Abstract
The t(4;14) translocation that occurs uniquely in a subset (15%) of patients with multiple myeloma (MM) results in the ectopic expression of the receptor tyrosine kinase (RTK), fibroblast growth factor receptor 3 (FGFR3). Inhibition of activated FGFR3 in MM cells induces apoptosis, validating FGFR3 as a therapeutic target in t(4;14) MM and encouraging the clinical development of FGFR3 inhibitors for the treatment of these patients, who have a poor prognosis. We describe here the characterization of a novel, small-molecule inhibitor of class III, IV, and V RTKs, CHIR-258, as an inhibitor of FGFR3. CHIR-258 potently inhibits FGFR3 with an inhibitory concentration of 50% (IC50) of 5 nM in in vitro kinase assays and selectively inhibited the growth of B9 cells and human myeloma cell lines expressing wild-type (WT) or activated mutant FGFR3. In responsive cell lines, CHIR-258 induced cytostatic and cytotoxic effects. Importantly, addition of interleukin 6 (IL-6) or insulin growth factor 1 (IGF-1) or coculture on stroma did not confer resistance to CHIR-258. In primary myeloma cells from t(4;14) patients, CHIR-258 inhibited downstream extracellular signal-regulated kinase (ERK) 1/2 phosphorylation with an associated cytotoxic response. Finally, therapeutic efficacy of CHIR-258 was demonstrated in a xenograft mouse model of FGFR3 MM. These studies support the clinical evaluation of CHIR-258 in MM.
Introduction
Autologous stem cell transplantation (ASCT) and advances in supportive care have had a significant impact on multiple myeloma (MM) and long-term survival.1,2 However, patients invariably have relapses, and MM remains a universally fatal disease. The identification of nonrandom chromosomal translocations in MM has resulted in the development of powerful prognostic tools and the identification of novel molecular targets. Nearly half of patients with MM overexpress a putative oncogene, dysregulated by one of 5 recurrent immunoglobulin heavy (IgH) translocations: 11q13 (cyclin D1), 6p21 (cyclin D3), 4p16 (fibroblast growth factor receptor 3 [FGFR3] and MMSET), 16q23 (c-maf), and 20q11 (mafB).3,4 These translocations likely represent an early and possibly seminal event in the development of MM. More recently, it has become clear that these specific IgH translocations impart prognostic significance. The t(4;14) translocation, in particular, which occurs in 15% of patients, appears to confer a particularly poor prognosis for MM, with no apparent therapeutic benefit from ASCT.5-8 Clearly, novel treatment approaches are required for these patients.
The t(4;14) translocation is unusual in that it appears to dysregulate 2 potential oncogenes, MMSET on der(4) and fibroblast growth factor receptor 3 (FGFR3) on der(14).9,10 Whether dysregulation of either or both of these genes is critical for MM pathogenesis is not known; however, several lines of evidence support a role for FGFR3 in tumor initiation and progression. Activation of wild-type (WT) FGFR3, a receptor tyrosine kinase (RTK), promotes proliferation and survival in myeloma cells and is weakly transforming in a hematopoietic mouse model.11-13 Subsequent acquisition of activating mutations of FGFR3 in some MMs are associated with progression to late-stage myeloma and are strongly transforming in several experimental models.12,14 In vitro studies suggest that FGFR3 can impart chemoresistance,13 an observation supported by clinical data that demonstrate poor responses to conventional chemotherapy5,6 and shortened median survival5-8 of t(4;14) MM patients. These findings suggest that ectopic expression of FGFR3 may play a significant, albeit not a singular role, in myeloma oncogenesis, thus making this RTK a target for molecular-based therapy.
Others and we have previously demonstrated that inhibition of FGFR3 in t(4;14) MM cell lines induces cytotoxic responses demonstrating that these cells remain dependent on FGFR3 signaling despite the complexity of genetic alterations in these cells derived from patients with end-stage disease.15-17 These observations are congruent with the results of receptor tyrosine inactivation in a range of human malignancies where clinical successes have been documented18-21 and encourage the clinical development of FGFR3 inhibitors for the treatment of these patients, who have a poor prognosis. In the present study, we identify the small-molecule RTK inhibitor, CHIR-258, as a potent inhibitor of FGFR3-transformed hematopoietic cell lines and human MM cell lines expressing either WT or mutant FGFR3. In addition, we demonstrate activity of CHIR-258 in a mouse model of FGFR3-mediated MM and against primary myeloma cells from t(4;14) patients. Together, these data indicate that CHIR-258 may have therapeutic value in MM, in particular in those patients with FGFR3 expression.
Patients, materials, and methods
Chemical compounds and biologic reagents
CHIR-258 (Chiron, Emeryville, CA) was dissolved in dimethyl sulfoxide (DMSO) at a stock concentration of 20 mM. For animal experiments CHIR-258 was formulated in 5 mM citrate buffer. FLT3, c-KIT, and human epidermal growth factor receptor 2 (HER2) RTKs were obtained from ProQuinase (Freiberg, Germany), epidermal growth factor receptor 1 (EGFR1) from Calbiochem (San Diego, CA) and FGFR3 and hepatocyte growth factor receptor (c-Met) from Upstate Biotechnology (Lake Placid, NY). EGFR2, FGFR1, platelet-derived growth factor receptor β (PDGFR-β), Flt1, Flt4, and vascular endothelial growth factor receptor 2 (VEGFR2) RTKs used in isolated enzyme assays were purified from insect cell lysates infected with recombinant baculovirus containing the respective kinase domains. All kinases contained WT amino acid sequences. Acidic FGF (aFGF) and heparin were purchased from R&D Systems (Minneapolis, MN) and Sigma (St. Louis, MO), respectively. FGFR3 antibodies (C15, H100, and B9) were from Santa Cruz Biotechnology (Santa Cruz, CA), and 4G10 was from Upstate Biotechnology.
In vitro kinase assays
The inhibitory concentration of 50% (IC50) values for the inhibition of RTKs by CHIR-258 were determined in a time-resolved fluorescence (TRF) or radioactive format, measuring the inhibition by CHIR-258 of phosphate transfer to a substrate by the respective enzyme. The kinase domains of FGFR3, FGFR1, PDGFR-β, and VEGFR1-3 were assayed in 50 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), pH 7.0, 2 mM MgCl2, 10 mM MnCl2 1 mM NaF, 1 mM dithiothreitol (DTT), 1 mg/mL bovine serum albumin (BSA), 0.25 μM biotinylated peptide substrate (GGGGQDGKDYIVLPI), and 1 to 30 μM adenosine triphosphate (ATP) depending on the Km for the respective enzyme. ATP concentrations were at or just below Km. For c-KIT and FLT3 reactions the pH was raised to 7.5 with 0.2 to 8 μM ATP in the presence of 0.25 to 1 μM biotinylated peptide substrate (GGLFDDPSYVNVQNL). Reactions were incubated at room temperature for 1 to 4 hours and the phosphorylated peptide captured on streptavidin-coated microtiter plates (Thermo Labsystems, Franklin, MA) containing stop reaction buffer (25 mM EDTA [ethylenediaminetetraacetic acid], 50 mM HEPES, pH 7.5). Phosphorylated peptide was measured with the DELFIA TRF system (Perkin Elmer Lifesciences, Boston, MA) using a Europium-labeled antiphosphotyrosine antibody PT66. The concentration of CHIR-258 for IC50 was calculated using nonlinear regression with XL-Fit data analysis software version 4.1 (IDBS, Guilford, United Kingdom). Inhibition of colony-stimulating factor-1 receptor (CSF-1R), PDGFR-α, insulin receptor (InsR), and insulin-like growth factor receptor 1 (IGFR1) kinase activity was determined using the IC50 Profiler Express service of Upstate Biotechnology at ATP concentrations close the Km for ATP.
Cell lines and tissue culture
All human MM cell lines and B9 cells were maintained in Iscove modified Dulbecco medium (IMDM) supplemented with 5% fetal calf serum (FCS), 100 μg/mL penicillin, and 100 μg/mL streptomycin (Hyclone, Logan, UT) and 1% interleukin 6 (IL-6)-conditioned medium (B9 cells only). Bone marrow stromal cells (BMSCs) were derived from MM patients and prepared as previously described.22 Briefly, mononuclear cells (MNCs) were cultured in 10 mL IMDM supplemented with 20% FCS in 10-cm2 plates. Subsequent to the first week the medium was replenished every 3 days. When an adherent cell monolayer developed, the cells, which show predominantly fibroblast morphology, were trypsinized, harvested, washed, and collected by centrifugation. For the purposes of viability assays, BMSCs were irradiated with 20 Gy after plating on 96-well plates.
FGFR3 expression vectors and B9 cell transfectants
B9 cells expressing WT FGFR3 (B9-WT), FGFR3-K650E (B9-K650E), and empty retrovirus (B9-MINV) have been described previously.11 Full-length FGFR3 cDNAs, containing F384L, Y373C, or J807C (a gift from Marta Chesi, Weill Medical College of Cornell, New York, NY) were cloned into an MSCV-based retroviral vector containing a green fluorescent protein (GFP) cassette. A construct carrying the G384D mutation was created from the FGFR3-WT by replacing the Pml I-Bgl II fragment between amino acid 290 and 413 with the same fragment obtained from the KMS18 as previously described.23 The constructed retroviral vectors were transfected into GP+E ecotropic packaging cells. The resulting retroviruses were used to introduce FGFR3 into the IL-6-dependent murine myeloma cell line, B9. A limiting cell dilution was further performed to generate single-cell clones. A high-expressing clone for each construct (B9-F384L, B9-Y373C, B9-G384D, and B9-J807C) was cryopreserved.
Viability assay
Cell viability was assessed by 3-(4,5-dimethylthiazol)-2,5-diphenyl tetrazolium (MTT) dye absorbance according to the manufacturer's instructions (Boehringer Mannheim, Mannheim, Germany). Cells were seeded in 96-well plates at a density of 5000 (B9 cells) or 20 000 (MM cell lines) cells per well. Cells were incubated with 30 ng/mL aFGF and 100 μg/mL heparin or 1% IL-6 where indicated and increasing concentrations of CHIR-258. For each concentration of CHIR-258, 10-μL aliquots of drug or DMSO diluted in culture medium was added. For drug combination studies, cells were incubated with 0.5 μM dexamethasone, 100 nM CHIR-258, or both simultaneously where indicated. To evaluate the effect of CHIR-258 on growth of MM cells adherent to BMSCs, 10 000 KMS11 cells were cultured on BMSC-coated 96-well plates in the presence or absence of CHIR-258. Plates were incubated for 48 to 96 hours. For assessment of macrophage colony-stimulating factor (M-CSF)-mediated growth, 5000 M-NFS-60 cells/well were incubated with serial dilutions of CHIR-25 ZS8 with 10 ng/mL M-CSF and without granulocyte-macrophage colony-stimulating factor (GM-CSF). After 72 hours cell viability was determined using Cell Titer-Glo Assay (Promega, Madison, WI). Each experimental condition was performed in triplicate.
Intracellular phosphoprotein staining
Determination of ERK1/2 phosphorylation by flow cytometry has been described previously.24,25 Briefly, cells were serum-starved overnight and then stimulated with 30 ng/mL aFGF and 10 μg/mL heparin for 10 minutes at 37°C. The cells were immediately fixed by adding 10% formaldehyde directly into the culture medium to obtain a final concentration of 2%. Cells were incubated in fixative for 10 minutes at 37°C then on ice for an additional 2 minutes. The cells were permeabilized by adding ice-cold methanol (final concentration of 90%) and incubated on ice for 30 minutes. Cells were stained with anti-ERK1/2 (Cell Signaling Technology, Beverly, MA) for 15 minutes and labeled with fluorescein isothiocyanate (FITC)-conjugated goat antirabbit and anti-CD138-phycoerythrin (PE; (PharMingen, San Diego, CA) where indicated. Malignant cells were identified as CD138-bright. Flow cytometry was performed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) and analyzed using CellQuest software (BD Biosciences).
Apoptosis analysis
For studies of apoptosis, cells were seeded at an initial density of 2 × 105/mL medium supplemented with DMSO or 100 nM or 500 nM CHIR-258 and cultured for up to 96 hours. The medium and drug were replenished every 3 days, and the cell density was adjusted to 2 × 105/mL. Apoptosis was determined by annexin V staining (Boehringer Mannheim, Indianapolis, IN) and analyzed by flow cytometry.
Primary patient samples
Patients identified for the study were determined to possess a t(4;14) translocation by fluorescence in situ hybridization (FISH). Expression of FGFR3 was confirmed by flow cytometry as described previously.12 Briefly, BM MNCs were incubated on ice for 30 minutes with rabbit anti-FGFR3 (H100) or rabbit preimmune serum. The cells were stained with FITC-conjugated goat antirabbit IgG and mouse anti-CD138-PE to identify MM cells.
All t(4;14)+ samples were further analyzed for the presence of FGFR3 or Ras mutations. Four pairs of primers were designed to amplify the regions of FGFR3 containing codons of the extracellular (EC) domain, transmembrane (TM) domain, tyrosine kinase (TK) domain, and stop codon (SC), known hot spots for activating mutations. Two pairs of primers were designed to amplify regions of codons 12, 13, and 61 of N-ras and K-ras.12 A first polymerase chain reaction (PCR) was performed on gDNA extracted from CD138-purified myeloma cells and amplicons were used for denaturing high-performance liquid chromatography (DHPLC) analysis. Results were confirmed by sequence analysis of the PCR products.
For cell death analysis, MNCs were plated at a cell density of 5 × 105 cells/mL in IMDM with 20% FCS, 30 ng/mL aFGF, 10 mg/mL heparin, and in the presence of DMSO or 500 nM CHIR-258 for up to 12 days. The medium, aFGF/heparin, and drug were replenished every 3 days. After 3, 7, and 12 days, cells were triple stained with anti-CD38-PE, anti-CD45-CyChrome (PharMingen), and FITC-conjugated annexin V as previously described.26 Controls included unstained cells, isotype control stained cells, and single-stained cells. Malignant plasma cells were defined as cells that express high levels of CD38 and no or low levels of CD45 (CD38++/CD45-). Samples were analyzed by FACScan analysis using CellQuest software. BM aspirates were obtained by consent under a protocol approved by the University Health Network Research Ethic Board (Toronto, ON, Canada).
Xenograft mouse model
The xenograft mouse model was prepared as previously described.27 Briefly, 6- to 8-week-old female BNX mice obtained from Frederick Cancer Research and Development Centre (Frederick, MD) were inoculated subcutaneously into the right flank with 3 × 107 KMS11 cells in 150 μL IMDM, together with 150 μL Matrigel basement membrane matrix (Becton Dickinson, Bedford, MA). Treatment was initiated when tumors reached volumes of 200 mm3 at which time mice were randomized to receive 10, 30, or 60 mg/kg CHIR-258 or 5 mM citrate buffer. Dosing was performed daily for 21 days by gavage. Eight to 10 mice were included in each treatment group. Caliper measurements were performed twice weekly to estimate tumor volume, using the formula: 4π/3 × (width/2)2 × (length/2). One-way analysis of variance was used to compare differences between vehicle- and CHIR-258-treated groups.
Immunoprecipitation and immunoblotting
Immunoprecipitation and immunoblotting were performed as described previously.28 Tumors from killed mice were immediately homogenized on ice and lysed in detergent buffer. Clarified cell extracts (1 mg/sample) were incubated for 6 hours with C15 FGFR3 antibody, then protein A/G agarose (Santa Cruz Biotechnology) was added for 2 hours. Immunoblotting was performed with antiphosphotyrosine antibody, 4G10, to assess phosphorylated FGFR3, or with anti-FGFR3 (B9) to measure total FGFR3.
Histopathology and immunohistochemical analysis
Tissue samples were fixed in 10% formalin and embedded in paraffin, from which 5-mm histologic sections were cut and stained with hematoxylin and eosin. Immunohistochemistry studies were performed by indirect immunoperoxidase staining of paraffin tissue sections using a TechMate500 BioTek automated immunostainer (Ventana Medical Systems, Tucson, AZ) and antibodies recognizing FGFR3 (C15), Ki-67 (Zymed, San Francisco, CA), and cleaved caspase 3 (Cell Signaling Technology) as previously described.29
Micrographs were acquired using a Nikon OptiPHOT transmitted fluorescence microscope (Nikon, Melville, NY) and a Coolsnap Pro color camera (Media-Cybernetics, San Diego, CA).
Results
Selective kinase inhibition of CHIR-258
A novel, multitargeted RTK inhibitor of the benzimidalzole-quinolinone class was identified by chemical libraries screening. The ability of the optimized analog, CHIR-258, to inhibit exogenous substrate phosphorylation was tested against a wide range of kinases. The concentration of CHIR-258 resulting in IC50 is reported in Table 1. CHIR-258 inhibited members of the class III RTKs including FLT3, c-Kit, CSF-1R, and PDGFRα/β with IC50 values of 0.001 to 0.21 mM as assessed by in vitro kinase assays. In addition, CHIR-258 potently inhibited class IV (FGFR1 and 3) and class V (VEGFR1-4) RTKs with IC50 values of 0.008 to 0.013 mM. When similar kinase assays for InsR, EGFR, c-Met, EphrinA2 (EphA2), Tie2, IGFR1, and HER2 were performed, significant inhibition was observed only at more than 10-fold higher concentrations. These studies demonstrated that CHIR-258 is a selective but multitargeted inhibitor of class III, IV, and V RTKs with high potency against FGFRs.
Specificity and potency of CHIR-258 kinase inhibitor
Receptor tyrosine kinases . | CHIR-258 IC50, μM . |
|---|---|
| FLT3 | 0.001 |
| c-KIT | 0.002 |
| CSF-1R/c-fms | 0.036 |
| FGFR1 | 0.008 |
| FGFR3 | 0.009 |
| VEGFR1/Flt1 | 0.01 |
| VEGFR2/Flk1 | 0.013 |
| VEGFR3/Flt4 | 0.008 |
| PDGFRβ | 0.027 |
| PDGFRα | 0.21 |
| InsR | 2 |
| EGFR1 | 2 |
| c-Met | > 3 |
| EphA2 | 4 |
| Tie2 | 4 |
| IGFR1 | > 10 |
| HER2 | > 10 |
Receptor tyrosine kinases . | CHIR-258 IC50, μM . |
|---|---|
| FLT3 | 0.001 |
| c-KIT | 0.002 |
| CSF-1R/c-fms | 0.036 |
| FGFR1 | 0.008 |
| FGFR3 | 0.009 |
| VEGFR1/Flt1 | 0.01 |
| VEGFR2/Flk1 | 0.013 |
| VEGFR3/Flt4 | 0.008 |
| PDGFRβ | 0.027 |
| PDGFRα | 0.21 |
| InsR | 2 |
| EGFR1 | 2 |
| c-Met | > 3 |
| EphA2 | 4 |
| Tie2 | 4 |
| IGFR1 | > 10 |
| HER2 | > 10 |
CHIR-258 inhibits the growth of WT and mutant FGFR3-transformed cells
We next tested the ability of CHIR-258 to inhibit constitutively activated FGFR3 mutants identified in MM patients (Y373C, G384D, K650E, J807C).12,29 Stable expression of these cDNAs conferred IL-6-independent growth to B9 cells, demonstrating that these mutants retain biologic activity and providing a platform for testing potential FGFR3 inhibitors against various classes of FGFR3 mutations. To determine the effect of CHIR-258 on FGFR3-mediated cell growth, B9 cells expressing FGFR3-WT, FGFR3-F384L (a nontransforming polymorphism), and the FGFR3-activated mutants were grown in increasing concentrations of inhibitor for 48 hours following which viability was determined by MTT assay (Figure 1). As expected, CHIR-258 potently inhibited the FGF-stimulated growth of WT and F384L-FGFR3-expressing B9 cells with IC50 values of 25 nM. In addition, CHIR-258 inhibited proliferation of B9 cells expressing each of the various activated mutants of FGFR3. Interestingly, there were minimal observed differences in the sensitivity of the different FGFR3 mutations to CHIR-258, with the IC50 ranging from 70 to 90 nM for each of the various mutations. IL-6-dependent B9 cells containing vector only (B9-MINV) were used to detect nonspecific toxicity. B9-MINV cells were resistant to the inhibitory activity of CHIR-258 at concentrations up to 1 μM. These data demonstrate inhibition of FGFR3 by CHIR-258 and indicate a lack of nonspecific cytotoxicity within the effective range of drug concentration. These results also indicate that CHIR-258 has potent activity against a variety of activated mutants of FGFR3 described in MM.
CHIR-258 inhibits the viability of FGFR3-expressing B9 cells but not parental IL-6-stimulated cells. Cells were exposed to CHIR-258 for 48 hours, and viability was assessed by an MTT-based assay. ⋄ indicates J807C cells; □, Y373C; ▵, K650E; ○, G384D; ▪, wild type (WT); ▴, F384L; and +, B9-MINV cells stimulated with IL-6. Untreated cells were defined as 100%. Values represent mean ± SD of 4 independent experiments.
CHIR-258 inhibits the viability of FGFR3-expressing B9 cells but not parental IL-6-stimulated cells. Cells were exposed to CHIR-258 for 48 hours, and viability was assessed by an MTT-based assay. ⋄ indicates J807C cells; □, Y373C; ▵, K650E; ○, G384D; ▪, wild type (WT); ▴, F384L; and +, B9-MINV cells stimulated with IL-6. Untreated cells were defined as 100%. Values represent mean ± SD of 4 independent experiments.
CHIR-258 is cytotoxic to FGFR3-expressing myeloma cells
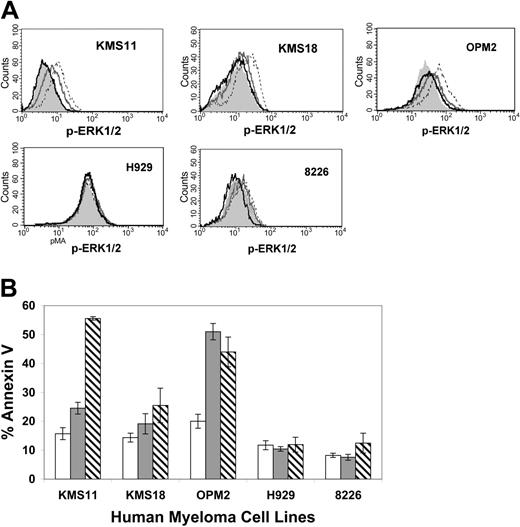
To assess the potential of CHIR-258 as a therapeutic agent in MM, we next studied the effect of CHIR-258 on the growth and survival of human myeloma cell lines. FGFR3+ cell lines (KMS11, KMS18, OPM2, H929) and the FGFR3- cell lines,6,12,15,16,22 U266 and 8226, were incubated with increasing concentrations of CHIR-258 and cell viability was monitored (Table 2). CHIR-258 inhibited cell proliferation of KMS11 (FGFR3-Y373C), OPM2 (FGFR3-K650E), and KMS18 (FGFR3-G384D) cells with IC50 of values of 90 nM (KMS11 and OPM2) and 550 nM, respectively. FGFR3- cell lines and H929 (FGFR3-WT), a cell line that harbors a downstream activating mutation of N-Ras,12 were resistant, requiring more than 5-fold higher concentrations to inhibit cell growth. Inhibition of cellular growth was associated with inhibition of downstream ERK1/2 phosphorylation as determined by flow cytometry. Pretreatment of responsive cell lines (KMS11, KMS18, OPM2) with CHIR-258 inhibited FGF-induced ERK1/2 phosphorylation (Figure 2A). In contrast, H929 cells, which displayed minimal cytostatic response to CHIR-258, demonstrated high basal levels of mitogen-activated protein (MAP) kinase activation as a result of constitutive Ras activation and showed no change in ERK1/2 phosphorylation, indicating that CHIR-258 is acting upstream of Ras.
IC50 values of CHIR-258 against human myeloma cell lines
Cell line . | t(4;14) . | FGFR3 genotype . | IC50, nM . |
|---|---|---|---|
| KMS11 | + | Y373C | 90 |
| KMS18 | + | G384D | 550 |
| OPM2 | + | K650E | 90 |
| H929 | + | WT | > 2500 |
| 8226 | — | ND | > 2500 |
| U266 | — | ND | > 2500 |
Cell line . | t(4;14) . | FGFR3 genotype . | IC50, nM . |
|---|---|---|---|
| KMS11 | + | Y373C | 90 |
| KMS18 | + | G384D | 550 |
| OPM2 | + | K650E | 90 |
| H929 | + | WT | > 2500 |
| 8226 | — | ND | > 2500 |
| U266 | — | ND | > 2500 |
Listed are MM cell lines and the presence (+) or absence (-) of the t(4;14) translocation and the FGFR3 mutations. The concentration of CHIR-258 that inhibits 50% viability (IC50) as compared with DMSO control (MTT assay or Cell Titer Glo) after 72 hours of incubation with CHIR-258 was determined.
WT denotes wild-type genotype; ND, not determined.
CHIR-258 inhibits FGF-mediated ERK1/2 phosphorylation and induces apoptosis of FGFR3-expressing human myeloma cell lines. (A) Flow cytometry analyses of ERK1/2 phosphorylation. Cells were serum-starved overnight (gray shaded curve) then stimulated with aFGF (dashed line), or starved overnight and pretreated for 2 hours with 100 nM (dark gray line) or 500 nM (black line) CHIR-258 and then stimulated with aFGF. (B) Cells were incubated with vehicle (□) or 100 nM (▦) or 500 nM (▧) CHIR-258 for 96 hours and apoptosis was assessed by means of flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent the mean ± SD of 4 independent experiments.
CHIR-258 inhibits FGF-mediated ERK1/2 phosphorylation and induces apoptosis of FGFR3-expressing human myeloma cell lines. (A) Flow cytometry analyses of ERK1/2 phosphorylation. Cells were serum-starved overnight (gray shaded curve) then stimulated with aFGF (dashed line), or starved overnight and pretreated for 2 hours with 100 nM (dark gray line) or 500 nM (black line) CHIR-258 and then stimulated with aFGF. (B) Cells were incubated with vehicle (□) or 100 nM (▦) or 500 nM (▧) CHIR-258 for 96 hours and apoptosis was assessed by means of flow cytometric assay of annexin V binding and propidium iodide exclusion. Values represent the mean ± SD of 4 independent experiments.
We also observed CHIR-258-induced apoptosis in responsive FGFR3-expressing cell lines. Treatment of KMS11, OPM2, and KMS18 cells with 500 nM CHIR-258 for 96 hours resulted in a significant increase in the percentage of annexin V-binding cells when compared with DMSO controls (Figure 2B). The delayed induction of apoptosis observed in some myeloma cell lines is similar to that previously reported with the more selective FGFR3 inhibitor, PD173074.15 Treatment of FGFR3- (U266 not shown) had no effect on annexin V binding, suggesting that class III and V RTKs that can potentially be inhibited by CHIR-258 are not expressed or are not essential for survival of these myeloma cells.
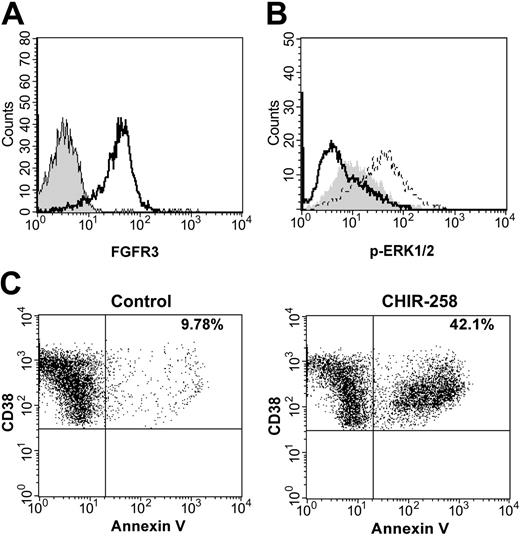
We next assessed the cytotoxic potential of CHIR-258 against primary human myeloma cells from patients previously identified by FISH as t(4;14) positive or negative.8 The presence or absence of FGFR3 expression was confirmed by flow cytometry (Figure 3A). Of the 5 t(4;14)+ samples, all but one demonstrated high-level expression of FGFR3 on CD138+ myeloma cells (Table 3). In addition, these samples were screened by DHPLC for FGFR3 mutations and downstream mutations of N- and K-Ras. No mutations were identified. FGF stimulation of primary cells in culture resulted in up-regulation of ERK1/2 phosphorylation in CD138+ myeloma cells demonstrating biologic activity of FGFR3 in these cells (Figure 3B). CHIR-258 at 500 nM inhibited FGF-induced increases in ERK1/2 phosphorylation in all samples. In addition, MNCs were cultured with 500 nM CHIR-258 or DMSO vehicle and apoptosis was determined by annexin V staining. Four of 5 t(4;14) myeloma samples demonstrated a cytotoxic response after 7 to 12 days of exposure to CHIR-258 when compared with vehicle control, whereas none of the other myeloma samples were affected (Figure 3C; Table 3). Interestingly, the t(4;14)+ sample that demonstrated low-level FGFR3 expression was CHIR-258 resistant, implying that only a high level of WT FGFR3 expression can confer dependence. Support for this hypothesis is provided by studies of c-KIT30 in gastrointestinal tumors and FLT331 in acute myeloid leukemia (AML) where high-level expression of the WT receptor, as well as receptor mutation, led to constitutive activity and inhibitor sensitivity. Furthermore, sensitivity to trastuzumab in breast cancer correlates with the level of HER2/neu expression.32 Alternatively, MM cells from this patient may have activation of additional pathways that circumvent dependency on FGFR3 signaling.
CHIR-258 inhibits FGF-mediated ERK1/2 phosphorylation and induces cytotoxicity in FGFR3-expressing primary MM cells. (A) FGFR3 is expressed at high levels on the surface of t(4;14) primary myeloma cells. Cells were stained with FGFR3 antibody (open curve) or rabbit preimmune serum (shaded curve) and then stained with goat antirabbit FITC. Myeloma cells were identified by CD138 labeling. (B) Primary myeloma cells were incubated in the absence (shaded curve) or presence of aFGF (dashed line) or preincubated with 500 nM CHIR-258 (solid line) for 2 hours and then stimulated with aFGF. ERK1/2 phosphorylation was assessed by flow cytometric analysis. (C) Primary myeloma cells were cultured in growth medium in the presence of DMSO or 500 nM CHIR-258. Cells were harvested after 7 days and stained with annexin V-FITC and analyzed by flow cytometry. Myeloma cells were identified by CD38++/CD45-labeling. The total percentage of CD38++/CD45-/annexin V+cells is shown in the upper right quadrant. Shown are representative data from patient 3.
CHIR-258 inhibits FGF-mediated ERK1/2 phosphorylation and induces cytotoxicity in FGFR3-expressing primary MM cells. (A) FGFR3 is expressed at high levels on the surface of t(4;14) primary myeloma cells. Cells were stained with FGFR3 antibody (open curve) or rabbit preimmune serum (shaded curve) and then stained with goat antirabbit FITC. Myeloma cells were identified by CD138 labeling. (B) Primary myeloma cells were incubated in the absence (shaded curve) or presence of aFGF (dashed line) or preincubated with 500 nM CHIR-258 (solid line) for 2 hours and then stimulated with aFGF. ERK1/2 phosphorylation was assessed by flow cytometric analysis. (C) Primary myeloma cells were cultured in growth medium in the presence of DMSO or 500 nM CHIR-258. Cells were harvested after 7 days and stained with annexin V-FITC and analyzed by flow cytometry. Myeloma cells were identified by CD38++/CD45-labeling. The total percentage of CD38++/CD45-/annexin V+cells is shown in the upper right quadrant. Shown are representative data from patient 3.
Summary of expression of FGFR3 on primary MM cells in relation to sensitivity to CHIR-258
Patient no. . | FGFR3 by flow cytometry . | FGFR3 genotype . | N- and K-Ras genotype . | % annexin V, DMSO . | % annexin V, 500 nM CHIR-258 . | Increase in annexin V, % . |
|---|---|---|---|---|---|---|
| 1 | ND | WT | WT | 9.0 | 21.8 | 20.9 |
| 2 | 1.3 | WT | WT | 10.4 | 8.6 | –1.8 |
| 3 | 11.6 | WT | WT | 9.8 | 42.1 | 32.3 |
| 4 | 2.7 | WT | WT | 6.8 | 25.7 | 18.9 |
| 5 | 26.9 | WT | WT | 10.1 | 24.5 | 14.4 |
| 6 | 1.1 | ND | ND | 8.8 | 10.2 | 1.4 |
| 7 | 0.95 | ND | ND | 15.3 | 16.0 | 0.7 |
| 8 | 0.79 | ND | ND | 20.9 | 20.7 | –0.2 |
| 9 | 1.02 | ND | ND | 12.8 | 13.4 | 0.6 |
| 10 | 0.78 | ND | ND | 15.0 | 17.1 | 2.1 |
Patient no. . | FGFR3 by flow cytometry . | FGFR3 genotype . | N- and K-Ras genotype . | % annexin V, DMSO . | % annexin V, 500 nM CHIR-258 . | Increase in annexin V, % . |
|---|---|---|---|---|---|---|
| 1 | ND | WT | WT | 9.0 | 21.8 | 20.9 |
| 2 | 1.3 | WT | WT | 10.4 | 8.6 | –1.8 |
| 3 | 11.6 | WT | WT | 9.8 | 42.1 | 32.3 |
| 4 | 2.7 | WT | WT | 6.8 | 25.7 | 18.9 |
| 5 | 26.9 | WT | WT | 10.1 | 24.5 | 14.4 |
| 6 | 1.1 | ND | ND | 8.8 | 10.2 | 1.4 |
| 7 | 0.95 | ND | ND | 15.3 | 16.0 | 0.7 |
| 8 | 0.79 | ND | ND | 20.9 | 20.7 | –0.2 |
| 9 | 1.02 | ND | ND | 12.8 | 13.4 | 0.6 |
| 10 | 0.78 | ND | ND | 15.0 | 17.1 | 2.1 |
FGFR3 expression on CD138 primary MM cells was analyzed by flow cytometry and the values indicate fold increase in mean fluorescence over isotype control. CD138-selected cells were screened for the FGFR3 and N- and K-Ras mutations.
ND indicates not determined.
Effect of IL-6, IGF-1, and stroma on response of MM cells to CHIR-258
Given the known role of IL-633,34 and, more recently, IGF-135,36 in tumor cell proliferation, survival, and drug resistance in MM, we next examined whether exogenous IL-6 and IGF-1 could overcome the growth inhibitory effects produced by CHIR-258. Inhibition with CHIR-258 was still observed when KMS11 cells were grown in the presence of 50 ng/mL IL-6 or 50 ng/mL IGF-1 and was comparable to that of cells cultured in the presence of aFGF (Figure 4A). These studies highlight the critical role of FGFR3 function in the hierarchy of growth factor receptors in these cells.
CHIR-258 inhibits viability of KMS11 cells in the presence of IL-6, IGF-1, and BMSCs. (A) KMS11 cells were cultured in with DMSO (□) and with 100 nM (▦) or with 500 nM (▧) CHIR-258 in the presence of 30 ng/mL FGF, 50 ng/mL IL-6 or 50 ng/mL IGF-1. Cell viability after 48 hours was assessed by MTT assay. (B) BMSCs, KMS11 cells, or BMSCs together with KMS11 were cultured with DMSO (□), 100 nM (▦), or 500 nM (▧) CHIR-258 and viability after 96 hours was assessed by MTT assay. The data represent means of quadruplicate cultures ± SD.
CHIR-258 inhibits viability of KMS11 cells in the presence of IL-6, IGF-1, and BMSCs. (A) KMS11 cells were cultured in with DMSO (□) and with 100 nM (▦) or with 500 nM (▧) CHIR-258 in the presence of 30 ng/mL FGF, 50 ng/mL IL-6 or 50 ng/mL IGF-1. Cell viability after 48 hours was assessed by MTT assay. (B) BMSCs, KMS11 cells, or BMSCs together with KMS11 were cultured with DMSO (□), 100 nM (▦), or 500 nM (▧) CHIR-258 and viability after 96 hours was assessed by MTT assay. The data represent means of quadruplicate cultures ± SD.
Because the BM microenvironment has been shown to confer drug resistance in MM cells37,38 we studied the effect of CHIR-258 on MM cell growth in the BM milieu. CHIR-258 had no direct toxicity on BMSCs compared with DMSO control (Figure 4B). KMS11 cells were then cultured with or without BMSCs in the presence or absence of CHIR-258. BMSCs did confer a modest degree of resistance with 44.6% growth inhibition for cells treated with 500 nM CHIR-258 and cultured on stroma compared with 71.6% growth inhibition for cells grown without BMSCs. However, cell growth was still significantly inhibited by the CHIR-258 despite the presence of stroma.
CHIR-258 augments dexamethasone cytotoxicity in MM
We have previously demonstrated that FGFR3 expression results in increased phosphorylation of signal transducer and activator of transcription 3 (STAT3) and higher levels of Bcl-xL expression than that observed in parental B9 cells after IL-6 withdrawal.11,13 These findings were associated with inhibition of dexamethasone-induced apoptosis, a phenomenon that was reversed by Bcl-xL antisense oligonucleotide. We therefore postulated that treatment of FGFR3-expressing MM cells could overcome resistance to dexamethasone. As shown in Table 4, KMS11 cells are relatively resistant to dexamethasone; however, when combined with CHIR-258, synergistic inhibitory effects were observed. These data indicate a potential usefulness of combining dexamethasone with CHIR-258 as a therapeutic strategy.
Effect of CHIR-258 or dexamethasone or both on KMS11 viability
Treatment (concentration) . | Viability as % of control ± SD . |
|---|---|
| DMSO | 100% |
| Dexamethasone (0.5 μM) | 87% ± 4.74 |
| CHIR-258 (100 nM) | 49% ± 4.64 |
| Dexamethasone (0.5 μM) and CHIR-258 (100 nM) | 10% ± 6.48 |
Treatment (concentration) . | Viability as % of control ± SD . |
|---|---|
| DMSO | 100% |
| Dexamethasone (0.5 μM) | 87% ± 4.74 |
| CHIR-258 (100 nM) | 49% ± 4.64 |
| Dexamethasone (0.5 μM) and CHIR-258 (100 nM) | 10% ± 6.48 |
CHIR-258 inhibits M-CSF-mediated cell growth
Osteolytic bone loss is one of the major complications in MM. The major osteoclast-activating factors involved in bone resorption are IL-1β, IL-6, receptor activator of nuclear factor κB ligand (RANK-L), and M-CSF.39 MM cells, osteoblasts, and stromal cells in the BM express M-CSF, which together with RANK-L, is essential for osteoclast formation.40 Increased serum concentrations of M-CSF have been detected in MM patients.41 In vitro kinase assays demonstrate potent activity of CHIR-258 against CSF-1R, the only known receptor for M-CSF with an IC50 of 36 nM (Table 1). In a cell-based assay, CHIR-258 inhibited proliferation of M-NFS-60, an M-CSF growth-driven mouse myeloblastic cell line with a median effective concentration (EC50) of 220 nM (data not shown). It would appear, therefore, that in addition to inhibiting MM cell growth, CHIR-258 has the advantage of potentially inhibiting tumor-associated osteolysis.
Evaluation of CHIR-258 in vivo in a xenograft mouse model
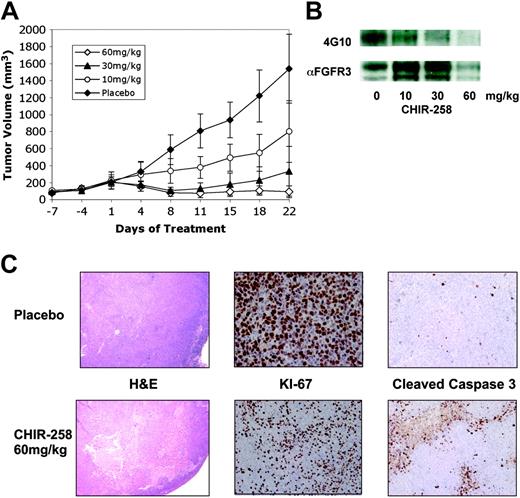
We tested the efficacy of CHIR-258 in a murine model in which KMS11 cells are injected subcutaneously into BNX mice.26,42 A similar plasmacytoma xenograft mouse model has been used in preclinical studies of bortezomib and immunomodulatory drugs (IMiDs) in MM. When the tumors reached approximately 200 mm3, mice were randomized (n = 8-10) to receive vehicle or CHIR-258 at 10 mg/kg, 30 mg/kg, and 60 mg/kg, administered by oral gavage once daily for 21 days. When compared with vehicle controls, a significant (P < .001) antitumor effect was observed in all 3 CHIR-258 dose groups with a minimum effective dose of 10 mg/kg/d (Figure 5A). Specifically, we calculated 48%, 78.5%, and 94% growth inhibition in the 10 mg/kg, 30 mg/kg, and 60 mg/kg treatment arms, respectively, compared with the placebo-treated mice. On the last day of dosing, 7 of 10 mice in the highest treatment group had achieved and maintained a partial remission with more than 50% reduction in tumor volumes as compared with day 1 of drug administration. Weight loss, as a marker of significant toxicity, was not observed in any of the treatment groups.
CHIR-258 inhibits FGFR3 phosphorylation and demonstrates antitumor effects in vivo. (A) When tumor size reached 200 mm3, mice were randomly assigned (8-10/group) to receive vehicle alone ( ) or varying doses of CHIR-258 (○, 10 mg/kg; ▴, 30 mg/kg; ⋄, 60 mg/kg) daily by oral gavage for 21 days. Results are tumor volume, mean ± SD mm3, plotted against time. (B) At the completion of treatment, mice from each treatment group were humanely killed, and the tumors were removed for assay. Tumors were immediately homogenized in ice-cold lysis buffer and FGFR3 was immunoprecipitated from 1 mg protein with antihuman FGFR3 and immunoblotting with antiphosphotyrosine antibody (4G10) was performed (top row). Then blot was stripped and probed with an anti-FGFR3 antibody (αFGFR3) as a loading control (bottom row). (C) KMS11-derived tumors were analyzed by hematoxylin and eosin (H & E) staining (10 × magnification) and immunostaining for Ki-67 and cleaved caspase 3 (40 × magnification). Shown are representative data from vehicle- and 60 mg/kg-treated mice.
) or varying doses of CHIR-258 (○, 10 mg/kg; ▴, 30 mg/kg; ⋄, 60 mg/kg) daily by oral gavage for 21 days. Results are tumor volume, mean ± SD mm3, plotted against time. (B) At the completion of treatment, mice from each treatment group were humanely killed, and the tumors were removed for assay. Tumors were immediately homogenized in ice-cold lysis buffer and FGFR3 was immunoprecipitated from 1 mg protein with antihuman FGFR3 and immunoblotting with antiphosphotyrosine antibody (4G10) was performed (top row). Then blot was stripped and probed with an anti-FGFR3 antibody (αFGFR3) as a loading control (bottom row). (C) KMS11-derived tumors were analyzed by hematoxylin and eosin (H & E) staining (10 × magnification) and immunostaining for Ki-67 and cleaved caspase 3 (40 × magnification). Shown are representative data from vehicle- and 60 mg/kg-treated mice.
CHIR-258 inhibits FGFR3 phosphorylation and demonstrates antitumor effects in vivo. (A) When tumor size reached 200 mm3, mice were randomly assigned (8-10/group) to receive vehicle alone ( ) or varying doses of CHIR-258 (○, 10 mg/kg; ▴, 30 mg/kg; ⋄, 60 mg/kg) daily by oral gavage for 21 days. Results are tumor volume, mean ± SD mm3, plotted against time. (B) At the completion of treatment, mice from each treatment group were humanely killed, and the tumors were removed for assay. Tumors were immediately homogenized in ice-cold lysis buffer and FGFR3 was immunoprecipitated from 1 mg protein with antihuman FGFR3 and immunoblotting with antiphosphotyrosine antibody (4G10) was performed (top row). Then blot was stripped and probed with an anti-FGFR3 antibody (αFGFR3) as a loading control (bottom row). (C) KMS11-derived tumors were analyzed by hematoxylin and eosin (H & E) staining (10 × magnification) and immunostaining for Ki-67 and cleaved caspase 3 (40 × magnification). Shown are representative data from vehicle- and 60 mg/kg-treated mice.
) or varying doses of CHIR-258 (○, 10 mg/kg; ▴, 30 mg/kg; ⋄, 60 mg/kg) daily by oral gavage for 21 days. Results are tumor volume, mean ± SD mm3, plotted against time. (B) At the completion of treatment, mice from each treatment group were humanely killed, and the tumors were removed for assay. Tumors were immediately homogenized in ice-cold lysis buffer and FGFR3 was immunoprecipitated from 1 mg protein with antihuman FGFR3 and immunoblotting with antiphosphotyrosine antibody (4G10) was performed (top row). Then blot was stripped and probed with an anti-FGFR3 antibody (αFGFR3) as a loading control (bottom row). (C) KMS11-derived tumors were analyzed by hematoxylin and eosin (H & E) staining (10 × magnification) and immunostaining for Ki-67 and cleaved caspase 3 (40 × magnification). Shown are representative data from vehicle- and 60 mg/kg-treated mice.
To demonstrate that the observed responses correlated with FGFR3 inhibition, mice were humanely killed 4 hours after receiving the last dose of CHIR-258 and tumors were harvested for analysis of in vivo inhibition of FGFR3 phosphorylation. FGFR3 was immunoprecipitated from tumor cell lysates and the level of expression and phosphorylation was determined on immunoblots (Figure 5B). In vivo inhibition of FGFR3 was observed, with near complete inhibition of FGFR3 occurring at the 60 mg/kg dose. Histopathologic examination of the tumors from representative animals (Figure 5C) further supported the interpretation of tumor reduction in the drug-treated mice compared with the placebo controls. Tumors from the drug-treated mice showed large areas of tumor necrosis. Immunohistochemistry for expression of the proliferative antigen, Ki-67, and for cleaved caspase 3, demonstrated that CHIR-258 inhibited cell growth and induced apoptosis. These findings suggest that CHIR-258 induces both cytostatic and cytotoxic responses in vivo resulting in regression of FGFR3-expressing tumors.
Discussion
The identification of recurrent cytogenetic abnormalities in MM and characterization of the translocation partners has identified novel molecular targets and presents the potential for molecular targeted therapy for this universally fatal disease.3,9 Nearly 20% of patients with newly diagnosed MM harbor the t(4;14) translocation as detected by the presence of IgH-MMSET hybrid transcript,43 the presence of which has generally been reported to be associated with a poor prognosis.5-8 FGFR3 is expressed in approximately 70%6,40 of these cases and 10%44 of patients will acquire an activating mutation of FGFR3 with disease progression.
An understanding of the genetic defects that are causally implicated in oncogenesis has led to targeted therapy for the treatment of a number of cancers.18-21 Most notably, the inhibition of BCR-ABL kinase activity by imatinib mesylate has produced major cytogenetic remissions in chronic myelogenous leukemia (CML).18 Inhibition of activated c-Kit in gastrointestinal stromal tumors by imatinib mesylate has also been effective against this chemoresistant tumor.19 In addition, trastuzumab, a monoclonal antibody targeting HER2/neu, has resulted in improved chemotherapy responses and prolonged survival of patients with breast cancer.20 A similar kinase inhibitor strategy targeting FLT3 in AML is also showing promising results in phase 2 clinical trials.21 Preclinical studies of FGFR3 inhibition in t(4;14) myeloma have likewise identified this RTK as a plausible candidate for targeted therapy. Two antagonists of FGFR3, PD173074 and SU5402, inhibited the growth and induced apoptosis of MM cells expressing mutant FGFR3.15-17 Together these studies support the clinical development of FGFR3 inhibitors for these patients.
CHIR-258, a novel benzimidazole-quinolinone, is a potent inhibitor of FGFR3 and class III, IV, and V RTKs, including FGFR1, VEGFR1/2/3, PDGFR, FLT3, c-KIT, and CSF-1R. In this study, we identified CHIR-258 as a highly active inhibitor of both WT and mutant FGFR3 tyrosine kinases. The activity of this inhibitor against a broad spectrum of RTKs implies that CHIR-258 requires less stringent conformation requirements for binding to the kinase domain and is consistent with the retained activity of CHIR-258 against many FGFR3 mutants. CHIR-258 treatment selectively induced apoptotic cell death of MM cell lines and primary patient samples that harbor FGFR3. The potential clinical application of CHIR-258 for the treatment of MM was further validated using a xenograft mouse model in which CHIR-258 treatment inhibited FGFR3 activity in vivo and produced tumor regression.
Although our data suggest that FGFR3 is the primary target of CHIR-258 in MM cells, it is important to note that OPM2 cells responded to this broadly active RTK inhibitor when they did not respond to the more selective FGFR3 inhibitor PD173074.15,16 Consistent with our results, Grand et al demonstrated that the multitargeted RTK inhibitor, SU5402, induced cytotoxic responses in OPM2 cells, whereas PD173074 failed to induce apoptosis.17 These findings therefore raise the possibility that CHIR-258 is targeting other, as yet to be defined, targets important for myeloma cell viability, a fact that is of further relevance given the demonstration that FGFR3 is sometimes lost during disease progression and may therefore be supplanted by other downstream signaling mediators.
With the latter point in mind, it is important to note that the clinical relevance of FGFR3 in t(4;14) MM has been questioned by observations that the der(14) chromosome is lost in some patients with MM, suggesting that FGFR3 is dispensable and that MMSET is the true causal target of t(4;14) in MM.6,43 Moreover, studies in model systems indicate that WT FGFR3 is not dominantly transforming, requiring additional cooperating oncogenic events to complement transformation.12,14 Our data, however, indicate that primary MM cells that definitively express FGFR3 remain dependent on this pathway for survival despite the presence of additional genetic events. It is likely that FGFR3 acts in concert with MMSET providing survival signals through the stimulation by FGF ligands expressed in the BM microenvironment. Along these lines, FLT3 mutations and high-level expression of FLT3 have been described in acute lymphoblastic leukemia where MLL, a gene similar to MMSET, is also expressed.31 These observations suggest a possible mechanism of complementation between tyrosine kinases and trithorax group proteins.
Our prior studies of FGFR3 inhibition in MM cell lines indicated that only cell lines expressing the constitutively active receptor responded to FGFR3 inhibition.15,16 This highlights the limitation of using MM cell lines that grow independently of the BM microenvironment and thus are no longer reliant on FGF produced by the stroma for growth and survival. Studies using primary patient material are therefore critical. The cytotoxic effect demonstrated by primary MM cells exposed to CHIR-258 predicts that this drug will be an effective therapy in patients expressing either WT or mutant FGFR3. Nevertheless, the only modest and delayed cytotoxic response to CHIR-258 observed in primary MM cells may imply that inhibition of WT FGFR3 does not itself introduce proapoptotic signal but more likely results in the withdrawal of strong antiapoptotic signals. This may suggest that CHIR-258 might have a limited role as single agent and that the most effective use of CHIR-258 will be in combination with chemotherapeutic agents such as dexamethasone as demonstrated in KMS11 cells. Modeling combination therapies of CHIR-258 with additional therapeutics that have shown single-agent antimyeloma activity are ongoing.
The importance of the BM microenvironment in supporting tumor growth is becoming increasingly clear.36,37 In particular, cytokines such as IL-6 and IGF-1 and direct interaction with BMSCs have been shown to confer drug resistance. Our in vitro experiments demonstrate that these paracrine factors failed to overcome the antitumor effects of CHIR-258. Given its target profile, CHIR-258 has the potential to affect host-derived tumor-associated cells within the BM that have implications in supporting tumor growth. CHIR-258 exhibits potent antiangiogenic activity in several angiogenesis assays including endothelial cell migration and tube formation on fibrin gels as well as in the ex vivo rat aortic ring assay.45 Accordingly, tumors from CHIR-258-treated mice grossly appeared less vascular when compared with controls (data not shown). We have further demonstrated that CHIR-258 inhibits CSF-1R activity, the receptor for M-CSF, an osteoclast-activating factor that may contribute to pathogenesis of bone disease in MM. Taken together, the data suggest that CHIR-258 can potentially target both the MM cell within the BM milieu and the BM microenvironment directly.
In summary, CHIR-258 represents a novel and potent small-molecule inhibitor of FGFR3 for the treatment of t(4;14) myeloma. The cytotoxic effects of CHIR-258 on MM cell lines and primary patient samples and a target profile that suggests the potential to favorably modulate the BM milieu suggest that this will be an effective therapy in this group that has a poor prognosis. Furthermore, the multitargeted specificity of CHIR-258 makes it a promising candidate for clinical development in a variety of solid and hematologic malignancies.
Prepublished online as Blood First Edition Paper, December 14, 2004; DOI 10.1182/blood-2004-10-3913.
Supported by grants from the Multiple Myeloma Research Foundation (S.T.), American Society of Hematology (ASH) Scholar Award (S.T.), Eli Lilly/Cancer Care Ontario (CCO)/Canadian Institutes of Health Research (CIHR) Hollenberg Award (S.T.), National Cancer Institute of Canada (A.K.S.), and Canadian Institutes for Health Research (A.K.S.). M.W. and C.H. are employed by a company (Chiron Corporation) whose compound CHIR-258 was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Mehrdad Yazdanpanah for his assistance with the FGFR3 and Ras mutation analysis and Kevin So for the histopathology and immunohistochemical preparation of tumor specimens (UniversityHealth Network, Toronto, ON, Canada). We are grateful to Marta Chesi (Weill Medical College, New York, NY) for providing the FGFR3 cDNAs and to Gena Lapointe and Isabelle Lee (Chiron Corporation, Emeryville, CA) for their technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal