Abstract
Hemophilia A is a lead candidate for treatment by gene therapy because small increments in the missing secreted protein product, coagulation factor VIII (FVIII), would result in substantial clinical amelioration. Clinically relevant therapy might be achieved by stably delivering a human FVIII cDNA to correct the bleeding disorder. We used the Sleeping Beauty (SB) transposon, delivered as naked plasmid DNA by tail-vein injection, to integrate B-domain–deleted FVIII genes into the chromosomes of hemophilia A mice and correct the phenotype. Since FVIII protein is a neoantigen to these mice, sustaining therapeutic plasma FVIII levels was problematic due to inhibitory antibody production. We circumvented this problem by tolerizing 82% of neonates by a single facial-vein injection of recombinant FVIII within 24 hours of birth (the remaining 18% formed inhibitors). Achievement of high-level (10%-100% of normal) FVIII expression and phenotypic correction required co-injection of an SB transposase-expressing plasmid to facilitate transgene integration in immunotolerized animals. Linker-mediated polymerase chain reaction was used to clone FVIII transposon insertion sites from liver genomic DNA, providing molecular evidence of transposition. Thus, SB provides a nonviral means for sustained FVIII gene delivery in a mouse model of hemophilia A if the immune response is prevented.
Introduction
Hemophilia A is an X-linked, recessive, genetic disorder that is caused by insufficient coagulation factor (F) VIII synthesis resulting in sustained bleeding after trauma or injury.1 Recombinant FVIII protein is currently used to treat bleeding episodes at the cost of approximately $55 000 per person year and is not available in many parts of the world.2 Gene therapy for hemophilia has recently been an intense area of study because even modest levels (2%-5% normal) of FVIII or FIX can improve clinical outcomes. Considerable progress has been made in developing both nonviral and viral vectors to this end. Viral vectors have been extremely effective in delivering FVIII and FIX transgenes, in some cases curing animal models of hemophilia A and B, respectively (reviewed in Nathwani et al3 ).
However, problems related to triggering of the host inflammatory response have resulted in cessation of clinical trials with both adenovirus4 and adeno-associated virus.5 Accordingly, improved adenoviral vectors are being developed that may be less immunogenic (reviewed in Ritter et al6 ), and the use of alternative serotypes also has shown promise in adeno-associated virus re-administration.7 Nevertheless, these kinds of problems, along with large-scale vector production challenges, have fueled intense interest in development of nonviral approaches for the treatment of hemophilia by gene therapy.
The benefits of nonviral vectors include simplicity, ease of storage, and amenability to large-scale manufacture.3,8 They are also potentially less immunogenic than viral vectors, which could allow for safer administration and/or re-administration. Integrating and nonintegrating plasmid-based vectors have been developed and successfully tested in animal models of hemophilia. Miao and colleagues have developed liver-specific plasmid vectors that are capable of mediating high-level FVIII expression for at least 6 months in immunodeficient mice without integration.9 However, similar studies using the integrating Sleeping Beauty (SB) transposable element or the phage ΦC31 integrase have shown that integration boosts the level of stable FIX gene expression.10,11 The long-term stability of episomal plasmids in humans has yet to be fully elucidated and warrants investigation.
Immune response to FVIII has been a persistent clinical problem for clotting factor replacement therapy12 and gene therapy studies in animal models.9,13-15 In FVIII vector-treated hemophilia A mice, anti-FVIII antibodies rapidly clear FVIII from the circulation, thereby complicating the assessment of the efficacy of gene therapy vectors.13-15 Prevention of the immune response often requires the use of immunosuppressive drugs, which are not always effective at preventing anti-FVIII antibody formation and complicate safety assessment.14,15 To circumvent this problem, we tolerized16,17 neonatal hemophilia A mice by a facial vein injection of recombinant B-domain–deleted (BDD) human (h)FVIII to prevent inhibitor formation. Madoiwa et al previously characterized the lack of inhibitory antibody formation and proliferative T-cell response to hFVIII in neonates tolerized in this manner.17 This study expands the use of tolerization to evaluate the efficacy of SB-mediated FVIII gene delivery in tolerized animals.
SB is a synthetic “cut-and-paste” transposable element that mediates precise integration of the transposon and its cargo into a TA dinucleotide at random.18,19 Herein we used the SB transposable element to integrate large (6.4 kb) FVIII expression transposons and achieve long-term FVIII expression along with phenotypic correction in immunotolerized hemophilia A mice. Achieving high-level, long-term expression required neonatal tolerization to prevent inhibitor formation and co-injection of an SB-expressing transposase plasmid to facilitate integration.
Materials and methods
Plasmid vectors and transfection
The pSBT series of Sleeping Beauty transposons was created by first making preSBT(-3) by removing the lacZ, multiple cloning site, and the T3/T7 primer binding sites from pBluescript. This was done by digesting with PvuII and filling with adapter 3 (CTGGATCCAGATCTGGTACCATTTAAAT), which creates a PvuII, BamHI, BglII, KpnI, SwaI mini-cloning site. Polymerase chain reaction (PCR) amplification of pT2/BH (sequence available at http://www.cbs.umn.edu/labs/perry/) using KJC-T2out1 (CCATCATTTTCTGGAATTTTCCAA GCTG) and KJC-T2out2 (CCAAGTTAAACAATTTAAAGGCAATGCTACC) was ligated to create preSBT(–2), replacing internal cloning sequences of pT2/BH with a single MscI site. PreSBT(–1) was made by moving the BamHI fragment containing the pT2 inverted repeat/direct repeat (IR/DR) sequences from preSBT(–2) into the BglII site of preSBT(–3). PSBT/RV was then made by opening preSBT(-1) with an MscI digest and cloning in adapter 4 (TCTCCCTATAGTGA GTCGTATTAGATATCAATTAACCCTCACTAAAGGGAGA) containing outward-facing T7 primer binding site, an EcoRV site, and an outward-facing T3 primer binding site. pSBT-SE was made by opening pSBT/RV with an EcoRV digest and cloning in adapter 5 (CCCGGGATTACTAGTTTAAACCTAGGATGCATACCGGTCAAGCTAGCGAT) to add a multicloning site with a SmaI, SpeI, PmeI, AvrII, NsiI, AgeI, NheI, EcoRV.
We constructed a shortened CAG promoter20 we call “mini-Caggs.” The mini-Caggs promoter was made by digesting pCAGGS20 with XbaI and ApaI, followed by Klenow treatment to blunt both sites and ligation. This removed 760 bp from the CAG intron, creating a mini-intron of only 248 bp that was functional in regulating hFVIII expression in vitro and in vivo. A SpeI to PstI fragment of pminiCAGGS that contained the cytomegalovirus (CMV) enhancer, beta-actin promoter, noncoding exon, mini-intron, and rabbit beta-globin 3′ untranslated region (UTR) and poly(A) signal were cloned into pSBT/SE from AvrII to NsiI to create pSBT/mCAG.
Modifications to the translational initiation context were made to the human factor VIII B-domain–deleted (BDD) cDNA21 by amplifying an N-terminal portion with KJC-FVIII*-F1 (TTCCTCGAGCCACCATGGCAATAGAGCTCTCCA) and KJC-FVIII*-R1 (GAGAGAAGCTCCTAGGTTCAATGGCA). The resulting N-terminal FVIII fragment was digested with XhoI and AvrII and cloned back into pT2/CHSQ that was also cut with XhoI and AvrII; the resulting vector pT2-CAG/F8 contained an optimal Kozak initiation sequence and resulted in a change of the second amino acid in the signal peptide fromaQtoanA. pT2-CAG/F8 was then cut with XhoI and BglII to move the F8 coding region into a smaller vector, pSBT-mCAG, which had been digested with BglII and XhoI, thus producing pSBT-mCAG/F8.
pT2/CHSQ was generated by ligating a human factor VIII BDD cDNA21 into pT2/CAGGS22 as an EcoRV, XhoI fragment. pCAGGS luciferase was made by cloning an EcoRI luciferase fragment into pCAGGS.20 pGFP was made by ligating a CMV promoter-GFP22 fragment into pCAGGS luciferase as a SnaBI, MscI fragment, replacing the chicken beta actin promoter and synthetic intron in CAGGS.20 pUB-SB10, containing a ubiquitin C-regulated SB10 sequence, was a kind gift from Paul Score (University of Minnesota).
HeLa cells were grown in Dulbecco modified Eagle medium media supplemented with 1 × nonessential amino acids (NEAA), Pen/Strep, and 10% fetal bovine serum at 5% CO2 in a humidified tissue culture incubator. For transfection studies 1 × 105 HeLa cells were seeded in 6-cm dishes and transfected with 1 μg pSBT/mCAGF8 with either 1 μg pUB-SB10 or pGFP using mirus LT1 transfection reagent (Mirus, Madison, WI). One milliliter media from transfected wells was collected every week, and hFVIII levels were measured by ELISA (enzyme-linked immunosorbent assay). The transfected cells were passaged 1:10 starting 1 week after transfection and every week thereafter.
Animal tolerization and plasmid injection
C57BL/6 hemophilia A (FVIII KO) mice were kindly provided by Dr Bianca Conti-Fine (University of Minnesota) and have been previously described.23 These mice express negligible FVIII (< 1% normal). Mice were maintained in a specific pathogen-free (SPF) facility according to the guidelines of the University of Minnesota Animal Care and Use Committee (IACUC). For tolerization studies, newborn pups were injected with 0.1 U g–1 recombinant hFVIII diluted in 100 μL 1 × phosphate-buffered saline within 24 hours of birth into the facial temporal vein with a 30-gauge needle. We used ReFacto, reconstituted according to the manufacturer's instructions, as a source of recombinant BDD hFVIII (Genetics Institute, Cambridge, MA). High pressure tail-vein injections24 were administered by injecting mice with the indicated amounts of plasmid (time = 6-9 seconds) diluted in sterile Ringer's solution to achieve a 1:10 weight-to-volume ratio (ie, 2 mL for a 20-g mouse).
Blood collection, FVIII analysis, and anti-hFVIII antibodies
For ELISA and COATEST assays blood was collected by retro-orbital puncture with 50-μL capillary tubes (Ram Scientific, Needham, MA) and immediately transferred to microcentrifuge tubes to a final concentration of 0.38% (vol/vol) sodium citrate. Two or three 50-μL capillary tubes were used for one blood collection to obtain 100 μL to 150 μL blood. Normal human pooled plasma (George King Biomedical, Overland Park, KS) was used to generate a standard curve for the FVIII ELISA (#F8C-EIA; Enzyme Research Laboratories, West Lafayette, IN) and COATEST (#K821918; Diapharma, West Chester, OH), assays which were performed according to the manufacturers' instructions and in either case were capable of detecting hFVIII as low as 1% of normal human levels.
A modified Bethesda assay was conducted to detect inhibitory anti-hFVIII antibodies in vitro. Equal volumes of pooled normal human plasma were mixed with plasma from vector-treated mice (50 μL:50 μL) and incubated for 2 hours at 37°C. After the 2 hours of incubation the plasma was analyzed by COATEST for residual FVIII activity as detailed in the previous paragraph. Bethesda units were determined by dilution of plasma, whereby each unit corresponded to a 50% reduction in expected residual hFVIII activity. Untreated hemophilia A mouse blood was used to dilute plasma from vector-treated mice; typically 25 μL of untreated hemophilic plasma was mixed with 25 μL of vector-treated plasma (50:50 dilution). Thus, the final dilution factor was 4, as plasma from vector-treated mice was diluted 50:50 with untreated hemophilic plasma, followed by addition of this 50 μL plasma to 50 μL of pooled normal human plasma. Untreated hemophilia A mouse blood always was used as a negative control for inhibitors.
Activated clotting time (ACT) of whole blood was measured using a Hemochron Jr Microcoagulation apparatus with the activated clotting time, low range (ACT-LR) cuvette (International, Technidyne, Edison, NJ). Similar application of this test to human hemophilia has been described.25 hFVIII level was quantified by generating a standard curve using hemophilia A mouse blood with known concentrations of added recombinant hFVIII (ReFacto). To generate the standard curve, ReFacto was reconstituted to a working concentration of 0.06 U/μL and was added to hemophilia A mouse blood in a dilution series (ie, 1 μL stock to 59 μL blood = 1 U/mL or 100% normal hFVIII level). Sixty microliters of blood was assayed, typically 3 to 4 replicates for each point. For blood collection, mice were sublethally anesthetized with avertin. Blood was collected directly from the heart with a syringe (27-gauge needle) and transferred to microcentrifuge tubes to a final concentration of 0.38% (vol/vol) sodium citrate; all samples were recalcified by addition of 0.4 M CaCl2 immediately prior to assaying in the ACT-LR cuvette. For blood from vector-treated mice, samples were incubated with or without sheep polyclonal anti-hFVIII antibody (Affinity Biologicals, ON, Canada) for 1 hour at room temperature prior to recalcification and assaying 60 μL of blood with the ACT-LR cuvette (anti-hFVIII antibody was added to a final concentration of 20 μg/mL).
FVIII transposon insertion cloning and mapping to the genome
FVIII transposons were cloned from the mouse genome using splinkerette PCR as described previously,26 with the only modification being BamHI digestion of the gel-purified primary PCR products. In brief, genomic DNA is digested with NlaIII and double-stranded oligos (splinkerettes) are ligated onto the restriction-endonuclease-treated genomic fragments. The splinkerettes serve as a primer binding site of known sequence, and the transposon IR serves as the other primer binding site. After primary PCR, the gel-purified primary PCR products were digested with BamHI (BamHI cuts outside of the IRs in pSBT/mCAGF8, thereby selecting against recovery of nonintegrated transposon clones). Secondary PCR was then performed on BamHI-treated primary PCR products as described.26 Secondary PCR products were gel-purified, sequenced, and analyzed.
Recovered flanking sequences (sequence outside of the transposon IR) were blasted against the mouse genome using the ENSEMBL database. Using ENSEMBL, insertions were mapped and annotated to the mouse genome. Transposons that integrated into known or predicted transcription units were determined to be within genes. Transposons that integrated outside of transcribed regions were not considered as insertions within genes. For insertions that were not within a gene, the nearest known or predicted transcription unit was considered the nearest gene.
Results
SB-mediated sustained FVIII expression in vitro
We cloned a BDD human (h)FVIII cDNA into a SB transposon (pSBT/F8) under the control of a chimeric CMV enhancer/chicken beta actin promoter with a mini-intron (mini-Caggs, Figure 1).
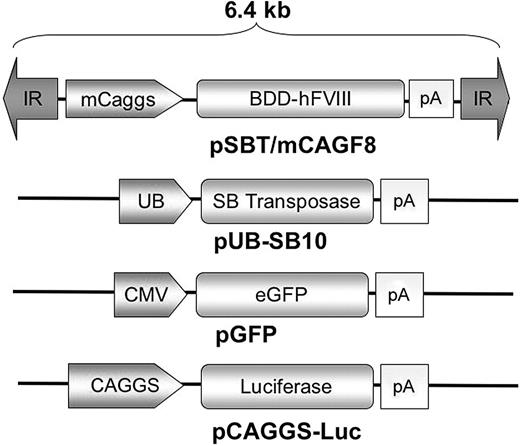
Plasmid vectors. pSBT/mCAGF8 contains a cytomegalovirus (CMV) enhancer/beta-actin chimeric promoter with a mini-intron (mini-Caggs; see Materials and methods) regulating expression of a BDD hFVIII cDNA followed by a rabbit beta-globin polyadenylation signal. The inverted repeat (IR) sequences serve as transposase binding sites and thus form the termini of the integrating unit, which is roughly 6.4 kb in length. pUB-SB10 contains a first generation “SB-10” transposase sequence regulated by a ubiquitin C promoter followed by an SV40 polyadenylation signal. pGFP is regulated by a CMV promoter, and pCAGGS-LUC is regulated by a full-length CAG promoter, each containing a rabbit beta-globin polyadenylation signal.
Plasmid vectors. pSBT/mCAGF8 contains a cytomegalovirus (CMV) enhancer/beta-actin chimeric promoter with a mini-intron (mini-Caggs; see Materials and methods) regulating expression of a BDD hFVIII cDNA followed by a rabbit beta-globin polyadenylation signal. The inverted repeat (IR) sequences serve as transposase binding sites and thus form the termini of the integrating unit, which is roughly 6.4 kb in length. pUB-SB10 contains a first generation “SB-10” transposase sequence regulated by a ubiquitin C promoter followed by an SV40 polyadenylation signal. pGFP is regulated by a CMV promoter, and pCAGGS-LUC is regulated by a full-length CAG promoter, each containing a rabbit beta-globin polyadenylation signal.
To assess the ability of the hFVIII transposon to mediate long-term gene transfer in mitotic cells, we transfected 1 μg of pSBT/F8 with 1 μg of an ubiquitin-regulated SB transposase-expressing plasmid (pUB-SB10) or with a GFP-plasmid (control for random integration) into HeLa cells. Culture supernatant was collected at 24 hours and every week thereafter, and the cells were passaged 1:10 starting 1 week after transfection, continuing for 4 weeks. Secreted hFVIII level in the culture supernatant was monitored by an hFVIII ELISA. We observed no difference in secreted hFVIII levels between the ± SB groups at 24 hours after transfection. However, by 2 weeks after transfection we could no longer detect hFVIII in supernatant of cells transfected without transposase (GFP control; Figure 2). In contrast, when SB transposase was co-transfected with the hFVIII transposon, hFVIII expression remained at roughly 4 ng/mL for 1 month (Figure 2). This indicates that stable gene transfer occurred in cells co-transfected with pUB-SB10 and an hFVIII transposon, resulting in a modest but sustained hFVIII secretion level of 4 ng/mL. This experiment demonstrates that SB boosted the level of long-term hFVIII expression in cultured cells under nonselective conditions.
SB mediates long-lasting hFVIII expression in vitro. 1 × 105 HeLa cells were seeded in 6-cm dishes and transfected with 1 μg pSBT/mCAGF8 with either 1 μg of pUB-SB10 (▦) or pGFP(▴; as a control for random integration). One milliliter of media was collected before transfection, 24 hours after transfection, and every week thereafter; hFVIII levels were measured by ELISA. The transfected cells were subcultured 1:10 starting 1 week after transfection and every week thereafter. Without SB transposase, hFVIII levels in the media were undetectable 2 weeks after transfection, thus showing an SB-dependent positive effect on hFVIII gene expression. (n = 3/group; error + SD).
SB mediates long-lasting hFVIII expression in vitro. 1 × 105 HeLa cells were seeded in 6-cm dishes and transfected with 1 μg pSBT/mCAGF8 with either 1 μg of pUB-SB10 (▦) or pGFP(▴; as a control for random integration). One milliliter of media was collected before transfection, 24 hours after transfection, and every week thereafter; hFVIII levels were measured by ELISA. The transfected cells were subcultured 1:10 starting 1 week after transfection and every week thereafter. Without SB transposase, hFVIII levels in the media were undetectable 2 weeks after transfection, thus showing an SB-dependent positive effect on hFVIII gene expression. (n = 3/group; error + SD).
Without immunotolerization circulating FVIII is rapidly extinguished in vivo
Hemophilia A mice23 were given a high-pressure tail-vein injection24 of 200 μg pSBT/mCAGF8 with 80 μg of pUB-SB10 or a luciferase plasmid (control). hFVIII expression in plasma was assayed by COATEST or ELISA in separate cohorts (n = 3-4/group; Figure 3). hFVIII expression was maximal at 24 hours and rapidly declined to undetectable levels within 3 weeks. There was no significant difference in measured hFVIII expression with or without pUB-SB10 co-injection.
Immune response to hFVIII in vector-treated hemophilia A mice without immunotolerization. (A) Hemophilia A mice were given a high-pressure tail-vein injection of 200 μg pSBTF8 with 80 μg of UB-SB10 (▦) or a luciferase plasmid (▴;n = 4/group), and plasma was subsequently assayed for hFVIII by ELISA. (B) Same as in A, except n = 3/group, and hFVIII activity in plasma was assayed by COATEST. (C) Equal volume of pooled normal human plasma was mixed with plasma from vector-treated mice (in panel B) and incubated for 2 hours at 37°C. Bethesda units were determined by dilution of plasma, whereby each unit corresponded to a 50% reduction in hFVIII activity measured by COATEST. Circulating hFVIII activity declined as anti-hFVIII antibody level increased, thus showing a humoral immune response to hFVIII. (A-C, error + SD).
Immune response to hFVIII in vector-treated hemophilia A mice without immunotolerization. (A) Hemophilia A mice were given a high-pressure tail-vein injection of 200 μg pSBTF8 with 80 μg of UB-SB10 (▦) or a luciferase plasmid (▴;n = 4/group), and plasma was subsequently assayed for hFVIII by ELISA. (B) Same as in A, except n = 3/group, and hFVIII activity in plasma was assayed by COATEST. (C) Equal volume of pooled normal human plasma was mixed with plasma from vector-treated mice (in panel B) and incubated for 2 hours at 37°C. Bethesda units were determined by dilution of plasma, whereby each unit corresponded to a 50% reduction in hFVIII activity measured by COATEST. Circulating hFVIII activity declined as anti-hFVIII antibody level increased, thus showing a humoral immune response to hFVIII. (A-C, error + SD).
A modified Bethesda inhibitor assay was conducted to determine if anti-hFVIII antibodies were responsible for accelerating clearance of hFVIII from the circulation. As expected, inhibitors could be detected by 2 weeks after injection, and by 3 weeks the anti-hFVIII antibody titer increased further to approximately 5 Bethesda units (Figure 3C). This level of inhibitor appears relatively low compared to that reported by Ye et al, where high-pressure tail-vein injection was used,14 but similar to that level reported by Kootstra et al using lentiviral vectors.13 Many factors, including the vector, promoter, mouse strain, and differences in the Bethesda assay, are likely to cause discrepancy in anti-FVIII antibody levels reported in gene therapy studies. Nevertheless, consistent with previous studies,9,13-15 we were unable to achieve sustained hFVIII expression in hemophilia A mice due to an immune response. Repeated injections of pSBT/mCAGF8 plasmids (± co-injection of SB-encoding plasmids) were carried out in an attempt to induce immune tolerance. However, hFVIII expression was consistently undetectable 1 week after the second injection, suggesting an adaptive immune response accelerated clearance of hFVIII from circulation after the second injection (data not shown).
Immunotolerization and SB-mediated gene transfer achieve long-term FVIII expression in vivo
Roughly 30% of patients with severe hemophilia A will develop anti-FVIII inhibitory antibodies when treated with recombinant FVIII infusions to achieve hemostasis.12 Inhibitors complicate treatment of bleeding episodes by rapidly inactivating infused FVIII, but in many cases this problem can be overcome by induction of immune tolerance via daily infusions of high-dose FVIII.12,27 Immune tolerance induction can be achieved in hemophilia A mice by injecting neonates with recombinant hFVIII within 24 hours of birth,16,17 when the immune system is very susceptible to immune tolerance induction.16,17,28-30 Therefore, we tolerized newborn (1-day-old) hemophilia A mice by injection of 0.1 U g–1 recombinant hFVIII into the facial temporal vein to evaluate the efficacy of gene delivery. This delivery technique for achieving tolerization has been documented to block both B- and T-cell–mediated immunity against hFVIII challenge.17
Tolerized mice (8-12 weeks old) were given 2 injections of 200 μg pSBT/mCAGF8 with 80 μg of pUB-SB10 or a luciferase plasmid (control for random integration) spaced 4 weeks between the first and second injection. Two mice that lost detectable hFVIII expression due to inhibitor formation (assayed by the Bethesda method, Figure 4C) after the first plasmid injection were presumably not tolerized and were dropped from the study (leaving n = 3/group in Figure 4A). Madoiwa et al observed that 1 of 9 mice injected with 0.1 U g–1 recombinant hFVIII developed high titer inhibitors when challenged with hFVIII as adults17 ; we observed a similar frequency using plasmid DNA to challenge adults that had been injected with 0.1 U g–1 recombinant hFVIII as neonates (2 of 11 formed inhibitors, Figure 4C).
Long-term hFVIII expression in SB-treated hemophilia A mice that were tolerized as neonates. (A) Neonatal hemophilia A mice were tolerized within 24 hours of birth by a facial vein injection of 0.1 U g–1 recombinant hFVIII. Pups were aged for 8 to 12 weeks and given 2 high-pressure tail-vein injections of 200 μg pSBT/F8 with 80 μg UB-SB10 (▦) or a luciferase plasmid (▴) (initial n = 4/group; 2 mice formed inhibitors and were dropped, leaving n = 3/group). Arrow shows time of each injection. Plasma was subsequently assayed for hFVIII activity by COATEST. Without SB transposase, hFVIII rapidly declined, whereas animals co-administered an SB-encoding plasmid exhibited significantly higher hFVIII activity (*P ≤ .05 by Student t test; error ± SD). (B) Same as in panel A, except all mice (n = 3) received transposase (80 μg pUB-SB10) and were assayed for 180 days for hFVIII activity by COATEST. (C) Bethesda assays on mice from Figure 4A-B. Anti-hFVIII inhibitory antibodies were detected in 2 of 11 mice injected with 0.1 U g–1 recombinant hFVIII in the facial vein within 24 hours of birth. Inhibitors were assayed for 3 weeks after the first plasmid injection and in both cases (2 of 11) coincided with undetectable hFVIII activity by COATEST (not shown). Thus, 2 of 11 mice were presumably not tolerized to hFVIII (one with and one without pUB-SB10 co-injection, both from Figure 4A). The majority of plasmid-treated mice (9 of 11) were tolerized, as they did not form detectable inhibitors to hFVIII and had sustained hFVIII activity assayed by COATEST (panels A and B).
Long-term hFVIII expression in SB-treated hemophilia A mice that were tolerized as neonates. (A) Neonatal hemophilia A mice were tolerized within 24 hours of birth by a facial vein injection of 0.1 U g–1 recombinant hFVIII. Pups were aged for 8 to 12 weeks and given 2 high-pressure tail-vein injections of 200 μg pSBT/F8 with 80 μg UB-SB10 (▦) or a luciferase plasmid (▴) (initial n = 4/group; 2 mice formed inhibitors and were dropped, leaving n = 3/group). Arrow shows time of each injection. Plasma was subsequently assayed for hFVIII activity by COATEST. Without SB transposase, hFVIII rapidly declined, whereas animals co-administered an SB-encoding plasmid exhibited significantly higher hFVIII activity (*P ≤ .05 by Student t test; error ± SD). (B) Same as in panel A, except all mice (n = 3) received transposase (80 μg pUB-SB10) and were assayed for 180 days for hFVIII activity by COATEST. (C) Bethesda assays on mice from Figure 4A-B. Anti-hFVIII inhibitory antibodies were detected in 2 of 11 mice injected with 0.1 U g–1 recombinant hFVIII in the facial vein within 24 hours of birth. Inhibitors were assayed for 3 weeks after the first plasmid injection and in both cases (2 of 11) coincided with undetectable hFVIII activity by COATEST (not shown). Thus, 2 of 11 mice were presumably not tolerized to hFVIII (one with and one without pUB-SB10 co-injection, both from Figure 4A). The majority of plasmid-treated mice (9 of 11) were tolerized, as they did not form detectable inhibitors to hFVIII and had sustained hFVIII activity assayed by COATEST (panels A and B).
Tolerized mice that received hFVIII transposon without transposase (luciferase-injected) exhibited similar hFVIII activity at 24 hours after injection compared to mice that received transposase-encoding plasmid to facilitate integration (Figure 4A). However, 7 to 28 days after the first plasmid injection a noticeable difference in hFVIII activity between animals that received SB-encoding plasmid and those that did not is apparent, and this trend was more pronounced after a second plasmid injection.
Eighty-four days after the first plasmid injection SB-treated mice averaged 16% normal hFVIII activity, whereas mice that received luciferase (random integration control) averaged less than 1% hFVIII activity (P = .005, Figure 4A). These data demonstrate that immunotolerization allowed measurement of the efficiency of hFVIII gene transfer and expression by SB-mediated transposition in hemophilia A mice. Furthermore, co-administration of the SB-encoding plasmid (pUB-SB10) clearly enhanced the level of stable hFVIII gene expression. In the absence of SB, sustained hFVIII activity of more than 1% was not observed (Figure 4A).
To provide molecular evidence of transposase-mediated integration of the hFVIII transposon, we performed splinkerette PCR26 on liver genomic DNA to clone transposon insertions. The background of episomal plasmids recovered from using this approach was high but was reduced when an additional BamHI restriction digest was performed prior to PCR. BamHI cuts outside of the IRs in pSBT/mCAGF8, thereby selecting against recovery of nonintegrated transposon clones. Using this approach we were able to clone 9 insertions of the hFVIII transposon into TA dinucleotides (Table 1). Of 9 insertions of the hFVIII transposon, 3 were within genes (transcribed regions), in agreement with previous studies that indicated that SB does not prefer to integrate into genes.18,22,26 Of 9 insertions, 6 did not map within a transcription unit, with the average distance from the nearest gene being 177 kb. These data provide molecular evidence that SB-mediated transposition occurred in mice that were co-administered an SB-encoding plasmid (pUB-SB10).
Molecular evidence of transposition: genomic annotations of 9 insertions of the FVIII transposon
Transposon IR: Target TA → Flanking sequence . | Map position . | Within gene? . | Integration annotation . |
|---|---|---|---|
| GACTATTTGACGTTCACATTGACCTTTTTAGTTTTCCATG | 6-B1 | Yes | Intron of Sec811 |
| GACTAATCTGCCGCCAAACGCAGACACCATTGCATACACT | 3-E1 | No | 176 kb from Rap2b |
| GACTACCAAAGTTGGGCAGGTCACTGGGCTGCCTTTCCTT | 3-H1 | No | 424 kb from Ttc5 |
| GACTACCAAACCTAGTCTATAGTCAACCACACAGAATTTA | 16-B2 | Yes | Intron of Il1rap |
| GACTACCTAACCCAGCACCCCACTCCCCTAGTCCCGACGG | 4-D2 | No | 1.5 kb from NM144941 |
| GACTATGTACCCGACTTGGATCCAGAGGGTGTATATGTAT | X-A5 | No | 248 kb from novel gene |
| GACTACTGGGAATGATGGACAGTCCCACACTCAGGATCAC | 13-D1 | No | 56 kb from Foxd1 |
| GACTATTTAGAAAATGTAGGCATTTACAAGAATATCCAAA | 7-B5 | No | 156kb from 008905 |
| GACTATATATTTAGAGAGCTTTAGAAAGTTACTGAAGCTT | 1-H1 | Yes | Intron of NM013862 |
Transposon IR: Target TA → Flanking sequence . | Map position . | Within gene? . | Integration annotation . |
|---|---|---|---|
| GACTATTTGACGTTCACATTGACCTTTTTAGTTTTCCATG | 6-B1 | Yes | Intron of Sec811 |
| GACTAATCTGCCGCCAAACGCAGACACCATTGCATACACT | 3-E1 | No | 176 kb from Rap2b |
| GACTACCAAAGTTGGGCAGGTCACTGGGCTGCCTTTCCTT | 3-H1 | No | 424 kb from Ttc5 |
| GACTACCAAACCTAGTCTATAGTCAACCACACAGAATTTA | 16-B2 | Yes | Intron of Il1rap |
| GACTACCTAACCCAGCACCCCACTCCCCTAGTCCCGACGG | 4-D2 | No | 1.5 kb from NM144941 |
| GACTATGTACCCGACTTGGATCCAGAGGGTGTATATGTAT | X-A5 | No | 248 kb from novel gene |
| GACTACTGGGAATGATGGACAGTCCCACACTCAGGATCAC | 13-D1 | No | 56 kb from Foxd1 |
| GACTATTTAGAAAATGTAGGCATTTACAAGAATATCCAAA | 7-B5 | No | 156kb from 008905 |
| GACTATATATTTAGAGAGCTTTAGAAAGTTACTGAAGCTT | 1-H1 | Yes | Intron of NM013862 |
Nine insertions of the hFVIII transposon were cloned by linker-mediated PCR. Liver genomic DNA from the animals in Figure 4B was used as template for splinkerette PCR reactions. Recovered flanking sequences were used to search the ENSMBL database to determine their map positions in the mouse genome (as described in “Materials and methods”). Of 9 transposons cloned from the genome, 3 were in genes (predicted transcribed regions), and all 3 were in introns.
A separate cohort of tolerized animals (8-12 weeks old, n = 3) were administered 2 injections of 200 μg pSBT/F8 with 80 μg of pUB-SB10 spaced 4 weeks between the first and second injection (Figure 4B). Circulating hFVIII activity was assayed for 180 days (6 months) by COATEST. The variability of hFVIII activity was high in this cohort, therefore the data are plotted for individual mice. Inhibitors were not detected, indicating that the humoral immune response was unlikely to be causing the variability (Figure 4C). The first of 2 plasmid injections were less effective than we usually achieve in 2 of 3 mice in this cohort, resulting in peak transgene expression of less than 10% normal hFVIII activity that may have contributed to the variability (Figure 4B). Nevertheless, we observed sustained hFVIII expression in all 3 mice for 6 months after the first injection. hFVIII activities ranged from 8% to 160% of normal levels as assayed by COATEST (day 180) and 10% to 100% (day 200) as assayed by a ACT-LR whole-blood clotting assay (Figure 4B, Table 2). Thus, SB-mediated gene transfer facilitates long-term, high-level hFVIII expression in hemophilia A mice if the humoral immune response is prevented.
ACT-LR whole-blood clotting assay demonstrates phenotypic correction of murine hemophilia A
. | Clotting time, seconds . | . | Estimated FVIII, % normal human . | . | . | ||
|---|---|---|---|---|---|---|---|
| Mouse treatment group . | -hFVIII Ab . | +hFVIII Ab . | -hFVIII Ab . | +hFVIII Ab . | Phenotype, by clotting time . | ||
| Wild-type C57BL/6J | 120-140 | NA | at least 100% | NA | Normal | ||
| FVIII Knockout C57BL/6J-FVIII-/- | more than 400 | more than 400 | less than 0.5% | less than 0.5% | Severe hemophilia | ||
| FVIII Knockout C57BL/6J-FVIII-/- FVIII transposon/SB (▪, Figure 4B) | 187 (± 14) | 248 (± 10) | 10% | 2% | Mild hemophilia | ||
| FVIII Knockout C57BL/6J-FVIII-/- FVIII transposon/SB (▴, Figure 4B) | 186 (± 8) | 350 (± 30) | 10% | less than 0.5% | Mild hemophilia | ||
| FVIII Knockout C57BL/6J-FVIII-/- FVIII transposon/SB (♦, Figure 4B) | 138 (± 10) | 400 (± 12) | 100% | 0.0% | Normal | ||
. | Clotting time, seconds . | . | Estimated FVIII, % normal human . | . | . | ||
|---|---|---|---|---|---|---|---|
| Mouse treatment group . | -hFVIII Ab . | +hFVIII Ab . | -hFVIII Ab . | +hFVIII Ab . | Phenotype, by clotting time . | ||
| Wild-type C57BL/6J | 120-140 | NA | at least 100% | NA | Normal | ||
| FVIII Knockout C57BL/6J-FVIII-/- | more than 400 | more than 400 | less than 0.5% | less than 0.5% | Severe hemophilia | ||
| FVIII Knockout C57BL/6J-FVIII-/- FVIII transposon/SB (▪, Figure 4B) | 187 (± 14) | 248 (± 10) | 10% | 2% | Mild hemophilia | ||
| FVIII Knockout C57BL/6J-FVIII-/- FVIII transposon/SB (▴, Figure 4B) | 186 (± 8) | 350 (± 30) | 10% | less than 0.5% | Mild hemophilia | ||
| FVIII Knockout C57BL/6J-FVIII-/- FVIII transposon/SB (♦, Figure 4B) | 138 (± 10) | 400 (± 12) | 100% | 0.0% | Normal | ||
NA indicates not attempted. Mice (Figure 4B) were sublethally anesthetized with avertin. Blood was collected directly from the heart with a needle and syringe and incubated with or without polyclonal anti-hFVIII antibody for 1 hour at room temperature. Clotting activity in whole blood was then assayed using the ACT-LR method. Anti-hFVIII antibody ablated all (1 of 3) or most (2 of 3) of clotting activity, revealing an hFVIII-dependent effect on clotting time and ruling out significant tissue factor contamination. All mice displayed improved clotting times, indicating partial (2 of 3) or complete (1 of 3) phenotypic correction of murine hemophilia A.
Correction of clotting function in murine hemophilia A by SB-mediated gene transfer
To assess clotting function, we used a modified whole blood clotting assay (ACT-LR) that was shown to be accurate and sensitive to low levels of FVIII or FIX in whole blood in a recent study.25 We have found that the ACT-LR is particularly useful for mouse blood, because it can accurately quantify low-level hFVIII activity (0.5%-5% normal) and is less prone to yield false positives relative to other clotting assays. A standard curve was generated by adding increasing amounts of recombinant hFVIII protein to hemophilia A mice mouse blood to reconstitute clotting function (0.5%-100% normal hFVIII levels; Figure 5). To determine if clotting function had been restored in vector-treated mice, blood was collected by intracardiac withdrawal from the 3 mice discussed in Figure 4B 200 days after the first plasmid injection. Intracardial puncture was conducted to minimize tissue factor contamination, which could yield false positive results in global clotting assays. As a control for hFVIII-specific clotting activity, we incubated whole blood with or without a polyclonal anti-hFVIII antibody prior to conducting the ACT-LR whole-blood clotting assay. All 3 mice in this cohort showed an improvement in clotting function, with clotting times indicating a range of 10% to 100% hFVIII activity without hFVIII antibody and less than 1% to 2% following incubation with anti-hFVIII antibody (Table 2). Severe hemophilia A is defined as less than 1% FVIII activity, moderate as 1% to 5%, and mild as 5% to 30% (reviewed in Kay and High1 ). Therefore, even after correcting for potential tissue factor contamination, the phenotype of 2 of 3 mice was partially corrected (10% normal; mild hemophilia) and one mouse (1 of 3) had completely normal clotting times (100% normal; Table 1). In sum, these results indicate that SB-mediated gene transfer is a nonviral means to correct the phenotype of murine hemophilia A when the immune response to hFVIII is prevented. Moreover, the effect of gene transfer is durable as evidenced by clotting assays performed 200 days after the first plasmid injection (171 days after the second injection).
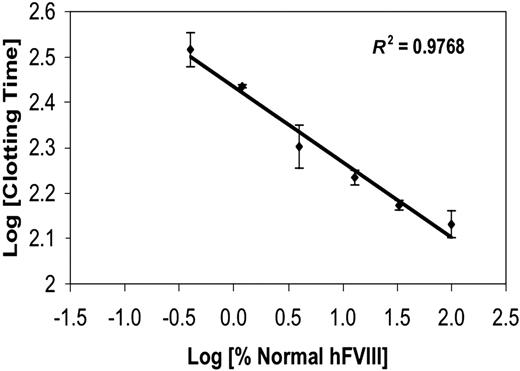
ACT-LR whole-blood clotting assay quantifies hFVIII levels by clotting time in hemophilia A mouse blood. A standard curve was generated by collecting hemophilia A mouse blood and adding recombinant hFVIII at 6 different known concentrations: 0.5%, 1.2%, 3.3%, 13.3%, 33.3%, and 100% normal human levels. A clean blood sample (free of significant tissue factor) was used to generate the standard curve; sample integrity was verified by blood that would not clot for more than 400 seconds (assay upper limit) without addition of hFVIII. The log of the clotting time (seconds) has a linear relationship with the amount of hFVIII in the blood (R2 = 0.97; error ± SD).
ACT-LR whole-blood clotting assay quantifies hFVIII levels by clotting time in hemophilia A mouse blood. A standard curve was generated by collecting hemophilia A mouse blood and adding recombinant hFVIII at 6 different known concentrations: 0.5%, 1.2%, 3.3%, 13.3%, 33.3%, and 100% normal human levels. A clean blood sample (free of significant tissue factor) was used to generate the standard curve; sample integrity was verified by blood that would not clot for more than 400 seconds (assay upper limit) without addition of hFVIII. The log of the clotting time (seconds) has a linear relationship with the amount of hFVIII in the blood (R2 = 0.97; error ± SD).
Discussion
In this study, we evaluated the SB transposon system as a means of achieving long-term hFVIII expression and improvement of clotting dysfunction in a mouse model of hemophilia A. In vitro experiments indicated that SB was capable of boosting the level of hFVIII expressed in transfected cells. As previously observed in immunocompetent animals,9,14 circulating hFVIII activity was rapidly extinguished after tail-vein injection of plasmids containing the hFVIII gene. However, circulating hFVIII activity was sustained in animals that had been tolerized against hFVIII by intravenous injection of recombinant hFVIII protein at birth (82% of mice were tolerized; 18% formed inhibitors). The sustained expression of hFVIII in these animals led to reduced clotting times, thus demonstrating for the first time the usefulness of the SB transposon system in mediating long-term correction of clotting function in this animal model of hemophilia A.
Unlike adeno-associated virus, SB does not have strict limitations on maximal transgene size. In vitro studies have shown maximal transposition efficiency is achieved with smaller (< 3 kb) transposons and drops to less efficient levels as transposon size increases, yet still retains 50% of maximal efficiency with a 6-kb transposon and can integrate a transposon near 11 kb in size.31
However, these studies were conducted using selective conditions. Here we used hFVIII for an in vivo study to address whether SB could integrate a large (6.4 kb) BDD-hFVIII transposon to produce high-level protein expression in a therapeutic setting. We did not use the recently described hyperactive SB transposase mutants32,33 in this study, so it is possible that the gene expression observed is an underestimate of what SB can achieve. These hyperactive mutants apparently increase the efficiency of transposition of large (> 10 kb) transposons.32 Moreover, an optimized combination of improved transposon vector with a hyperactive transposase apparently increases SB-mediated gene transfer by 7-fold in mouse liver.33 Likewise, cis vectors, where the SB expression cassette resides on the same plasmid as the transposon, can result in greater levels of long-term gene expression.34
We did not observe sustained hFVIII activity of more than 1% normal without co-injection of an SB-encoding plasmid (Figure 4A), whereas co-delivery of SB-encoding plasmid resulted in sustained hFVIII activity up to 160% of normal human levels assayed by COATEST (Figure 4B). Therefore, the majority of sustained hFVIII gene expression was likely transposition-dependent. This trend has been observed previously when an hFIX or human α-1—antitrypsin transposon was delivered by high-pressure tail-vein injection.11,34 For example, when the FIX transposon was injected then re-injected with and without active transposase, only mice that received transposase had high-level FIX expression long-term,11 similar to what we observed here with hFVIII transposons.
We repeated plasmid injections twice to determine the efficacy of repeated administration. In nontolerized animals, repeated injections resulted in complete loss of circulating hFVIII activity regardless of the dose of pT2/mCAGF8 (± SB-encoding plasmid) we administered (not shown). Circulating hFVIII activity was consistently undetectable by 3 weeks after the first injection and 1 week after the second injection, indicating that an adaptive immune response accelerated clearance of hFVIII from the plasma after the second injection. In contrast, when neonatally tolerized mice were given 2 injections of pT2/mCAGF8, plasma-hFVIII activity remained near 1% normal without co-injection of SB-encoding plasmid and ranged from 8% to 160% normal when pUB-SB10 was co-injected (Figure 4A-B). There are several possible reasons why the second injection of plasmids increased long-term hFVIII expression only when SB-encoding plasmid was co-administered.
High-pressure tail-vein injection has been shown to cause some liver damage35 and transiently stimulate mitosis in mouse liver.36 Perhaps hepatocytes already harboring hFVIII transposon insertions from the first injection were stimulated to divide by the second injection, thereby increasing the number of hepatocytes secreting hFVIII. Alternatively, the second co-injection of pT2/mCAGF8 with pUB-SB10 may have resulted in transposition of episomal or integrated hFVIII transposons already present after the first injection, resulting in increased hFVIII expression. Finally, the increase in hFVIII expression due to the second plasmid co-injection may just be an additive effect; whereby more SB-mediated integrations of FVIII transposon occurred and therefore the total hFVIII activity in the plasma increased. In any case, these results indicate that SB-mediated repeat administration is efficacious and tolerated in this animal model of hemophilia A.
Our results differ from those obtained by Miao and colleagues using similar delivery methods and naked plasmid DNA, which resulted in sustained FVIII expression in immunodeficient mice9 or in immune-suppressed mice14 without apparent transgene integration. There are several possible explanations for the difference between our study and these previously published studies. Miao et al9 and Ye et al14 used liver-specific regulatory sequences and an optimized plasmid for high-level gene expression,9,14 whereas we used a ubiquitous CAG promoter.13,20 Recent studies have shown that plasmid backbones replete with CpG dinucleotides are prone to silencing.37,38 Therefore, it is possible that transposition of the hFVIII cDNA away from the plasmid backbone into genomic DNA contributes to the benefit of SB we observed, in part, by inhibiting silencing. Another benefit of SB is certainly integration, so that mitotic cells retain the transgene upon cell division. It would be informative to study an optimized liver-specific hFVIII expression cassette9 within an SB transposon, combined with improvements in the plasmid backbone, to assess the benefit of integration and optimize plasmid-based methods for hemophilia gene therapy.
We have shown that neonatal tolerization is useful in a gene therapy setting, as the humoral immune response to a neoantigen can be prevented, thereby allowing long-term vector expression to be evaluated. This approach may be useful in developing gene therapy for a variety of recessive genetic disorders when immunecompetent animal models are used. In particular, gene therapy for hemophilia A has been difficult to study in mice using viral13,15,39 and nonviral9,14 vectors due to anti-FVIII antibody formation. Hemophilia gene therapy studies could benefit from this tolerizing technique, which is simple and requires only one neonatal injection to achieve tolerization.
However, we cannot exclude the possibility that noninhibitory anti-hFVIII antibodies were present at low levels in animals presumed to be tolerized, because the Bethesda assay we conducted detects only antibodies that inhibit hFVIII activity. Nonetheless, tolerization was absolutely required to allow long-term hFVIII activity in the plasma to be assessed and to demonstrate correction of clotting dysfunction. In addition, we cannot exclude the possibility of T-cell–mediated killing of transfected cells in tolerized animals in this study, but if this were occurring, one would expect hFVIII levels to have gradually declined. The method we used to achieve tolerization has been described previously and blocked both inhibitory antibody formation and proliferative T-cell response against hFVIII challenge.17 Furthermore, the immune response to hFVIII in hemophilia A mice has already been documented to be T-cell–dependent,15,40-42 as it can be blocked by anti-CD4 antibody15 or anti–CTLA4-Ig40 and does not occur in mice deficient in the T-cell costimulatory ligand B7-2.40 The immunogenicity of the SB transposase itself remains obscure and warrants investigation.
Although nonviral gene therapy for hemophilia holds much promise, safety studies addressing the risk of insertional mutagenesis (or other DNA rearrangements) with the integrating nonviral systems such as SB and the phage ΦC31 integrase are lacking and need to be conducted. Preliminary studies suggest that SB integrates at random18 and that the phage ΦC31 integrase may be fairly site-specific,10,43 whereas integrating viral vectors show a strong preference to integrate near/in genes44 or are associated with deletions/translocations upon integration.45,46 Since hemophilia is currently treatable by recombinant protein infusion, gene therapy approaches must be carefully considered, balancing the risks against the benefits. This reinforces the need for rigorous safety assessment of all vectors currently under development for gene therapy for hemophilia.
Considerable progress has been made in developing viral and nonviral approaches for the treatment of hemophilia by gene therapy. Within the past decade the first gene therapy trials for hemophilia have shown evidence of gene transfer and transient circulating clotting factor, however, sustained gene expression is still an elusive goal. Further study is needed to address how vector-induced inflammatory responses and inhibitor formation can be prevented and how all vectors can be made safer. SB-mediated integration of the FVIII gene could allow for stable gene transfer and long-term expression in patients with hemophilia A, while possibly avoiding/reducing inflammatory response. However, effective methods for delivering plasmid vectors that are clinically relevant are still lacking. Methods to deliver plasmid DNA in vivo are under development, including electroporation,47,48 ultrasound,49 chemical conjugates,22,50 and catheter-mediated high-pressure injection into the hepatic vein.51 Considering the rate at which progress in the areas of vector improvement and administration have been made, it is conceivable that effective gene therapy for hemophilia will be achieved in the foreseeable future.
Prepublished online as Blood First Edition Paper, December 2, 2004; DOI 10.1182/blood-2004-09-3496.
Supported by National Institutes of Health grants NIH/NHLBI 1R43 HL072593-01 and HL65578 (N.S.K.).
The Sleeping Beauty transposon system has been licensed to a biotechnology company called Discovery Genomics, Inc. located in Minneapolis, MN. D. A. L., R. S. M., and P. B. H. are co-founders of DGI and part owners of the company, as is the University of Minnesota. K. J. C. is an employee of DGI.
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank the Arnold and Mabel Beckman Foundation and all members of the Beckman Center for Transposon Research. We thank Dr Bianca Conti-Fine for providing hemophilia A mice and Dr Robert Hebbel for the hFVIII cDNA.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal