dramatically change following proteasome inhibition, however, the antiapoptotic Mcl-1 is the apparent exception. Mcl-1 is a protein with a rapid turnover that soon accumulates after proteasome inhibition. The authors set out to investigate how this accumulation of Mcl-1 could influence apoptosis in various tumor cells in response to proteasome inhibitors. Using Jurkat cells they demonstrated that a variety of proteasome inhibitors promoted apoptosis. Fas-associated death domain (FADD)–deficient Jurkat cells were just as sensitive as the parental Jurkat to the apoptotic effects of proteasome inhibition, ruling out any involvement of death receptors in this apoptotic process. By contrast, Jurkat cells transfected with Bcl-2 were highly resistant to the apoptotic effects of proteasome inhibitors, suggesting that activation of the intrinsic apoptotic pathway involving mitochondrial disruption was a crucial component of the apoptotic signaling. Since Mcl-1 levels were also increased in these Jurkat cells, it was clear that a reduction of Mcl-1 was not essential for apoptosis to occur in response to proteasome inhibition. To study the effects of Mcl-1 in more detail, Nencioni and colleagues reduced levels of this protein using specific shRNAs. Surprisingly for Jurkat cells the reduction of Mcl-1 alone proved lethal. To investigate the effect of silencing Mcl-1 on apoptosis induced by proteasome inhibition, the authors used HeLa cells, where a clear increase in apoptosis resulted following proteasome inhibition in cells where Mcl-1 levels were reduced. Therefore, the fact that proteasome inhibitors also increased Mcl-1 somewhat compromised the strength of their proapoptotic effects. A number of important questions still remain. What is the proapoptotic signal(s) transmitted to the mitochondria following proteasome inhibition? Does Mcl-1 reduction additionally unleash Bcl-2 interacting mediator of cell death (Bim), a proapoptotic Bcl-2 family member thought to regulate leukocyte cell numbers? Nonetheless, this study does provide a good indication that a rational combination of agents targeting different components of the apoptotic pathway(s) may significantly increase the therapeutic index above that of either agent alone. ▪FIG1
A model for Mcl-1 regulation in apoptosis promoted by proteasome inhibitors. See the complete figure in the article beginning on page 3255.
A model for Mcl-1 regulation in apoptosis promoted by proteasome inhibitors. See the complete figure in the article beginning on page 3255.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal