Abstract
Previous studies suggest that cells within the CD34+ hematopoietic stem cell compartment are endowed with immune regulatory activity. Furthermore, it is possible to expand the human regulatory cells upon short-term culture of purified CD34+ cells with an early-acting cytokine cocktail. We now show that addition of anti-CD28, anti-CD2, interleukin-2 (IL-2), anti–IL-10, or IL-12 to the bulk mixed lymphocyte reaction (MLR) cannot reverse the inhibitory activity of the CD34+ cells, ruling out anergy-based mechanisms or mechanisms involving Th1-Th2 skewing. Furthermore, phenotyping of cells present after addition of CD34+ cells to the bulk MLR ruled out potential induction of plasmacytoid dendritic precursors, known to be endowed with regulatory activity. In contrast, the inhibitory activity of CD34+ cells could be reversed by adding the caspase inhibitor BD-FMK to the bulk MLR, indicating a deletion-based mechanism. The deletion can be inhibited by anti–tumor necrosis factor-α (anti–TNF-α) and not by anti–transforming growth factor-β (anti–TGF-β), suggesting a potential role for TNF-α in the regulatory activity of CD34+ cells.
Introduction
Studies in mice and humans demonstrate that transplantation of hematopoietic progenitors in numbers larger than commonly used (“megadose” transplants) overcomes major genetic barriers (reviewed by Reisner and Martelli1,2 and Martelli et al3 ). Megadose haploidentical transplants, mismatched at 3 HLA loci, engraft rapidly and durably without induction of graft versus host disease in patients with acute leukemia.4-6 In vitro studies suggest that cells within the CD34+ cell fraction are endowed with immune regulatory “vetolike” activity that could facilitate this favorable out-come.7,8 Thus, when purified CD34+ cells were added to bulk mixed lymphocyte reactions (MLRs) they suppressed the development of cytotoxic T lymphocytes (CTLs) against CD34+ donor-origin stimulators but not against stimulators from a third party. Furthermore, short-term ex vivo expansion of CD34+ cells by early-acting cytokines resulting in myeloid differentiation also showed that early myeloid CD33+ cells exhibit similar specific regulatory activity, inhibiting the generation of host donor-reactive CTLs.8 This tolerizing activity was shown to depend on cell contact.8 Murine hematopoietic Sca-1+Lin- bone marrow progenitor cells also exhibit immune regulatory activity, and transplantation of megadose grafts surmounts resistance to engraftment posed by the numerous lymphocytes that survive sublethal conditioning.9-11 Consequently, an allogeneic chimera, generated by transplantation of large numbers of Sca-1+Lin- cells, permanently accepts allogeneic skin grafts derived from the donor of the hematopoietic progenitors.10
In the present study, we investigated potential mechanisms that could be mediating the specific inhibitory activity of early hematopoietic cells, and we demonstrate that donor-reactive cytotoxic T lymphocyte precursors (CTL-p) are likely deleted and not anergized by CD34+ cells. While Fas-FasL apoptosis is associated with the deletion of effectors by veto CD8+ T cells, our data indicate that the regulatory activity of CD34+ cells is likely mediated by tumor necrosis factor-α (TNF-α).
Materials and methods
Monoclonal antibodies and other reagents
Purified antihuman HLA-A, -B, and -C monoclonal antibody (mAb) (immunoglobulin G1 [IgG1], k mouse; clone G46-2.6), IgG1 mouse antihuman CD28 mAb, and IgG1 mouse antihuman CD2 were purchased from Pharmingen (San Diego, CA). Human recombinant interleukin-2 (IL-2) was obtained from EuroCetus (Amsterdam, The Netherlands). Antihuman IL-10 mAb (IgG2a rat) and IgG1 rat antihuman IL-4 mAb were purchased from Pharmingen. Recombinant human IL-10 and recombinant human IL-12 were purchased from R&D Systems (Minneapolis, MN). R-phycoerythrin (R-PE)–conjugated antihuman CD13 (mouse IgG2a) was purchased from Ancell (Bayport, MN). Fluorescein isothiocyanate (FITC)–conjugated antihuman CD34 (mouse IgG1) was purchased from Pharmingen. R-PE–conjugated antihuman granulocyte-macrophage colony-stimulating factor receptor α (GM-CSFRα) (CD116, mouse IgG2a) was purchased from Serotec (Oxford, England). FITC-conjugated antihuman IL-3 receptor α (IL-3Rα) (mouse IgG1) was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). BD-FMK (FK-011 inhibitor, Boc-Asp (OMe)-fluoromethyl ketone) was purchased by Enzyme Systems Products (Livermore, CA). The antihuman CD95 mAb (clone DX2, IgG1, k mouse) was purchased from Pharmingen. The IgG1 mouse antihuman TNF-α mAb was purchased from Pharmingen. The antihuman transforming growth factor-β1 (TGF-β1) mAb (IgG1 mouse) was obtained from R&D Systems. For testing the role of leukocyte function-associated antigen-1 (LFA-1), supernatants from the culture of the hybridoma clone TS1/18.1.2.11 12,13 were used as described in “Results.”
Peripheral blood progenitor-cell collection, processing, and CD34+ cell purification
Peripheral blood progenitor cells (PBPCs) were collected from consenting healthy related allogeneic donors enrolled in our ongoing transplantation programs, following mobilization with standard doses of recombinant human granulocyte colony-stimulating factor (rhG-CSF) (institutional review board [IRB] approval 2382/01-04, granted by Chaim Sheba Medical Center, Tel Hashomer, Israel, included an informed consent). Low-density mononuclear cells were isolated over Ficoll-paque Plus (Amersham Pharmacia Biotech, Uppsala, Sweden). CD34+ cells were purified by magnetic cell sorting using magnetic beads linked to anti-CD34 mAb (Miltenyi Biotec, Bergisch Gladbach, Germany). The purity of CD34+ cells was analyzed by flow cytometry.
CD34+ regulatory-cell assay
As previously described,8 immune down-regulation activity was evaluated by the CD34+ cell–mediated inhibition of cytotoxic T lymphocyte precursors (CTL-p), induced by MLR against stimulator cells of the CD34+ cell donor. Peripheral blood mononuclear cells (PBMCs) that have been used as responders and as third-party stimulators have been selected from healthy volunteers' buffy coats (G-CSF unstimulated). Stimulator PBMCs from the CD34+ cell donor were collected before G-CSF treatment. HLA class I mismatching between each other and the respective CD34+ donor cells has been ascertained by HLA typing using serologic methods. HLA-A and -B types for the responder and stimulator cells used in each experiment are presented in Table 1.
HLA class I determinants of responder and stimulator cells
. | . | Stimulator cells . | . | |
|---|---|---|---|---|
| Figure or table . | Responder cells . | Donor CD34+ cells . | Third-party cells . | |
| Table 2 | ||||
| A1/23(9),B7/44(12) | A11/31(19),B52(5)/8 | A24,B35/50 | ||
| A24/69(28),B14 | A1/30(19),B8/13 | A2/3,B44(12) | ||
| A23(9)/69(28),B35/51(5) | A2/3,B41/55(22) | A24(9)/26(10),B14/38(16) | ||
| - | A30(19)/1,B44(12)/57(17) | A2/24(9),B14/40 | ||
| A11/24(9),B41B51(5) | A1/24(9),B8/38(16) | A2/30(19),B18/44(12) | ||
| A24(9)/32(19),B35/53 | A1/69(28)/B55(22)/8 | A23(9)/26(10),B38(16)/41 | ||
| A1/29(19),B7/57(17) | A2/68(28),B7/44(12) | A24(9)/33(19),B14/35 | ||
| A1/11,B52(5)/57(17) | A1/68(28),B37/B35 | A25(10)/29(19),B44(12)/39(16) | ||
| Figure 1 | ||||
| A69(28),B14 | A1/30(19),B8/13 | A2/3,B44(12) | ||
| A2,B18/41 | A26(10)/68(28),B38(16)/56(22) | A1/23(9),B35/49(21) | ||
| Figure 2 | ||||
| A2,B61(40) | A1/30(19),B8/13 | A24(9)/68(28),B18/38(16) | ||
| A2/26(10),B38(16)/47 | A2/30,B13/18 | A1/3,B52(5)/38(16) | ||
| A26(10)/11,B52/35 | A2/30,B13/18 | A23(9)/69(28),B8/14 | ||
| A3/68(28),B7/14 | A1/30(19),B8/13 | A24(9)/33(19),B35/52(5) | ||
| Figure 3 | ||||
| A29(19)/30(19),B13/7 | A1/24(9),B8/38(16) | A68(28),B14/44(12) | ||
| Figure 4 | ||||
| A2/24(9),B14/40 | A1/68(28),B37/35 | A3/24(9),B13/63(15) | ||
| A3/32(19),B8/44(12) | A1/2,B44(12)/63(15) | A30(19)/B35/53 | ||
| A24(9),B18/52(5) | A2/3,B7/35 | A68(28)/33(19),B17/38(16) | ||
| Figure 5 | ||||
| A26(10),B14 | A2/3,B7/35 | A24(9)/26(10),B44/38(16) | ||
| Figure 6 | ||||
| - | A3/30,B13/22 | A1/2,B7/53 | ||
| Figure 8 | ||||
| A24(9),B18/52(5) | A2/3,B7/35 | A68(28)/33(19),B17/38(16) | ||
| A26(10),B14 | A2/3,B7/35 | A24(9)/26(10),B44/38(16) | ||
| A2/24(9),B52(5)/44(12) | A26(10)/32(19)/B35/65(14) | A2/11,B14/18 | ||
. | . | Stimulator cells . | . | |
|---|---|---|---|---|
| Figure or table . | Responder cells . | Donor CD34+ cells . | Third-party cells . | |
| Table 2 | ||||
| A1/23(9),B7/44(12) | A11/31(19),B52(5)/8 | A24,B35/50 | ||
| A24/69(28),B14 | A1/30(19),B8/13 | A2/3,B44(12) | ||
| A23(9)/69(28),B35/51(5) | A2/3,B41/55(22) | A24(9)/26(10),B14/38(16) | ||
| - | A30(19)/1,B44(12)/57(17) | A2/24(9),B14/40 | ||
| A11/24(9),B41B51(5) | A1/24(9),B8/38(16) | A2/30(19),B18/44(12) | ||
| A24(9)/32(19),B35/53 | A1/69(28)/B55(22)/8 | A23(9)/26(10),B38(16)/41 | ||
| A1/29(19),B7/57(17) | A2/68(28),B7/44(12) | A24(9)/33(19),B14/35 | ||
| A1/11,B52(5)/57(17) | A1/68(28),B37/B35 | A25(10)/29(19),B44(12)/39(16) | ||
| Figure 1 | ||||
| A69(28),B14 | A1/30(19),B8/13 | A2/3,B44(12) | ||
| A2,B18/41 | A26(10)/68(28),B38(16)/56(22) | A1/23(9),B35/49(21) | ||
| Figure 2 | ||||
| A2,B61(40) | A1/30(19),B8/13 | A24(9)/68(28),B18/38(16) | ||
| A2/26(10),B38(16)/47 | A2/30,B13/18 | A1/3,B52(5)/38(16) | ||
| A26(10)/11,B52/35 | A2/30,B13/18 | A23(9)/69(28),B8/14 | ||
| A3/68(28),B7/14 | A1/30(19),B8/13 | A24(9)/33(19),B35/52(5) | ||
| Figure 3 | ||||
| A29(19)/30(19),B13/7 | A1/24(9),B8/38(16) | A68(28),B14/44(12) | ||
| Figure 4 | ||||
| A2/24(9),B14/40 | A1/68(28),B37/35 | A3/24(9),B13/63(15) | ||
| A3/32(19),B8/44(12) | A1/2,B44(12)/63(15) | A30(19)/B35/53 | ||
| A24(9),B18/52(5) | A2/3,B7/35 | A68(28)/33(19),B17/38(16) | ||
| Figure 5 | ||||
| A26(10),B14 | A2/3,B7/35 | A24(9)/26(10),B44/38(16) | ||
| Figure 6 | ||||
| - | A3/30,B13/22 | A1/2,B7/53 | ||
| Figure 8 | ||||
| A24(9),B18/52(5) | A2/3,B7/35 | A68(28)/33(19),B17/38(16) | ||
| A26(10),B14 | A2/3,B7/35 | A24(9)/26(10),B44/38(16) | ||
| A2/24(9),B52(5)/44(12) | A26(10)/32(19)/B35/65(14) | A2/11,B14/18 | ||
Measurements were made by using serologic analysis. Values in parentheses denote parent antigens from which the specifications have split.
- indicates not done.
Briefly, the MLR was set up using 1 × 106/mL PBMCs as responders and 1 × 106/mL irradiated (30 Gy) PBMCs from the CD34+ cell donor or from a third-party donor as stimulators in 6 mL complete media (CM) plus 10% fetal calf serum (FCS; Biological Industries, Kibbutz Beit Haemek, Israel). CD34+ cells (0.4 × 106/mL to 0.5 × 106/mL, for a regulatory-to-responder cell range of ratios from 0.4:1 to 0.5:1) were added at the initiation of the culture. CM is RPMI 1640 containing 2 mM l-glutamine, 100 U/mL penicillin, 0.1 mg/mL streptomycin, 2 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, and 5 × 10-5 M 2-mercaptoethanol (Biological Industries).
After culture for 5 days, effector cells were harvested from the MLR culture and separated on Ficoll. The effector cells were plated out in different dilutions (range, 156 to 40 000 cells per well) in 96-well round-bottom plates (16 replicates per input number). Irradiated (30 Gy) stimulator cells from the original donor used in the bulk MLR (105) were also added to each well. In the limiting dilution cultures, in which the response against stimulator cells originated from the CD34+ cell donor was tested, PBMCs from the CD34- fraction, obtained after G-CSF treatment and CD34+ cell selection, were used. The limiting dilution cultures were maintained for 7 days in CM plus 10% FCS and 10 U/mL rhIL-2 (EuroCetus) in a final volume of 0.2 mL.
Estimate of CTL activity
Cytotoxic activity was measured in a standard 4-hour assay against 51Cr-labeled cells. Briefly, concanavalin A–prepared blasts (Sigma, St Louis, MO) from the same stimulator donors (as target cells) were labeled with 51Cr. Various dilutions of effector cells (100 μL of each limiting dilution analysis per well) were plated with 5 × 103 target cells in conical-bottom 96-well plates (Greiner, Frickenhausen, Germany) in a final volume of 200 μL. The mean radioactivity in the supernatants of 16 replicate samples was calculated, and the percentage of specific lysis was calculated by the following equation: 100 × (mean experimental release - mean spontaneous release)/(mean total release - mean spontaneous release). The release of 51Cr by target cells cultured in medium alone or lysed with 1% sodium dodecyl sulfate (SDS) was defined as spontaneous release or as total release, respectively.
Frequency calculation of CTL-p
To calculate the frequency from the limiting dilution culture readout, we used the following equation: ln y = -fx + ln a (which represents the zero-order term of the Poisson distribution14 ), in which y is the percentage of nonresponding cultures, x is the number of responding cells per culture, f is the frequency of responding precursors, and a is the y intercept theoretically equal to 100%. The mean plus 3 standard deviations of the 16 wells containing the target cells alone was determined as the cutoff value for background radioactivity. Experimental wells were scored positive for lysis when exceeding the cutoff value. The percent responding cultures was defined by calculating the percent of positive cultures. The CTL-p frequency (f) and standard error (SE) were determined from the slope of the line drawn utilizing linear regression analysis of the data. To evaluate the statistical significance of the difference between the slopes of 2 regression lines, t was calculated as follows: t = f1 - f2/√[SE2(f1) + SE2(f2)].8,15
The CTL-p weighted mean (WM) frequency (fWM)16 and the determination of the variance of fWM have been calculated according to the following formula of weighted arithmetic mean: Σfiwi/Σwi → f is obtained independently from each individual dose i. The weight wi for each estimate was chosen as the reciprocal of its variance divided by the sum of the weights. Thus, the WM frequency estimate fWM was calculated as follows:
When i is the number of groups of replicate cultures, ri is the number of negatively responding cultures of each dose i, and pi is ri/ni (the fraction of negatively responding cultures of each dose i), and xi is the value of an individual dose.
To exclude potential error that might be caused by differential expansion of CD34+ cells, the final CTL-p frequency was adjusted for the yield of responder CD3+ T cells in the primary bulk culture, defined by fluorescence-activated cell sorter (FACS) analysis at the end of the bulk MLR and prior to the limiting dilution analysis. The total CTL-p number was finally calculated by multiplying the total cell number recovered at the end of the bulk culture by the CTL-p frequency defined by the limiting dilution assay.
There is a substantial interexperimental variability when using the limiting dilution analysis (LDA) for CTL-p assay in humans. Therefore, in some experiments the LDA permitted the calculation of CTL-p frequency, while in other experiments we were only able to define the differences when analyzing the counts per minute (cpm) readouts as “percent specific lysis.”
Immobilization of anti-CD28 and anti-CD2 mAbs
Plates of 6-well (Nunc, Roskilde, Denmark) were coated by adding anti-CD28 (1 or 5 μg per well) or anti-CD2 (22 μg per well) mAbs (Pharmingen) in a final volume of 1 mL for 20 hours at 4°C. Coating was performed in a coating buffer: double-distilled water with boric acid (H3Bo3, 166 mM) and sodium chloride (NaCl, 120 mM), pH 8.5 (achieved with NaOH).
Inhibition of the immune down-regulatory effect of CD34+ cells by BD-FMK
Responder cells (4 × 106/mL to 5 × 106/mL) were cultured for 2 hours with 500 μM BD-FMK (Enzyme Systems Products) in CM at a final volume of 2 mL. Then, the cells were cultured in a 5-day MLR, and BD-FMK was added to adjust to a final concentration of 375 μM.
Results
Mechanism of action of CD34+ regulatory cells
The inhibitory activity of CD34+cells is not mediated by anergy-based or other nonapoptotic mechanisms. Several attributes of the CD34+ regulatory cells have been previously characterized,8 including (1) optimal regulatory-to-responder cell ratio, (2) the importance of cell contact, (3) demonstration that CD34+ cells are not effective if added later than 24 hours after initiation of MLR, (4) demonstration that removal of CD34+ cells at the end of the culture, prior to Cr-release assay, does not retract from the inhibitory activity of the CD34+ cells, and (5) demonstration that the specific inhibitory activity is exhibited when the stimulators of the donor and of the third party are incubated together in the bulk MLR. The ability of CD34+ cells to paralyze CTLs is best illustrated by flow cytometry of the bulk MLR after 5 days of culture, showing that the CD34+ cells are not killed by the allogeneic T cells.7 In contrast, when allogeneic responder cells (Table 1) were cultured with the CD34+ donor stimulators for 4 days and then the CD34+ cells were added for 5 days, the CD34+ cells were killed, as determined by flow cytometry (data not shown).
Anergy induction or other nonapoptotic mechanisms, such as Th1-to-Th2 skewing, could potentially explain the regulatory activity of CD34+ cells. Such mechanisms could be compatible with the observation that CD34+ cells exhibit HLA class I and class II in the absence of the costimulatory molecules B7-1 and B7-2.7 To address this question we tested several reagents that could either provide costimulation or inhibit signals that otherwise might drive the responder cells into an anergic state. A major problem in the design of these experiments is posed by the minute number of purified CD34+ cells and stimulator cells (PBMCs obtained prior to G-CSF mobilization) available from a given donor. In addition, the variability in antigen-presenting cell (APC) frequencies in different PBMC populations has led us to use a more lengthy assay in which the effector response is amplified by a secondary response against the stimulators. Thus, our data collection is largely based on a gathering of isolated individual experiments, each of which requires a 2-week assay. Despite this major difficulty, we were able in 9 experiments to test 5 different agents that can potentially reverse anergy or Th1-to-Th2 skewing. The former agents include anti-CD28, anti-CD2 antibody, exogenous IL-2, or anti–IL-10 antibody. The latter agents include IL-12. A neutralizing anti-HLA class I antibody served as a positive control, in line with early observations that the veto activity is dependent on class I triggering.17-19
The critical control for each experiment is the specificity control—namely, the inhibition of antidonor response in the absence of inhibition of anti–third-party response. Table 2 summarizes the results of different experiments in which the potential of several regulatory agents to neutralize the regulatory activity of donor CD34+ cells toward antidonor CTL-p was tested. The ability of CD34+ cells to inhibit the generation of CTL-p was evaluated in the absence or presence of the tested agent. For each experiment the number of positive and negative cultures (determined by scoring counts per minute [cpm] above a mean plus 3 standard deviation threshold obtained in the 16 spontaneous cultures) tested at the highest effector cell concentration (40 000 cells per culture) are shown along with the statistical significance obtained by the χ2 test for each comparison. As can be seen in Table 2, no significant difference could be found between MLR cultures carried out in the presence or absence of the tested regulatory agents. Thus, while anti-HLA class I was found to induce reversal of CD34+ inhibition, no such reversal could be detected in 3 experiments with anti-CD28, 1 with anti-CD2, and 2 with anti–IL-10. Likewise, reversal of the CD34+ cell regulatory activity was not found upon the addition of IL-2 (2 experiments) or IL-12 (2 experiments). Similar results were also found at an effector cell concentration of 20 000 per culture (data not shown).
Effect of different regulatory agents on the inhibitory activity of CD34+ cells
. | . | No. of responding/nonresponding cultures‡ . | . | Statistical significance§ . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Treatment* . | MLR cultures† . | Responding . | Nonresponding . | P value . | P range . | Significance . | |||
| Anti-HLA-1 | |||||||||
| A | 16 | 0 | ND | ND | ND | ||||
| B | 9 | 7 | 0.0004 | P < .001 | S | ||||
| A# | 16 | 0 | ND | ND | ND | ||||
| D | 15 | 1 | 0.3016 | P > .1 | NS | ||||
| Anti-IL-10 | |||||||||
| A | 16 | 0 | ND | ND | ND | ||||
| B | 7 | 9 | 5.7E-06 | P < .001 | S | ||||
| C | 16 | 0 | ND | ND | ND | ||||
| D | 3 | 13 | 8.3E-17 | P < .001 | S | ||||
| Anti-CD28∥ | |||||||||
| A | 37 | 11 | ND | ND | ND | ||||
| B | 9 | 39 | 3.9E-25 | P < .001 | S | ||||
| C | 30 | 2 | ND | ND | ND | ||||
| D | 5 | 43 | 1.4E-37 | P < .001 | S | ||||
| Anti-CD2 | |||||||||
| A | 6 | 10 | ND | ND | ND | ||||
| B | 2 | 14 | 0.0024 | .01 > P > .001 | S | ||||
| C | 10 | 6 | ND | ND | ND | ||||
| D | 1 | 15 | 1.4E-20 | P < .001 | S | ||||
| IL-2¶ | |||||||||
| A | 27 | 5 | ND | ND | ND | ||||
| B | 13 | 19 | 4.6E-07 | P < .001 | S | ||||
| C | 28 | 4 | ND | ND | ND | ||||
| D | 10 | 22 | 6.6E-12 | P < .001 | S | ||||
| IL-12¶ | |||||||||
| A | 12 | 20 | ND | ND | ND | ||||
| B | 3 | 29 | 4.8E-08 | P < .001 | S | ||||
| C | 31 | 0 | ND | ND | ND | ||||
| D | 7 | 25 | 3.8E-25 | P < .001 | S | ||||
. | . | No. of responding/nonresponding cultures‡ . | . | Statistical significance§ . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Treatment* . | MLR cultures† . | Responding . | Nonresponding . | P value . | P range . | Significance . | |||
| Anti-HLA-1 | |||||||||
| A | 16 | 0 | ND | ND | ND | ||||
| B | 9 | 7 | 0.0004 | P < .001 | S | ||||
| A# | 16 | 0 | ND | ND | ND | ||||
| D | 15 | 1 | 0.3016 | P > .1 | NS | ||||
| Anti-IL-10 | |||||||||
| A | 16 | 0 | ND | ND | ND | ||||
| B | 7 | 9 | 5.7E-06 | P < .001 | S | ||||
| C | 16 | 0 | ND | ND | ND | ||||
| D | 3 | 13 | 8.3E-17 | P < .001 | S | ||||
| Anti-CD28∥ | |||||||||
| A | 37 | 11 | ND | ND | ND | ||||
| B | 9 | 39 | 3.9E-25 | P < .001 | S | ||||
| C | 30 | 2 | ND | ND | ND | ||||
| D | 5 | 43 | 1.4E-37 | P < .001 | S | ||||
| Anti-CD2 | |||||||||
| A | 6 | 10 | ND | ND | ND | ||||
| B | 2 | 14 | 0.0024 | .01 > P > .001 | S | ||||
| C | 10 | 6 | ND | ND | ND | ||||
| D | 1 | 15 | 1.4E-20 | P < .001 | S | ||||
| IL-2¶ | |||||||||
| A | 27 | 5 | ND | ND | ND | ||||
| B | 13 | 19 | 4.6E-07 | P < .001 | S | ||||
| C | 28 | 4 | ND | ND | ND | ||||
| D | 10 | 22 | 6.6E-12 | P < .001 | S | ||||
| IL-12¶ | |||||||||
| A | 12 | 20 | ND | ND | ND | ||||
| B | 3 | 29 | 4.8E-08 | P < .001 | S | ||||
| C | 31 | 0 | ND | ND | ND | ||||
| D | 7 | 25 | 3.8E-25 | P < .001 | S | ||||
ND indicates not done; S, significant; and NS, not significant.
The tested regulatory agents were added at the initiation of the MLR to the culture medium, at their optimal concentration, according to the manufacturer's recommendation and according to preliminary titration experiments, except for the anti-HLA-1. This agent was preincubated with the CD34+ cells and washed prior to their addition to the MLR culture
MLR cultures in which responder cells were stimulated against allogeneic PBMCs from the CD34+ cells' donor, in the absence (A and C) or presence (B and D) of CD34+ cells, were established
The potential of different agents to reverse the inhibitory regulatory activity of CD34+ cells was evaluated by comparing the inhibition in the presence (C and D) and in the absence (A and B) of the specific agent. Briefly, a 5-day MLR was established in which the responder cells were then recultured for an additional 7 days under limiting dilution in microtiter plates. For each experiment, the number of positive and negative cultures, tested at the highest effector cell concentration (40 000 cells per well), are shown. Wells were scored positive for CTL activity when Cr release exceeded the mean spontaneous release value by at least 3 standard deviations of the mean. The regulatory activity of CD34+ cells was evaluated by their capacity to inhibit alloreactive CTL-p clones in the MLR to which they were added at a ratio of 0.5:1 CD34+/responder cell. The addition of CD34+ cells to MLR against third-party stimulators has not led to a significant inhibition (P > .1). Thus, in a total of 5 experiments carried out in the absence of CD34+ cells, 3 of 96 anti-third-party MLR culture wells were scored negative while 6 of 96 were scored negative in the presence of CD34+ cells
The results were statistically analyzed by the χ2 test
The results represent a total of 3 experiments
The results represent a total of 2 experiments
The role of anti-HLA-1 antibody was tested by incubating the antibody with CD34+ cells prior to their addition to the MLR. Therefore, for the statistical analysis of results in culture D we used as a reference the results of culture A
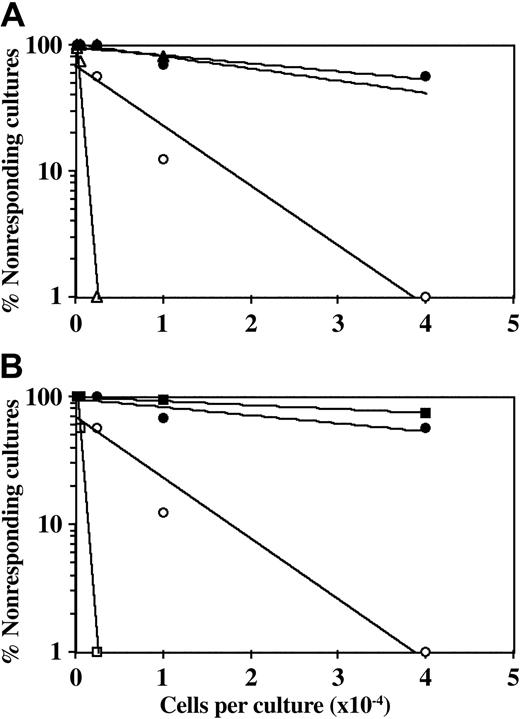
Figure 1 shows a typical experiment in which anti-CD28 was added to the MLR in the presence or absence of CD34+ cells. To exclude potential error that might be caused by differential expansion of CD34+ cells, the final CTL-p frequency (fWM) was adjusted for the yield of responder CD3+ T cells at the end of the primary bulk culture (defined by FACS analysis), prior to the limiting dilution analysis. Engaging the surface CD28 molecules by the anti-CD28 antibody can transduce the second signal provided generally by interaction with the B7 molecules.20-22 Thus, the frequency of CTL-p is generally enhanced upon the addition of anti-CD28 antibody. However, addition of anti-CD28 antibody to the bulk culture did not reverse the inhibition exhibited by CD34+ cells. Thus, at concentrations of 1 μg/mL and 5 μg/mL anti-CD28 antibody (Figure 1), fWM in the absence of CD34+ cells was 1.2 × 10-3 and 2.3 × 10-4, respectively, and in the presence of CD34+ cells it was reduced to 1.0 × 10-4 and 3.1 × 10-5. This inhibition is similar to that found in control groups to which anti-CD28 antibody was not added (fWM in the absence and presence of CD34+ cells was 1.6 × 10-4 and 7.8 × 10-5, respectively).
CD34+ cell–mediated suppression of CTL responses is not prevented by addition of anti-CD28 mAb. A 5-day MLR was established in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor with (•, ▴, ▪) or without (○, ▵, □) the addition of CD34+ cells. Anti-CD28 mAb was added (▵, ▴, □, ▪) or not added (○, •) at the beginning of the culture at a concentration of 1 μg/mL (A) or 5 μg/mL (B). The cells were then recultured for 7 days under limiting dilution, and the CTL activity was determined by 51Cr-release assay. Three experiments were carried out; 1 experiment is presented. The CTL-p frequency (fWM) was calculated as described in “Materials and methods.” The inhibition of CTL-p–fWM mediated by CD34+ cells in the presence of anti-CD28 mAb is significant (P < .005 and P < .001 for the concentration of 1 μg/mL and 5 μg/mL, respectively). V(fWM) values were below 5 × 10-8. Sloping line indicates CTL-p frequency.
CD34+ cell–mediated suppression of CTL responses is not prevented by addition of anti-CD28 mAb. A 5-day MLR was established in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor with (•, ▴, ▪) or without (○, ▵, □) the addition of CD34+ cells. Anti-CD28 mAb was added (▵, ▴, □, ▪) or not added (○, •) at the beginning of the culture at a concentration of 1 μg/mL (A) or 5 μg/mL (B). The cells were then recultured for 7 days under limiting dilution, and the CTL activity was determined by 51Cr-release assay. Three experiments were carried out; 1 experiment is presented. The CTL-p frequency (fWM) was calculated as described in “Materials and methods.” The inhibition of CTL-p–fWM mediated by CD34+ cells in the presence of anti-CD28 mAb is significant (P < .005 and P < .001 for the concentration of 1 μg/mL and 5 μg/mL, respectively). V(fWM) values were below 5 × 10-8. Sloping line indicates CTL-p frequency.
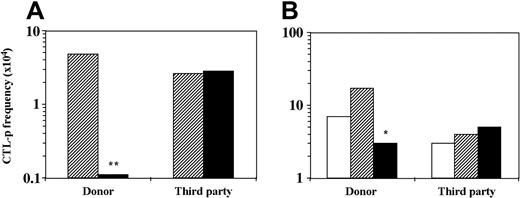
Suppressor T cells are not induced in MLR in the presence of CD34+cells. The role of suppressor T cells in tolerance induction both in vitro and in vivo has been well documented (reviewed by Waldmann and Cobbold23,24 ). Thus, as has been shown, suppressor CD4+ T cells can be induced by anti-CD4 or anti-CD8 antibodies or by other suppressor T cells (“infectious tolerance”)25 or, as was shown later, by anergy-inducing protocols.22,26 Therefore, suppressor T cells could potentially be induced by the addition of CD34+ cells to the MLR. To address this question, T cells were isolated by E rosetting with sheep red blood cells from 6-day bulk MLR against stimulators of the CD34+ donor or a third-party donor with or without CD34+ cell addition. The isolated T cells were added to another MLR containing different responder cells (at a ratio of 1:1 T–responder cell) and stimulators of the CD34+ cells' origin or of the third-party origin. As can be seen in Figure 2, only T cells isolated from bulk MLR against CD34+ donor stimulators in the presence of CD34+ cells inhibit the CTL-p frequency of the second responders. However, this reduction (2.3-fold) is compatible with the expected dilution factor (1:1) and is short of a more robust inhibition. Thus, while the T cells incubated in the initial MLR are deficient of CTL-p against the donor of the CD34+ cells, they did not transfer suppression to another MLR with naive responder cells from a different individual.
T cells cultured in the presence of CD34+ cells do not exhibit suppressive activity in a secondary MLR. A 6-day MLR was established in which responder cells (1 × 106/mL) were cultured with irradiated allogeneic stimulator cells (1 × 106/mL) of the CD34+ cell donor or a third-party donor in the absence (▨) or presence (▪) of CD34+ cells (0.5 × 106/mL). The cultures were then harvested. (A) A fraction of the recovered cells were recultured for 7 days in limiting dilution cultures, and the CTL activity was determined by 51Cr-release assay. The CTL-p frequency (f) was calculated as described in “Materials and methods.” In the second fraction of the recovered cells, T cells were isolated using E rosetting with sheep red blood cells. (B) A second 6-day MLR was carried out in which naive responder cells (1 × 106/mL) from a new donor were stimulated against stimulator cells (1 × 106/mL) of the CD34+ cell donor or the primary third-party donor. The isolated T cells from the primary MLR were added (▨, ▪) or not (□) to the secondary MLR at a T–responder cell ratio of 1:1. The responder cells were then recultured for 8 days under limiting dilution, and the CTL-p frequency was determined. The statistical significance of the difference between the slopes (f) of 2 regression lines was evaluated as described in “Materials and methods.” Asterisks indicate statistical significance. **P < .001 when compared by t test with control cultures without CD34+ cells; *P < .005 when compared by t test with control cultures without addition of isolated T cells. The total CTL-p number (n) per primary bulk culture was calculated according to the yield of effector cells by the end of the primary culture period. (A) MLR against donor stimulators. In the absence of CD34+ cells (▨)n = 1162, in the presence of CD34+ cells (▪)n = 48. MLR against third-party stimulators. In the absence of CD34+ cells (▨)n = 621, in the presence of CD34+ cells (▪) n = 1749. (B) MLR against donor stimulators. In the absence of T cells (□) n = 1540, in the presence of T cells from culture without stem cells (▨) n = 11 560, in the presence of T cells from stem cell culture (▪) n = 1518. MLR against third-party stimulators. In the absence of T cells (□)n = 966, in the presence of T cells from culture without stem cells (▨)n = 1588, in the presence of T cells from stem cell culture (▪)n = 1765. The fWM was calculated as described in “Materials and methods”: (A) MLR against donor stimulators. In the absence of CD34+ cells (▨) fWM = 1.9 × 10-4, in the presence of CD34+ cells (▪) fWM = 1.1 × 10-5. MLR against third-party stimulators. In the absence of CD34+ cells (▨) fWM = 2.8 × 10-4, in the presence of CD34+ cells (▪) fWM = 2.4 × 10-4. V(fWM) values were below 4.5 × 10-9. (B) MLR against donor stimulators. In the absence of T cells (□) fWM = 5.5 × 10-4, in the presence of T cells from culture without stem cells (▨) fWM = 1.4 × 10-3, in the presence of T cells from stem cell culture (▪) fWM = 2.6 × 10-4. MLR against third-party stimulators. In the absence of T cells (□) fWM = 8.6 × 10-5, in the presence of T cells from culture without stem cells (▨) fWM = 3.2 × 10-4, in the presence of T cells from stem cell culture (▪) fWM = 3.7 × 10-4. V(fWM) values were below 1.2 × 10-7. Results represent 1 experiment.
T cells cultured in the presence of CD34+ cells do not exhibit suppressive activity in a secondary MLR. A 6-day MLR was established in which responder cells (1 × 106/mL) were cultured with irradiated allogeneic stimulator cells (1 × 106/mL) of the CD34+ cell donor or a third-party donor in the absence (▨) or presence (▪) of CD34+ cells (0.5 × 106/mL). The cultures were then harvested. (A) A fraction of the recovered cells were recultured for 7 days in limiting dilution cultures, and the CTL activity was determined by 51Cr-release assay. The CTL-p frequency (f) was calculated as described in “Materials and methods.” In the second fraction of the recovered cells, T cells were isolated using E rosetting with sheep red blood cells. (B) A second 6-day MLR was carried out in which naive responder cells (1 × 106/mL) from a new donor were stimulated against stimulator cells (1 × 106/mL) of the CD34+ cell donor or the primary third-party donor. The isolated T cells from the primary MLR were added (▨, ▪) or not (□) to the secondary MLR at a T–responder cell ratio of 1:1. The responder cells were then recultured for 8 days under limiting dilution, and the CTL-p frequency was determined. The statistical significance of the difference between the slopes (f) of 2 regression lines was evaluated as described in “Materials and methods.” Asterisks indicate statistical significance. **P < .001 when compared by t test with control cultures without CD34+ cells; *P < .005 when compared by t test with control cultures without addition of isolated T cells. The total CTL-p number (n) per primary bulk culture was calculated according to the yield of effector cells by the end of the primary culture period. (A) MLR against donor stimulators. In the absence of CD34+ cells (▨)n = 1162, in the presence of CD34+ cells (▪)n = 48. MLR against third-party stimulators. In the absence of CD34+ cells (▨)n = 621, in the presence of CD34+ cells (▪) n = 1749. (B) MLR against donor stimulators. In the absence of T cells (□) n = 1540, in the presence of T cells from culture without stem cells (▨) n = 11 560, in the presence of T cells from stem cell culture (▪) n = 1518. MLR against third-party stimulators. In the absence of T cells (□)n = 966, in the presence of T cells from culture without stem cells (▨)n = 1588, in the presence of T cells from stem cell culture (▪)n = 1765. The fWM was calculated as described in “Materials and methods”: (A) MLR against donor stimulators. In the absence of CD34+ cells (▨) fWM = 1.9 × 10-4, in the presence of CD34+ cells (▪) fWM = 1.1 × 10-5. MLR against third-party stimulators. In the absence of CD34+ cells (▨) fWM = 2.8 × 10-4, in the presence of CD34+ cells (▪) fWM = 2.4 × 10-4. V(fWM) values were below 4.5 × 10-9. (B) MLR against donor stimulators. In the absence of T cells (□) fWM = 5.5 × 10-4, in the presence of T cells from culture without stem cells (▨) fWM = 1.4 × 10-3, in the presence of T cells from stem cell culture (▪) fWM = 2.6 × 10-4. MLR against third-party stimulators. In the absence of T cells (□) fWM = 8.6 × 10-5, in the presence of T cells from culture without stem cells (▨) fWM = 3.2 × 10-4, in the presence of T cells from stem cell culture (▪) fWM = 3.7 × 10-4. V(fWM) values were below 1.2 × 10-7. Results represent 1 experiment.
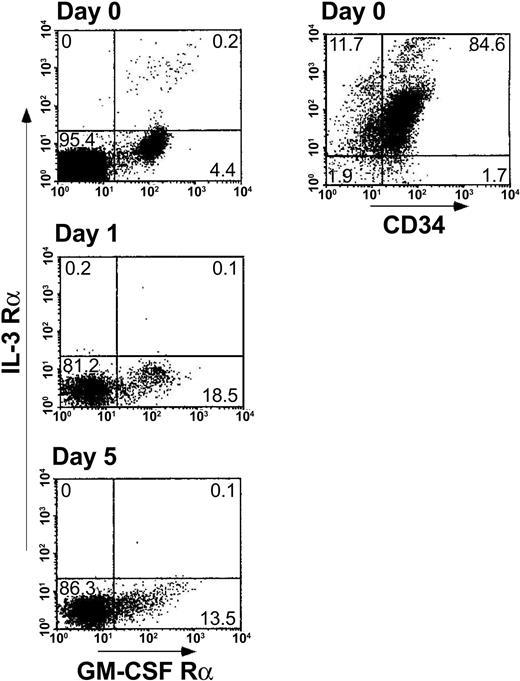
DC2s are not dominant in MLR with regulatory CD34+cells. Another potential explanation for the observed inhibitory activity of CD34+ cells could be based on skewing of the effector T cells toward the Th2 phenotype by DC2 dendritic cells (DCs). The plasmacytoid DC2 precursors (pDC2) are characterized by lack of myeloid lineage antigens and the expression of pre–T-cell receptor α (pre-TCRα) chain, low GM-CSFRα, and high CD123 (IL-3Rα).27-29 In contrast, DC1 precursors (pDC1), which induce generation of Th1 T cells, express myeloid lineage antigens, such as CD11c, CD13, and CD33, and express high GM-CSF receptor α (GM-CSFRα) and low IL-3 receptor α (IL-3Rα).27-29 Rissoan et al27 found that stimulation of allogeneic naive T cells with DC1 resulted in generation of Th1 cells secreting IFN-γ, whereas DC2 generated IL-4–producing Th2 cells. To address the possibility that CD34+ cells might differentiate during the MLR toward a DC2 subset deviating the response to the Th2 type, CD34+ cells were characterized for their CD13 myeloid antigen expression and GM-CSFRα versus IL-3Rα expression. As can be seen in Figure 3, the predominant phenotype of fresh CD34+ cells is CD34+CD13+ (84.6%) with low frequency of GM-CSFRα–expressing cells (4.4%) but without a trace of IL-3Rα–expressing cells. Furthermore, along the MLR period, the frequency of GM-CSFRα–expressing cells (pDC1) was increased (13.5% to 18.5%), while staining for IL-3Rα–expressing cells (pDC2) remained negative. This analysis is based on CD34+ cells present in the myeloid gate (defined by their forward scatter/side scatter [FSC/SSC] image); however, few CD34+ cells are present in the lymphocyte FSC/SSC gate (about 8% versus about 80% in the myelogate), and it could be argued that the CD34+ cells in the lymphogate might give rise to the DC2 subset. However, even in the lymphocyte gate the DC1 subset prevailed. Thus, only low expression of IL-3Rα was found along the MLR (0.1% to 0.8%) while the expression of GM-CSFRα in the lymphoid population was 2.1% to 2.5% (data not shown). Taken together these results suggest that CD34+ cells give rise to the pDC1/DC1 myeloid phenotype, which induces Th1 differentiation, ruling out the possibility that tolerance could be mediated by DC2 progeny of the CD34+ cells.
Cellular levels of pDC1 (GM-CSFRα chain, CD13) versus pDC2 (IL-3Rα chain) induced by the addition of CD34+ cells to the bulk MLR. A 5-day MLR was established in which responder cells (1 × 106/mL) were cocultured with irradiated allogeneic stimulator cells (1 × 106/mL) from the CD34+ cell donor in the presence of CD34+ cells (0.5 × 106/mL). At the indicated days of the MLR, cells were harvested and the expression of pDC1/pDC2 antigens was measured by immunofluorescent staining. CD34+ cells were analyzed in myelogate based on their FSC/SSC profile. The percentages of the cell subpopulations are indicated in the relevant area of the dot plot. Results represent 1 experiment.
Cellular levels of pDC1 (GM-CSFRα chain, CD13) versus pDC2 (IL-3Rα chain) induced by the addition of CD34+ cells to the bulk MLR. A 5-day MLR was established in which responder cells (1 × 106/mL) were cocultured with irradiated allogeneic stimulator cells (1 × 106/mL) from the CD34+ cell donor in the presence of CD34+ cells (0.5 × 106/mL). At the indicated days of the MLR, cells were harvested and the expression of pDC1/pDC2 antigens was measured by immunofluorescent staining. CD34+ cells were analyzed in myelogate based on their FSC/SSC profile. The percentages of the cell subpopulations are indicated in the relevant area of the dot plot. Results represent 1 experiment.
The role of TNF-α–mediated apoptosis
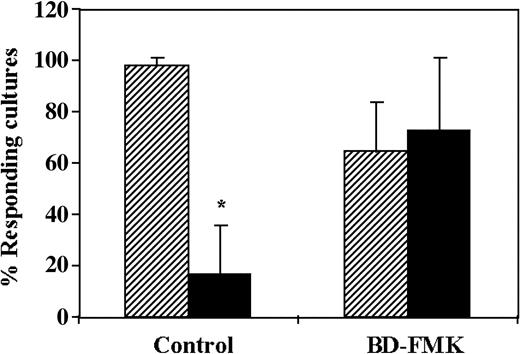
The regulatory activity of CD34+cells can be reversed by the caspase inhibitor BD-FMK. Considering that several veto cells exert their activity via apoptosis of CTL-p upon the interaction with the latter cells,30-32 we investigated the role of apoptosis by addition of BD-FMK, a broad-spectrum caspase inhibitor. BD-FMK, a cell-permeable peptide fluoromethyl ketone that blocks CPP-32–like proteases, was shown to be an effective inhibitor of apoptotic cell death of effector T cells. Thus, Sarin et al33 have shown that anti-CD3, Fas-induced death or death induced by IL-2 withdrawal or dexamethasone was inhibited in T lymphocytes by BD-FMK. As can be seen in Figure 4, the average antidonor CTL activity in 3 different experiments was reduced by 5.9-fold upon addition of the CD34+ cells to the MLR. In contrast, addition of the inhibitor BD-FMK (400 μM) to the MLR was found to completely reverse the regulatory activity of CD34+ cells, indicating that the effect of CD34+ cells is mediated by apoptosis of the antidonor CTL-p clones. The reversal of the inhibitory activity of the CD34+ cells by the BD-FMK cannot be attributed to enhancement of the CTL activity, as shown in the control in which it was added in the absence of CD34+ cells (97% activity versus 64% activity, in the absence and presence of BD-FMK, respectively).
CD34+ cell–mediated suppression of CTL responses is prevented by the caspase inhibitor BD-FMK. A 5-day MLR was established in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor in the presence (▪) or absence (▨) of CD34+ cells. In some cultures, the responder cells were precultured for 2 hours with the caspase inhibitor BD-FMK (500 μM) and were then added to the MLR. The BD-FMK inhibitor was also added to the MLR at a final concentration of 250 to 375 μM. The CTL activity was determined by the end of 7-day limiting dilution cultures. Data show the average (± standard deviation) of 3 experiments at cell concentrations of 4 × 104 and 1 × 104 cells per well, calculated as described in “Materials and methods.” *Statistically significant (P < .05 when compared by t test with control cultures without CD34+ cells).
CD34+ cell–mediated suppression of CTL responses is prevented by the caspase inhibitor BD-FMK. A 5-day MLR was established in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor in the presence (▪) or absence (▨) of CD34+ cells. In some cultures, the responder cells were precultured for 2 hours with the caspase inhibitor BD-FMK (500 μM) and were then added to the MLR. The BD-FMK inhibitor was also added to the MLR at a final concentration of 250 to 375 μM. The CTL activity was determined by the end of 7-day limiting dilution cultures. Data show the average (± standard deviation) of 3 experiments at cell concentrations of 4 × 104 and 1 × 104 cells per well, calculated as described in “Materials and methods.” *Statistically significant (P < .05 when compared by t test with control cultures without CD34+ cells).
The regulatory activity of CD34+cells can be reversed by the anti–TNF-α mAb. Recent studies have indicated a role for Fas-FasL–mediated apoptosis in the veto activity of CD8+ CTL lines.32,34,35 In addition, the simultaneous involvement of CD8 molecules on host-nonreactive CTL veto cells, which interact with α3 domain of HLA class I molecules on the responder cells, was also documented.32 However, the addition of anti-FasL antibody to MLR at a concentration of 10 μg/mL36 did not lead to reversal of the inhibitory activity of the CD34+ cells. Thus, antidonor CTL-p frequency in MLR with CD34+ cells and without anti-FasL antibody was 1.5 × 10-5 (percent responding cultures upon plating 40 000 and 20 000 cells per well was 12.5% and 18.75%, respectively) while in the presence of the antibody it was 1.1 × 10-5 (percent responding cultures upon plating 40 000 and 20 000 cells per well was 12.5% and 12.5%, respectively). Thus, CD34+ cell inhibition of CTL activity does not involve Fas-mediated cytotoxicity, indicating that death ligands other than FasL, expressed on the CD34+ cells, might be responsible for their regulatory effect. One could argue that the anti-Fas mAb might mediate the death of the CTL-p. However, the CTL-p frequency in the presence of the mAb and the absence of CD34+ cells was not reduced (f = 11.4 × 10-5 and percent responding cultures = 100%, 87.5% in the presence of the anti-Fas mAb; f = 6.6 × 10-5 and percent responding cultures = 93.75%, 56.25% in the absence of the mAb). Considering the indication that the CD34+ cell–mediated apoptosis may not be mediated by FasL, it was of interest to test the role of TNF-α, which could potentially mediate apoptosis by a similar mode of action. Likewise, it was of interest to test the potential role of TGF-β previously implicated in the veto activity of a CD3-CD8+CD16+ bone marrow subpopulation.31,37
As can be seen in a typical experiment, while neutralizing mAb to TGF-β (5 μg/mL) was not found to exhibit an appreciable effect on the regulatory activity, a marked reversal of the inhibitory activity was found when anti–TNF-α was added together with the CD34+ cells (Figure 5). Moreover, addition of anti–TGF-β mAb together with anti–TNF-α mAb could not improve the neutralization of the CD34+ cell activity induced by the anti–TNF-α (data not shown).
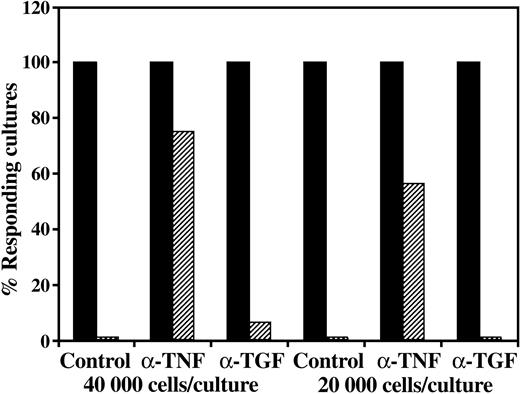
CD34+ cell–mediated suppression of CTL responses is prevented by addition of anti–TNF-α but not by the addition of anti–TGF-β1. A 5-day MLR was established in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor in the presence (▨) or absence (▪) of CD34+ cells. Anti–TNF-α or TGF-β1 neutralizing mAbs were added at the beginning of the MLR at a concentration of 5 μg/mL. The CTL activity was determined by the end of 7-day limiting dilution cultures. Data show the percent responding cultures at cell concentrations of 4 × 104 and 2 × 104 cells per well, calculated as described in “Materials and methods.”
CD34+ cell–mediated suppression of CTL responses is prevented by addition of anti–TNF-α but not by the addition of anti–TGF-β1. A 5-day MLR was established in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor in the presence (▨) or absence (▪) of CD34+ cells. Anti–TNF-α or TGF-β1 neutralizing mAbs were added at the beginning of the MLR at a concentration of 5 μg/mL. The CTL activity was determined by the end of 7-day limiting dilution cultures. Data show the percent responding cultures at cell concentrations of 4 × 104 and 2 × 104 cells per well, calculated as described in “Materials and methods.”
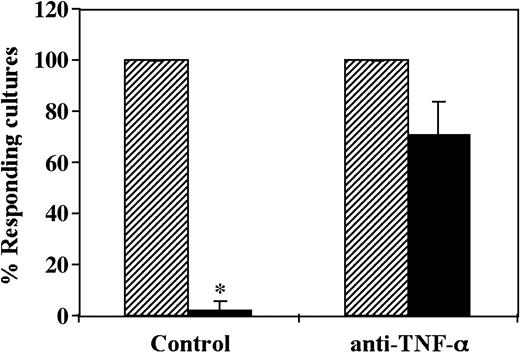
Taken together, in 3 different experiments the CD34+ cell–mediated decrease of the antidonor CTL-p cells was inhibited by 75% ±13.5% (mean ± SD) by a neutralizing anti–TNF-α mAb added at the initiation of the MLR (Figure 6).
CD34+ cell–mediated suppression of CTL responses is prevented by addition of neutralizing anti–TNF-α mAb. A 5-day MLR in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor in the presence (▪) or absence (▨) of CD34+ cells was established. Anti–TNF-α mAb was added at the beginning of the culture at a concentration of 5 μg/mL. The CTL activity was determined by the end of 7-day limiting dilution cultures. Data show the average percent responding cultures (± standard deviation) of 3 experiments at cell concentrations of 4 × 104 cells per well. * Indicates statistical significance.
CD34+ cell–mediated suppression of CTL responses is prevented by addition of neutralizing anti–TNF-α mAb. A 5-day MLR in which responder cells were stimulated against allogeneic cells from the CD34+ cell donor in the presence (▪) or absence (▨) of CD34+ cells was established. Anti–TNF-α mAb was added at the beginning of the culture at a concentration of 5 μg/mL. The CTL activity was determined by the end of 7-day limiting dilution cultures. Data show the average percent responding cultures (± standard deviation) of 3 experiments at cell concentrations of 4 × 104 cells per well. * Indicates statistical significance.
Discussion
The immune regulatory role of CD34+ cells was first documented by Rachamim et al.7 Several characteristics of these cells were further described recently,8 and it was also documented that early myeloid CD33+ cells, harvested 7 to 12 days after ex vivo expansion of CD34+ cells, are also endowed with marked inhibitory activity. In the present study, further attributes of the CD34+ cells were shown including the importance of the CD34+ HLA class I recognition for their activity.
A major mechanism for tolerance induction exerted by several drugs or cell subpopulations involves anergy induction in the responder T cells. Thus, anergy can be induced by costimulation blockade with cytotoxic T lymphocyte antigen 4 (CTLA4)–Ig,38,39 anti-CD40 ligand (anti-CD40L),40,41 or anti-B742 antibodies by cytokines such as IL-10 43,44 or by suppressor T cells such as CD4+CD25+ cells.45-49 In addition, stimulation with APCs of a DC2 subset27,50 or skewing the T-cell response into a Th2 type by appropriate cytokines such as IL-451 or IL-1052,53 creates an unfavorable setting for the development of alloreactive CTLs. Our studies using anti-CD28 mAb or the addition of exogenous IL-2 to bypass the requirement for costimulation via B7, as well as using anti–IL-10 blocking mAbs, have ruled out the possibility that CD34+ cells induce tolerance by such mechanisms. Furthermore, the demonstration that the only effective way to reverse the inhibitory activity of CD34+ cells was afforded by a caspase inhibitor such as BD-FMK, which induces resistance to apoptosis in effector T cells,33 strongly supports a deletion-based mechanism similar to that reported for other veto cells.19,30,32,54
It has been suggested that the specificity for the HLA displayed by the CD34+ cells, shown by the ability to inhibit CTL-p against donor but not against a third party, is very similar to that documented by veto cells.30,54-59 Two types of veto cells that have been widely characterized are the CD8+ CTL32,60,61 and CD8+ BM cells.34,37,62 In both instances it has been shown that FasL is likely involved in the killing of the effector cells by the veto cells. However, our failure to reverse CD34+ cell–mediated regulatory activity by anti-Fas antibody led us to investigate the role of other death ligands, such as TNF-α and TGF-β. In contrast to other studies showing that CD2+CD3-CD8+CD16+ veto cells in the monkey bone marrow mediate their effect through TGF-β, our present study indicates that the regulatory activity of CD34+ cells is likely mediated by TNF-α and not by TGF-β.
Previous insights on the veto mechanism of CD8+ veto T cells have indicated that both CD8 and FasL on the veto cells might be required to induce specific deletion of the effector cells. Such a mechanism involves initial recognition of the veto cell by the TCR of the effector cell, leading to expression of Fas upon activation and thereby allowing for Fas-FasL apoptosis to take place once inhibitory molecules, such as Fas-associated death domain–like IL-1B–converting enzyme (FLICE)–inhibitory protein (FLIP), are down-regulated in the effector cell. The extra affinity required to maintain the interaction between the effector cell and the veto cell might be afforded through binding between CD8 on the veto cell and class I α3 domain on the effector cell. Considering that human CD34+ cells do not express CD8 molecules, our results indicate that the extra affinity afforded by CD8 on CD8+ veto cells could be provided by other adhesion molecules on the CD34+ cells. Preliminary results suggest that LFA-1–intercellular adhesion molecule-1 (LFA-1–ICAM-1) might be involved in this context.
Taken together, we suggest the following working hypothesis. Recognition of the donor stem cell by CTL-p through the interaction between the TCR and major histocompatibility complex (MHC) class I induces up-regulation of TNF receptor 1 (TNFR1) (p55) surface expression on the effector T cells. Likewise, signaling in the opposing “activated” CD34+ stem cell leads to up-regulation of membrane TNF-α (mTNF-α) or to the secretion of soluble TNF-α (sTNF-α). The aggregation of the TNFR on the CTL-p by TNF-α binding results in the induction of the caspase cascade (which can be blocked by BD-FMK), leading to apoptosis of the CTL-p. It could be that the TCR-MHC stimulation is also needed to transmit an “ability to die” signal permitting the occurrence of the caspase cascade. Considering that activated T cells are initially protected from apoptosis by inhibitory molecules such as FLIP, the contact between the CTL-p and the stem cell should persist until FLIP will be down-regulated. Such prolonged contact could be facilitated by adhesion molecules such as LFA-1–ICAM-1.
The recent observations that Sca-1+Lin- mouse early progenitors exhibit potent regulatory activity similarly to human CD34+ cells9-11 could enable further elucidation of the mechanism of action of regulatory stem cells. In particular, such studies could be facilitated by the availability of different knock-out mice or mice mutated in death molecules of the TNF family.
Prepublished online as Blood First Edition Paper, October 7, 2004; DOI 10.1182/blood-2002-11-3463.
Supported by National Institutes of Health (NIH), Therapy of CML PO1 grant CA49369, Project 6 Tolerance Induction by Megadose Stem Cell Transplants: Implications for GVHD and GVL; grants from Mrs E. Drake; and the Gabriella Rich Center for Transplantation Biology Research.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal