Comment on Walters et al, page 2952
An siRNA approach targeting the FLT3 receptor with internal tandem duplication may increase the efficacy of the FLT3-specific tyrosine kinase inhibitor MLN518 in acute myeloid leukemias.
In recent years it has become evident that tyrosine kinases are common and frequent targets for mutations in human leukemias. Activating mutations have been associated directly with the transforming phenotype and found to be an ideal target for drug development. In acute myeloid leukemia (AML), Fms-like tyrosine kinase 3 (FLT3) has been identified as a frequent target of mutations with about 30% of the patients carrying constitutively active forms of the receptor tyrosine kinase. The activation occurs mainly by either internal tandem duplications (ITDs) in the juxtamembrane domain or by mutations in the activation loop of FLT3 (see Banerji and Sattler1 for review). The efficacy of FLT3 tyrosine kinase inhibitors has been shown in clinical trials, but there is insufficient information about the long-term effects of these drugs. Initial clinical data conducted with the FLT3 inhibitor PKC412 suggest that in analogy with imatinib mesylate, remission with an FLT3 tyrosine kinase inhibitor may not be permanent.2 Indeed, in vitro data have shown multiple lines of evidence for altered or variant drug efficacy toward activated FLT3, including up-regulation of receptor expression and activation loop mutations in the kinase domain.3,4 It seems necessary to identify targets that enable us to either increase the sensitivity of existing drugs, develop newer drugs with higher specificity at lower concentrations, or find targets that would complement the existing treatment. A reasonable approach is to target not only the oncogenic tyrosine kinase, but also an additional pathway that is required for transformation. For example, inhibiting mammalian target of rapamycin (mTOR) with rapamycin and PKC412 treatment synergistically inhibited cells expressing PKC412-sensitive or -resistant transforming FLT3 mutants.5 FIG1
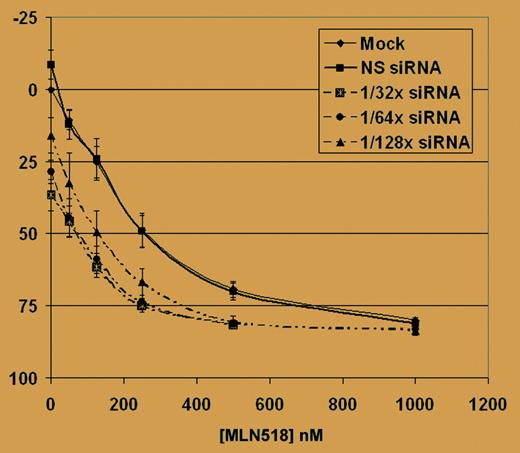
RNAi-induced down-regulation of FLT3 sensitizes Ba/F3 FLT3-ITD transfectants and Molm-14 cells to treatment with MLN518. See the complete figure in the article beginning on page 2952.
RNAi-induced down-regulation of FLT3 sensitizes Ba/F3 FLT3-ITD transfectants and Molm-14 cells to treatment with MLN518. See the complete figure in the article beginning on page 2952.
In this issue of Blood, Walters and colleagues report the feasibility of an siRNA approach targeting the FLT3 receptor with ITD mutation. FLT3-ITD inhibition with the FLT3 kinase inhibitor MLN518 was found to collaborate with targeted FLT3 siRNA. This approach increases the specificity of inhibiting FLT3 by targeting the tyrosine kinase at 2 levels. First, the degradation of FLT3-ITD mRNA, and thus the protein expression, is reduced by FLT3 siRNA. Second, the remaining enzyme activity is inhibited with MLN518. The surprising and interesting finding here is that FLT3 siRNA not only led to a decrease in proliferation, but also increased the efficacy of MLN518, reducing the 50% inhibitory concentration (IC50) by about 70% compared with treatment with the tyrosine kinase inhibitor alone. This method has great potential because RNAi is uniquely suitable to target the expression of a specific gene. The possibility for drug resistance based on genomic mutations in the FLT3 gene is low because FLT3 siRNA can be used as a pool targeting different regions in the mRNA of the receptor and still lead to degradation of the whole transcript. The next challenge will be to provide a suitable system to introduce FLT3 siRNA into leukemia cells. The RNAi technique relies on the introduction of double-stranded RNA molecules into the cell, usually by transfection. Double-stranded siRNA can be simulated by a vector-based shRNA approach. Lentivirus-based vectors may be suitable because they are able to infect target cells with high efficiency and are, in general, safe to use. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal