Abstract
The acute release of neutrophils from the bone marrow is a critical step in their trafficking to sites of inflammation. This process is stimulated by systemically acting inflammatory mediators, such as the CXC chemokines. In this study we have used a novel in situ perfusion system of the rat femoral bone marrow to directly investigate the role of specific adhesion molecules in chemokine-stimulated neutrophil mobilization. We show here that neutrophils mobilized in response to rat macrophage inflammatory protein-2 (MIP-2) shed l-selectin and expressed significantly higher levels of CD11b and CD49d. However, inhibition of l-selectin sheddase activity with KD-IX-73-4 had no effect on the number of neutrophils mobilized in response to rat MIP-2. Blockade of CD18, using a neutralizing monoclonal antibody (mAb), did not inhibit neutrophil mobilization but unexpectedly increased the rate and number of neutrophils released from the bone marrow in response to chemokine, suggesting that CD18 could play a role in neutrophil retention within the bone marrow. Blockade of CD49d using either a selective mAb or a specific antagonist resulted in a dramatic inhibition (> 75%) of the chemokine-stimulated neutrophil mobilization from the bone marrow. These data reveal contrasting roles for CD18 and CD49d in the retention and release of neutrophils from the bone marrow.
Introduction
There is a large storage pool of mature neutrophils within the bone marrow, termed the bone marrow reserve, which may be rapidly mobilized during inflammatory reactions, such as sepsis, ischemia reperfusion, and adult respiratory distress syndrome (ARDS). Release of neutrophils from the bone marrow results in a dramatic rise in circulating neutrophil numbers that thereby increases the number of neutrophils available for recruitment to sites of inflammation. The acute release of neutrophils from the bone marrow is therefore a critical step in their trafficking to sites of inflammation. Numerous inflammatory mediators, such as complement 5a (C5a), leukotriene B4 (LTB4), tumor necrosis factor α (TNF-α), and interleukin 8 (IL-8) have been shown to induce a blood neutrophilia by stimulating the release of neutrophils from the bone marrow reserve.1,2 In anatomical terms, neutrophils reside within the hematopoietic compartment of the bone marrow. Mobilization stimulated by blood-borne chemotactic factors therefore requires the migration of neutrophils out of the stroma and across the bone marrow sinusoidal endothelium in an abluminal-to-luminal direction to enter the circulation.3,4 The molecular mechanisms involved in this release process are poorly understood, although we have recently shown that CXCR4/CXCL12 interactions play an important role in neutrophil retention within the bone marrow.5 As adhesion molecules play a fundamental role in neutrophil trafficking from the blood into tissues, we reasoned that such adhesive interactions would also be critical for neutrophil release from the bone marrow. The aim of this study was, therefore, to identify which adhesion molecules play a role in this process.
To study the release process in isolation we have developed an in situ perfusion system of the rat femoral bone marrow. Using this system, the femoral bone marrow can be perfused by cannulation of the femoral artery, and leukocytes mobilized are collected via cannulation of the femoral vein. In this way we can directly and accurately assess the kinetics of neutrophil release and the absolute numbers of neutrophils released without the complications of neutrophil redistribution into other vascular compartments or trafficking into inflammatory sites. It has been reported that plasma levels of CXC chemokines rise during inflammatory reactions and contribute to the acute release of neutrophils from the bone marrow.6-11 Therefore, to mimic an inflammatory reaction we have infused a pulse of the CXC chemokine, rat macrophage inflammatory protein-2 (MIP-2) to stimulate an acute mobilization of neutrophils from the bone marrow.
Using this in situ perfusion system, for the first time, we have been able to directly investigate the functional importance of specific adhesion molecules in the mobilization process. Neutrophils mobilized during inflammatory reactions express reduced levels of CD62L (l-selectin) and elevated levels of CD11b (Mac1) and CD49d (very late antigen 4 [VLA4]), suggesting that these molecules may play a functional role in the release process.1,12-16 In this study we have examined whether the shedding of l-selectin is necessary for neutrophil release from the bone marrow and investigated the role of CD18 (β2 integrin subunit) and CD49d integrins in this process. Our results highlight the fact that the molecular mechanisms regulating neutrophil mobilization from the bone marrow are distinct from those involved in neutrophil extravasation from the blood into tissues.
Materials and methods
Animals
Male Sprague-Dawley rats (150-200 g) were obtained from Harlan Olac (Bicester, Oxfordshire, United Kingdom).
Materials
Rat recombinant MIP-2 was purchased from Biosource International (Oxford, United Kingdom) and PeproTech EC Ltd (London, United Kingdom). R-phycoerythrin (RPE)–conjugated anti–rat CD11a (WT.1, immunoglobulin G2a [IgG2a]) and CD11b (MRC OX-42, IgG2a) and nonbinding isotype-matched control monoclonal antibody (mAb; F-10-89-4) were purchased from Serotec (Oxford, United Kingdom). Fluorescein isothiocyanate (FITC)–conjugated anti–rat L-selectin (CD62L) (HRL-1) and isotype-matched control mAb were purchased from Biosource International. FITC-conjugated anti–rat VLA4 (CD49d) and isotype-matched control mAb were purchased from BD Biosciences (Heidelberg, Germany). Anti–CD18-integrin chain mAb, WT-3, IgG117 was a gift from Dr J Panes (Universidad de Barcelona, Spain), and isotype-matched control mAb was purchased from Sigma Chemical (Poole, United Kingdom). The l-selectin sheddase inhibitor, KD-IX-73-4, was a gift from Dr Takashi Kei Kishimoto, Boehringer Ingelheim (Ridgefield, CT).18 The specific anti-CD49d blocking mAb Max68P19 and CD49d antagonist CT562720 were gifts from Dr Tony Shock and Dr Roger Palframan at Celltech (Slough, United Kingdom).
Hanks balanced salt solution (HBSS) with and without Ca2+/Mg2+, phosphate-buffered saline (PBS), and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) were purchased from Life Technologies (Paisley, United Kingdom). Bovine serum albumin (BSA) was purchased from Sigma Chemical. Fentanyl citrate (Hypnorm; 0.315 mg/mL; fluanisone 10 mg/mL) was purchased from Janssen Pharmaceuticals (Oxford, United Kingdom). Midazolam (Hypnovel, 5 mg/mL) was purchased from Roche (Welwyn, United Kingdom). Heparin was purchased from Leo Laboratories Ltd (Bucks, United Kingdom). Sodium pentobarbitone (Expiral, 200 mg/mL) was purchased from May and Baker (Dagenham, United Kingdom). Fluorescence-activated cell sorting (FACS) lysing solution and FACS flow solution were purchased from Becton Dickinson (Franklin Lakes, NJ). Flow-count fluorospheres were purchased from Beckman Coulter (Bucks, United Kingdom). The Giemsa staining kit was purchased from BDH (Poole, United Kingdom).
Modified Krebs-Ringer bicarbonate buffer of the following composition was used in perfusion experiments: 10 mM d-Glucose, 2.50 mM CaCl2, 0.49 mM MgCl2 · 6H2O, 4.56 mM KCl, 120 mM NaCl, 0.7 mM Na2HPO4, and 24 mM NaHCO3, supplemented with Ficoll T-70 4% and BSA 0.1%.
In situ perfusion of the rat hind limb
The guinea pig and mouse hind limb perfusion technique previously described5,21,22 was adapted to a rat model. Briefly, rats were anesthetized, and the external iliac artery and veins were exposed. The following arteries and their satellite veins were ligated with 5/0 braided silk suture: caudal abdominal artery, superficial iliac circumflex artery, and pudendoepigastric trunk. The animals were killed with sodium pentobarbitone (Expiral; 250 mg/kg by cardiac puncture). Polyethylene cannulae (0.8 mm outer diameter [OD]; Portex, London, United Kingdom) were inserted into the femoral artery and vein and tied in. Modified Krebs-Ringer bicarbonate buffer was infused (1.8 mL/min) via the arterial cannula and removed from the venous cannula using a Minipuls peristaltic pump (Anachem, Luton, United Kingdom). Various test agents (chemokines, mAbs, and inhibitors) were infused at a rate of 0.07 mL/minute via a side port at the desired time using an infusion/withdrawal pump (Harvard Instruments, Edenbridge, United Kingdom). The final concentration of chemokines was calculated assuming a distribution into a total volume of 18.7 mL over the 10-minute infusion period. It should be noted that the final concentrations were not measured and are calculated assuming the chemokine is confined to the perfusion volume. Perfusate fractions were collected every 10 minutes onto ice. In some experiments a sample of the perfusate fraction was removed for analysis of adhesion molecule expression, as described in “Measurement of cell surface levels of adhesion molecules on neutrophils.” The remaining perfusate was centrifuged (200g for 10 minutes at 4°C). Erythrocytes present in the cell pellet were lysed using FACS lyse, and the leukocytes were then resuspended in FACS counting buffer for quantification of leukocyte numbers by flow cytometry. Cell counts were obtained by gating on the different leukocyte populations and the fluorospheres and acquiring events for a set time period of 30 seconds.
Measurement of cell surface levels of adhesion molecules on neutrophils
For preparation of bone marrow leukocytes from nonperfused femurs, male Sprague Dawley rats (150 g) were killed, the femurs were removed, and the femoral bone marrow was harvested by flushing with 5 mL HBSS buffer (HBSS without Ca2+/Mg2+ containing 30 mM HEPES and 0.25% BSA, pH 7.4). The erythrocytes were removed using hypotonic shock (addition of 10 mL 0.2% NaCl for 30 seconds followed by 10 mL 1.6% NaCl to restore isotonicity), and the leukocyte population was resuspended in HBSS buffer. Leukocytes collected onto ice from the in situ perfusion system were centrifuged (300g for 10 minutes at 4°C), and the cell pellet was resuspended at 2 × 106 leukocytes/mL in HBSS buffer. Leukocytes were incubated with mAb raised against l-selectin, CD11a, CD11b, and CD49d or an isotype-matched control mAb for 30 minutes on ice. Following washing, the cell pellet was resuspended in Cellfix for analysis using a FACScalibur flow cytometer. Bone marrow neutrophils were gated by the characteristic forward side scatter profile of these leukocytes using CellQuest (Becton Dickinson, San Jose, CA), as previously described.
Statistical analysis
The Mann-Whitney U test was used for 2 group comparisons in Figures 2C and 3F. The Kruskall-Wallis H test followed by a Noether test was used in all other figures to compare multiple groups to assess nonnormally distributed parameters. Data are presented as the mean ± standard error of the mean (SEM) for n experiments, except where stated.
Effect of inhibiting l-selectin sheddase on neutrophil mobilization from the bone marrow. The rat femoral bone marrow was perfused as before for a total of 100 minutes. At t = 10 minutes the bone marrow was infused with KD-IX-73-4 (50 μg/mL) or vehicle for 5 minutes, followed by a 10-minute infusion of vehicle or MIP-2 (1 nM). (A) Expression of l-selectin on neutrophils mobilized in response to vehicle or MIP-2 in the presence or absence of KD-IX-73-4 is shown by the solid histograms. Binding of an isotype-matched control mAb is shown by the dotted line. Histograms are representative of n = 3 to 7 separate perfusions with staining performed in triplicate. (B) Combined results are expressed as the mean of the specific geomeans ± SEMs. *P < .05. (C) The total number of neutrophils released during the 100-minute perfusion in the presence and absence of KD-IX-73-4 in response to vehicle or MIP-2, mean ± SEM (n = 4-7 separate perfusions). NS indicates not significant.
Effect of inhibiting l-selectin sheddase on neutrophil mobilization from the bone marrow. The rat femoral bone marrow was perfused as before for a total of 100 minutes. At t = 10 minutes the bone marrow was infused with KD-IX-73-4 (50 μg/mL) or vehicle for 5 minutes, followed by a 10-minute infusion of vehicle or MIP-2 (1 nM). (A) Expression of l-selectin on neutrophils mobilized in response to vehicle or MIP-2 in the presence or absence of KD-IX-73-4 is shown by the solid histograms. Binding of an isotype-matched control mAb is shown by the dotted line. Histograms are representative of n = 3 to 7 separate perfusions with staining performed in triplicate. (B) Combined results are expressed as the mean of the specific geomeans ± SEMs. *P < .05. (C) The total number of neutrophils released during the 100-minute perfusion in the presence and absence of KD-IX-73-4 in response to vehicle or MIP-2, mean ± SEM (n = 4-7 separate perfusions). NS indicates not significant.
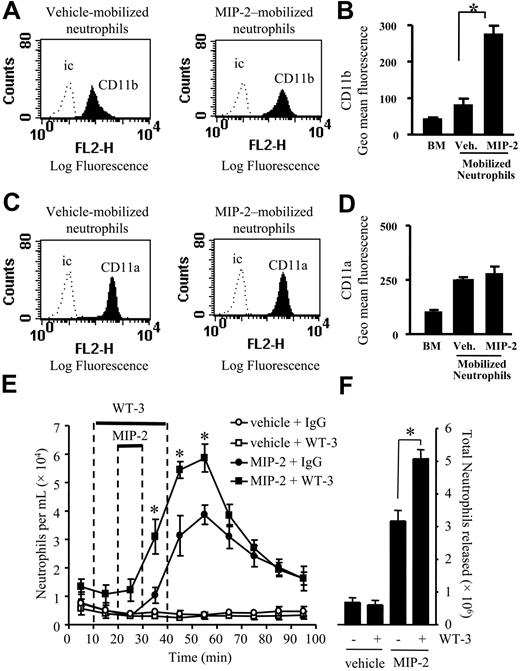
Effect of CD18 blocking mAb on neutrophil mobilization stimulated by MIP-2. (A-D) Cell surface expression of CD11b and CD11a was determined on neutrophils from a nonperfused bone marrow (BM) and those mobilized from the femoral bone marrow in response to a 10-minute infusion of vehicle (Veh.) or MIP-2 (1 nM) using the in situ perfusion system. (A,C) Expression of CD11b and CD11a is shown by the solid histogram, and binding of the isotype-matched control mAb (ic) is shown by the dotted line. Histograms are representative of n = 3 to 7 separate perfusions with staining performed in triplicate. (B,D) Combined results are expressed as the mean of the specific geomeans ± SEMs. (E,F) Using the in situ perfusion system the rat femoral bone marrow was infused with anti-CD18 mAb, WT-3 (10 μg/mL), or an isotype-matched control mAb (10 μg/mL) for 30 minutes, MIP-2 (1 nM) or vehicle were infused for 10 minutes, as indicated by the solid bars. (E) The kinetics of neutrophil mobilization (○, vehicle + IgG; □, vehicle + WT-3; •, MIP-2 + IgG; ▪, MIP-2 + WT-3) and (F) the total number of neutrophils mobilized, mean ± SEM (n = 4 separate perfusions). *P < .05 for all panels.
Effect of CD18 blocking mAb on neutrophil mobilization stimulated by MIP-2. (A-D) Cell surface expression of CD11b and CD11a was determined on neutrophils from a nonperfused bone marrow (BM) and those mobilized from the femoral bone marrow in response to a 10-minute infusion of vehicle (Veh.) or MIP-2 (1 nM) using the in situ perfusion system. (A,C) Expression of CD11b and CD11a is shown by the solid histogram, and binding of the isotype-matched control mAb (ic) is shown by the dotted line. Histograms are representative of n = 3 to 7 separate perfusions with staining performed in triplicate. (B,D) Combined results are expressed as the mean of the specific geomeans ± SEMs. (E,F) Using the in situ perfusion system the rat femoral bone marrow was infused with anti-CD18 mAb, WT-3 (10 μg/mL), or an isotype-matched control mAb (10 μg/mL) for 30 minutes, MIP-2 (1 nM) or vehicle were infused for 10 minutes, as indicated by the solid bars. (E) The kinetics of neutrophil mobilization (○, vehicle + IgG; □, vehicle + WT-3; •, MIP-2 + IgG; ▪, MIP-2 + WT-3) and (F) the total number of neutrophils mobilized, mean ± SEM (n = 4 separate perfusions). *P < .05 for all panels.
Results
MIP-2 stimulates a dose-dependent increase in neutrophil mobilization from the bone marrow
The femoral bone marrow was perfused with modified Krebs-Ringer bicarbonate buffer via the femoral artery, and 10-minute fractions were collected from the femoral vein. The perfusion was carried out for a total of 100 minutes with a 10-minute infusion of MIP-2 (148 or 444 ng) to give a final concentration of 1 or 3 nM, or vehicle (PBS/0.1% BSA) commencing after 20 minutes. A steady release of neutrophils (a total of 0.65 ± 0.12 × 106 in 100 minutes) was observed during perfusion under basal conditions (Figure 1). Infusion with MIP-2 induced a rapid and transient release of neutrophils into the perfusate. This peaked 30 minutes after starting the infusion of MIP-2 and declined rapidly thereafter (Figure 1). The release was dose-dependent with a total of 3.9 ± 0.5 × 106 and 9.1 ± 1.5 × 106 neutrophils released in response to the infusion of 1 and 3 nM MIP-2, respectively (Figure 1). Analysis of cytospin preparations revealed that the nuclei of the neutrophils released were both segmented and nonsegmented (data not shown).
Mobilization of neutrophils from the bone marrow stimulated by MIP-2. Using the in situ perfusion system the rat femoral bone marrow was infused with MIP-2 (1 and 3 nM) or vehicle (PBS/0/1% BSA) for 10 minutes as indicated by the solid bar (D). (A-B) Forward-scatter (FSC) and side-scatter (SSC) FACS plots of leukocytes mobilized from the bone marrow in response to infusion of vehicle or 1 nM MIP-2, respectively. (C) Gates used to determine neutrophil numbers: R1 gate for neutrophils, R2 gate for eosinophil, R3 gate for mononuclear cells, R4 gate for fluorescent beads. (D, left) The kinetics of neutrophil release (•, 3 nM MIP-2; ▴, 1 nM MIP-2; ○, vehicle) and (right) the total number of neutrophils released, mean ± SEM (n = 3-7 separate perfusions). *P < .05.
Mobilization of neutrophils from the bone marrow stimulated by MIP-2. Using the in situ perfusion system the rat femoral bone marrow was infused with MIP-2 (1 and 3 nM) or vehicle (PBS/0/1% BSA) for 10 minutes as indicated by the solid bar (D). (A-B) Forward-scatter (FSC) and side-scatter (SSC) FACS plots of leukocytes mobilized from the bone marrow in response to infusion of vehicle or 1 nM MIP-2, respectively. (C) Gates used to determine neutrophil numbers: R1 gate for neutrophils, R2 gate for eosinophil, R3 gate for mononuclear cells, R4 gate for fluorescent beads. (D, left) The kinetics of neutrophil release (•, 3 nM MIP-2; ▴, 1 nM MIP-2; ○, vehicle) and (right) the total number of neutrophils released, mean ± SEM (n = 3-7 separate perfusions). *P < .05.
l-selectin shedding is not required for neutrophil mobilization
To determine whether l-selectin shedding is required for neutrophil mobilization induced by MIP-2, the l-selectin sheddase inhibitor, KD-IX-73-4 (50 μg/mL),18 was infused into the femoral bone marrow for 5 minutes prior to a 10-minute infusion of MIP-2 (1 nM) or vehicle. The marrow was perfused for a total of 100 minutes. Samples of the neutrophils mobilized under basal conditions (vehicle) or in response to MIP-2 in the presence or absence of KD-IX-73-4 were collected onto ice, incubated with l-selectin–specific mAbs, and analyzed by flow cytometry. As shown in Figure 2A-B neutrophils released from the bone marrow under basal conditions expressed l-selectin. Shedding of l-selectin from the neutrophil cell surface was evident when neutrophils were mobilized by MIP-2 but was effectively inhibited by coinfusion of KD-IX-73-4 (Figure 2A-B). In contrast Figure 2C shows that under both basal conditions and in response to MIP-2, the infusion of KD-IX-73-4 had no effect on the total number of neutrophils mobilized from the bone marrow. Furthermore, no changes in the kinetics of release were recorded (data not shown).
Blockade of CD18 integrins increases neutrophil mobilization from the bone marrow
Expression of CD11a and CD11b was examined by FACS staining of neutrophils present in nonperfused bone marrow and neutrophils mobilized under basal conditions (vehicle), and in response to a 10-minute infusion of MIP-2 (1 nM) using the in situ perfusion system of the femoral bone marrow. While there was a marked up-regulation of CD11b on neutrophils mobilized from the bone marrow in response to MIP-2 (Figure 3A-B), levels of CD11a were not significantly different between the vehicle and MIP-2 mobilized neutrophils (Figure 3C-D).
To investigate the role of the CD18-integrins (CD11a/CD18 and CD11b/CD18) in neutrophil mobilization, we used the CD18-integrin blocking mAb, WT-3.17 Anti-CD18 mAb, or isotype-matched control mAb (10 μg/mL) was infused for 30 minutes, commencing 10 minutes prior to a 10-minute infusion of MIP-2 or vehicle. The basal release of neutrophils from the bone marrow was not altered by the infusion of anti-CD18 mAb (Figure 3B-C). However, the coinfusion of MIP-2 and anti-CD18 mAb caused a significant increase in the total number of neutrophils mobilized (Figure 3C). A detailed study of the mobilization kinetics showed that the release of neutrophils stimulated by MIP-2 was significantly increased by the coinfusion of anti-CD18 mAb from t = 30 to 60 minutes (Figure 3B).
CD49d plays a critical role in neutrophil mobilization stimulated by MIP-2
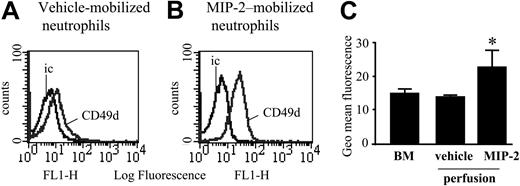
Expression of CD49d was examined by FACS staining of neutrophils present in nonperfused bone marrow and neutrophils mobilized under basal conditions or in response to a 10-minute infusion of MIP-2 (1 nM) using the in situ perfusion system of the femoral bone marrow. Levels of CD49d were significantly increased on neutrophils mobilized from the bone marrow in response to MIP-2 (Figure 4).
CD49d levels on blood, bone marrow, and MIP-2–mobilized neutrophils. Cell surface expression of CD49d on neutrophils from a nonperfused bone marrow, and those mobilized from the perfused femoral bone marrow following a 10-minute infusion of either vehicle or MIP-2 (1 nM). (A-B) Expression of CD49d and the isotype-matched control mAb (ic) is shown. Histograms are representative of n = 3 to 5 separate perfusions with staining performed in triplicate. (C) Combined results are expressed as the mean of the specific geomeans ± SEM (n = 3-5 separate rats or perfusions). *P < .05.
CD49d levels on blood, bone marrow, and MIP-2–mobilized neutrophils. Cell surface expression of CD49d on neutrophils from a nonperfused bone marrow, and those mobilized from the perfused femoral bone marrow following a 10-minute infusion of either vehicle or MIP-2 (1 nM). (A-B) Expression of CD49d and the isotype-matched control mAb (ic) is shown. Histograms are representative of n = 3 to 5 separate perfusions with staining performed in triplicate. (C) Combined results are expressed as the mean of the specific geomeans ± SEM (n = 3-5 separate rats or perfusions). *P < .05.
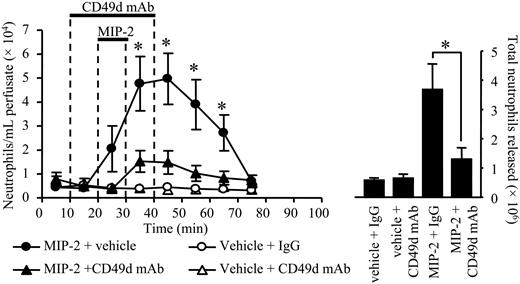
Two approaches were taken to examine the effect of CD49d blockade on neutrophil mobilization from the bone marrow. First, specific CD49d blocking mAb (Max 68P)19 or an isotype-matched control mAb (10 μg/mL) was infused into the bone marrow via the femoral artery for 30 minutes, commencing 10 minutes prior to a 10-minute infusion of MIP-2 (1 nM) or vehicle (PBS/0.1% BSA). As shown in Figure 5, infusion of the CD49d blocking mAb caused a marked inhibition of neutrophil release stimulated by MIP-2, while having no effect on the basal release of neutrophils. In terms of the total number of neutrophils mobilized by MIP-2, the CD49d blocking mAb inhibited release by 78%. Second, the femoral bone marrow was infused with the selective CD49d antagonist CT562720 (200 nM) for 90 minutes, commencing 20 minutes prior to a 10-minute infusion of MIP-2 (10 nM) or vehicle (PBS/0.1% BSA). As shown in Figure 6, the CD49d antagonist had no effect on the basal release of neutrophils, but significantly inhibited the release of neutrophils stimulated by MIP-2. In terms of the total number of neutrophils mobilized by MIP-2, the CD49d antagonist inhibited release by 86%.
Effect of anti-CD49d mAb on neutrophil mobilization stimulated by MIP-2. Using the in situ perfusion system of the rat femoral bone marrow, anti-CD49d mAb, Max68P, (10 μg/mL) or an isotype-matched control mAb (10 μg/mL) was infused for a total of 30 minutes, and MIP-2 (1 nM) or vehicle (PBS/0/1% BSA) was infused for 10 minutes, as indicated by the solid bars. (Left) The kinetics of neutrophil mobilization (•, MIP-2 + vehicle; ▴, MIP-2 + anti-CD49d mAb; ○, vehicle + IgG; ▵, vehicle + CD49d mAb) and (right) the total number of neutrophils mobilized are shown on the right-hand axis, mean ± SEM (n = 5 separate perfusions). *P < .05.
Effect of anti-CD49d mAb on neutrophil mobilization stimulated by MIP-2. Using the in situ perfusion system of the rat femoral bone marrow, anti-CD49d mAb, Max68P, (10 μg/mL) or an isotype-matched control mAb (10 μg/mL) was infused for a total of 30 minutes, and MIP-2 (1 nM) or vehicle (PBS/0/1% BSA) was infused for 10 minutes, as indicated by the solid bars. (Left) The kinetics of neutrophil mobilization (•, MIP-2 + vehicle; ▴, MIP-2 + anti-CD49d mAb; ○, vehicle + IgG; ▵, vehicle + CD49d mAb) and (right) the total number of neutrophils mobilized are shown on the right-hand axis, mean ± SEM (n = 5 separate perfusions). *P < .05.
Effect of a CD49d antagonist on neutrophil mobilization stimulated by MIP-2. Using the in situ perfusion system of the rat femoral bone marrow, the CD49d antagonist CT5627 (200 nM) or vehicle was infused for a total of 80 minutes, and MIP-2 (1 nM) or vehicle (PBS/0/1% BSA) was infused for 10 minutes, as indicated by the solid bars. (Left) The kinetics of neutrophil mobilization from the perfused femoral bone marrow (•, MIP-2 alone; ▴, MIP-2 + CT5627; ○, vehicle; ▵, vehicle + CT5627) and (right) the total number of neutrophils mobilized are shown on the right-hand axis, mean ± SEM (n = 5 separate perfusions). *P < .05 for both panels.
Effect of a CD49d antagonist on neutrophil mobilization stimulated by MIP-2. Using the in situ perfusion system of the rat femoral bone marrow, the CD49d antagonist CT5627 (200 nM) or vehicle was infused for a total of 80 minutes, and MIP-2 (1 nM) or vehicle (PBS/0/1% BSA) was infused for 10 minutes, as indicated by the solid bars. (Left) The kinetics of neutrophil mobilization from the perfused femoral bone marrow (•, MIP-2 alone; ▴, MIP-2 + CT5627; ○, vehicle; ▵, vehicle + CT5627) and (right) the total number of neutrophils mobilized are shown on the right-hand axis, mean ± SEM (n = 5 separate perfusions). *P < .05 for both panels.
Discussion
Adhesion molecules play a fundamental role in regulating neutrophil trafficking from the blood into tissues; however, little is known about the role of these molecules in the mobilization of neutrophils from the bone marrow into the blood. In this study, we have investigated the dynamic changes in adhesion molecule expression associated with the acute release of neutrophils from the bone marrow and examined the functional role of specific adhesion molecules in the mobilization process.
We used an infusion of the glutamate-leucine-arginine (ELR+) CXC chemokine, rat MIP-2, to model the acute neutrophilia associated with disease states such as acute sepsis, trauma, and ischemia reperfusion injury, which are associated with increased plasma CXC chemokine levels and a concomitant blood neutrophilia.6-11 Experiments were performed using an in situ perfusion system of the rat femoral bone marrow. Thus, the chemokine rat MIP-2 was infused directly into the femoral bone marrow via the femoral artery, and mobilized leukocytes were collected via cannulation of the femoral vein. In these experiments MIP-2 was shown to stimulate a dose-dependent, rapid release of neutrophils from the bone marrow.
Using this system we assessed changes in adhesion molecule expression on neutrophils mobilized directly from the bone marrow. These experiments revealed that neutrophils mobilized acutely in response to MIP-2 shed l-selectin and expressed significantly higher levels of both CD11b and CD49d, as compared with neutrophils mobilized under basal conditions. In contrast, levels of CD11a expression did not change. These results are consistent with experimental and clinical data showing that, during inflammatory episodes, circulating neutrophils express low levels of l-selectin and elevated levels of CD11b and CD49d.1,12-16 Further, the same changes in adhesion molecule expression have previously been reported following stimulation of neutrophils with chemotactic factors in vitro.23,24 Therefore, using the in situ perfusion system of the rat femoral bone marrow we sought to investigate the functional significance of these changes with respect to the mobilization process.
Selectins play an important role in mediating the first step in neutrophil trafficking from the blood into tissues, namely the tethering and rolling of leukocytes along the endothelium. Further, l-selectin shedding has been shown to regulate the velocity of neutrophils rolling along the endothelium.18 Thus, when shedding is inhibited the rolling velocity is reduced, and leukocyte recruitment from the blood into inflammatory sites is enhanced.18 Importantly, l-selectin shedding does not appear to be required for transendothelial migration of leukocytes that occurs during their extravasation from the blood into tissues (ie, in a luminal to abluminal direction).25 Mobilization of neutrophils from the bone marrow requires their migration across the bone marrow sinusoidal endothelium in an abluminal to luminal direction. It is unlikely that this process involves neutrophil rolling. However, it is commonly observed that under inflammatory conditions in both man and animals neutrophils rapidly mobilized from the bone marrow express very low levels of l-selectin.1,14,15 Furthermore, in a previous study a detailed immunohistochemical analysis of the bone marrow showed that neutrophils shed l-selectin as they were mobilized.26 Taken together these data led to the hypothesis that l-selectin was a retention factor for bone marrow neutrophils and that shedding of this molecule was a prerequisite for neutrophil mobilization. In this study, using a l-selectin sheddase inhibitor, we have prevented l-selectin shedding from neutrophils as they are mobilized and shown that this does not inhibit their release. Thus, we can conclude that l-selectin shedding is not required for neutrophil mobilization. Furthermore, our data do not support the hypothesis that l-selectin is a retention factor for bone marrow neutrophils.
To investigate the role of CD18 integrins in the release process we used the CD18-neutralizing mAb, WT-3. Infusion of WT-3 did not inhibit MIP-2–stimulated release of neutrophils from the bone marrow. This is the first direct demonstration that neutrophil release from the bone marrow is not inhibited by blockade of CD18. This finding is consistent with previous publications showing that the blood neutrophilia stimulated by an intravenous injection of lipopolysaccharide (LPS), C5a, or TNF-α is not inhibited by mAbs that block CD18.1,27 Unexpectedly, our data indicated that blockade of CD18 actually enhances the rate of neutrophil release from the bone marrow in response to MIP-2. The mechanism underlying this effect is unclear, but one explanation is that neutrophils are normally retained in the bone marrow in a CD18-dependent manner. Interestingly, a recent report has provided evidence that the retention of hemopoietic progenitor cells in the bone marrow is dependent on CD11b:CD18.28 Indeed hemopoietic progenitor cell mobilization in response to a variety of factors was shown to be enhanced by CD11b blockade and in CD11b-deficient mice.28 It is possible therefore that CD11b:CD18 plays a fundamental role in the retention of both immature and mature leukocytes within the bone marrow.
Rodent neutrophils express the α-4 integrin subunit, CD49d, and multiple studies have now established a role for CD49d-dependent adhesion in neutrophil recruitment into tissues during specific inflammatory reactions.23,29-32 Expression of CD49d on neutrophils is up-regulated in vivo during specific inflammatory reactions and in vitro when neutrophils migrate in response to chemoattractants.12,13,23 In this study we report that expression of CD49d is significantly increased as neutrophils are mobilized from the bone marrow. Moreover, we show here, for the first time, that neutrophil mobilization in response to an inflammatory stimulus, MIP-2, is CD49d dependent. Thus, using either a specific CD49d blocking mAb or a selective CD49d antagonist, chemokine-stimulated neutrophil mobilization was inhibited by 78% and 86%, respectively. The bone marrow sinusoidal endothelium and stromal elements express vascular cell adhesion molecule-1 (VCAM-1) constitutively.33,34 Indeed CD49d/VCAM-1 interactions have been shown to play an important role in the homing of progenitor cells back to the bone marrow.35,36 It has previously been shown that CD49d expressed on neutrophils mediates interactions with VCAM-129 ; therefore, it is likely that neutrophil release is VCAM-1 dependent. However, there is also a precedent in the literature, in a model of chronic vasculitis, for CD49d/CD29-mediated neutrophil adhesion to be independent of VCAM-1.32 At present, therefore, we do not know the ligand mediating the CD49d-mediated neutrophil mobilization. Furthermore, it has yet to be established whether the observed effect is due to inhibition of CD49d/CD29 or CD49d/beta7.
In addition to investigating the chemokine-stimulated acute mobilization of neutrophils from the bone marrow, the perfusion system allows us to monitor the basal, constitutive release of neutrophils from the bone marrow. Interestingly, when these results are considered it is clear that basal release of neutrophils from the bone marrow is independent of l-selectin shedding, CD18 or CD49d integrins. Thus, the mechanisms regulating the constitutive release of neutrophils are clearly distinct from those regulating the stimulated release.
In conclusion, our data show that l-selectin shedding is not required for neutrophil mobilization from the bone marrow and reveals contrasting roles for CD18 and CD49d in this process. This study shows, for the first time, that the role of specific adhesion molecules in neutrophil egress from the bone marrow is fundamentally different to their role in recruitment from the blood into tissues.
Prepublished online as Blood First Edition Paper, November 12, 2004; DOI 10.1182/blood-2004-08-3193.
Supported by grants from the Wellcome Trust.
P.C.E.B. and C.M. contributed equally to the data presented in this manuscript.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal