Abstract
Dendritic cells (DCs) are being evaluated for cancer immunotherapy due to their unique ability to induce tumor-directed T-cell responses. Here we report that the type of human DC, the mode of activation, and the strategy for delivery of antigen are 3 critical factors for efficient stimulation of tumor-specific CD8+ and CD4+ T cells. Only CD1c+ blood DCs and monocyte-derived DCs (MoDCs) were capable of presenting epitopes of the full-length tumor antigen NY-ESO-1 on both major histocompatibility complex (MHC) class I (cross-presentation) and MHC II, whereas plasmacytoid DCs were limited to MHC II presentation. Cross-presentation was inefficient for soluble protein, but highly efficient for antigen-antibody immune complexes (NY-ESO-1/IC) and for protein formulated with ISCOMATRIX adjuvant (NY-ESO-1/IMX). DC activation with CD40L further enhanced cross-presentation efficiency. The mode of antigen delivery was found to be a determining factor for cytosolic proteolysis by DCs. Immune complexes (ICs) targeted a slow, proteasome-dependent cross-presentation pathway, whereas ISCOMATRIX (IMX) targeted a fast, proteasome-independent pathway. Both cross-presentation pathways resulted in a long-lived, T-cell stimulatory capacity, which was maintained for several days longer than for DCs pulsed with peptide. This may provide DCs with ample opportunities for sensitizing tumor-specific T cells against a broad array of tumor antigen epitopes in lymph nodes.
Introduction
To achieve tumor cell killing by cytotoxic CD8+ T cells (CTLs), cancer vaccines target major histocompatibility complex (MHC) class I–restricted epitopes. CTL responses alone may not be sufficient for effective anticancer immunity, and additional help from CD4+ T cells is required for optimal CTL priming and memory induction.1-4 Avariety of vaccine strategies are being developed to generate an integrated CD4+ and CD8+ T-cell response. One strategy uses dendritic cells (DCs), which have the unique capacity to not only present exogenous antigen on MHC II, but also to “cross-present” these on MHC I.5 DC-based clinical trials have demonstrated “proof of concept” using primary DCs isolated directly from peripheral blood or DCs generated in vitro from monocytes (MoDCs) or CD34+ progenitors, pulsed with MHC I–restricted peptides (reviewed in Davis et al6 ). We and others have previously studied the functional profiles of different DC populations providing valuable insights into their potential clinical utility.7-9 Despite this, the rational design of DC-based cancer vaccines still lacks some critical information. What are the optimal approaches to achieve antigen presentation on both MHC I and MHC II? Which DC population is best suited for this purpose? How should these DCs be matured or activated?
We have used the tumor antigen NY-ESO-1 as a model antigen to address these questions. NY-ESO-1 is a 180 amino acid protein, which is absent in normal tissues apart from testis, but is expressed in a variety of common human cancers including melanoma, breast, lung, prostate tumors, and others.10,11 Patients with NY-ESO-1–expressing tumors frequently develop immune responses to this antigen, which are characterized by antibodies as well as T-cell responses.12-15 Numerous MHC I– and MHC II–restricted epitopes have been identified and T-cell responses against these epitopes have been induced with peptide vaccination.16 In contrast to vaccinating with defined peptides (with or without DCs), which restrict the T-cell repertoire, the advantage of using full-length protein is the potential to induce both CD8+ and CD4+ T-cell responses against multiple known as well as unknown epitopes. Although the delivery of protein-based vaccines provides a broader spectrum of MHC I and II epitopes, cross-presentation of soluble protein antigen by DCs is highly inefficient. Cross-presentation efficiency can be enhanced by targeting the antigen to Fcγ receptors (FcγRs) with antigen-antibody immune complexes (ICs).17-20 An alternative approach is to formulate the antigen with ISCOMATRIX (ISCOTEC AB, CSL Limited, Parkville, Victoria, Australia) adjuvant (IMX), which is being developed for use in human vaccines. IMX is based on the immuno-stimulatory complexes (ISCOM) technology, which combines an efficient antigen delivery system with the immuno-stimulatory activity of saponin. IMX can be combined with antigens to form vaccines that have been shown to promote humoral and cellular immune responses in a variety of experimental animal models (reviewed in Sjolander et al21 ). Formulating NY-ESO-1 with IMX induced tumor-specific CTLs and tumor protection in murine tumor models.22 In addition, a recently completed phase 1 clinical trial using an NY-ESO-1/IMX vaccine induced both antibody and CTL responses against multiple NY-ESO-1 epitopes in the majority of cancer patients.23
By studying presentation of MHC I and II epitopes from full-length NY-ESO-1 protein formulations to NY-ESO-1–specific CD8+ and CD4+ T-cell lines, we found that human DC populations differ substantially in their antigen-presenting capacities and requirement for maturation-inducing stimuli. In addition, we found that not the antigen itself, but the mode of antigen delivery, was a determining factor for cytosolic proteolysis by DCs, which occurred via 2 distinct cytosolic protease systems. These studies establish a rationale for developing effective DC-based tumor vaccines using recombinant protein antigens.
Materials and methods
Generation of recombinant NY-ESO-1 formulations
Full-length NY-ESO-1 protein was produced in E coli and purified under cGMP conditions.47 Endotoxin levels of NY-ESO-1 protein batches ranged between 3 EU/0.1 mg and 31 EU/0.1 mg protein (limit < 175 EU/0.1 mg protein). NY-ESO-1 was reactive with anti–NY-ESO-1 mAbs E978 and ES121,12 by Western blot analysis. Immune complexes (NY-ESO-1/IC) were generated by mixing NY-ESO-1 protein with anti–NY-ESO-1 mAb at a 1:1 molar ratio in serum-free RPMI at 37°C for 30 minutes.20 Alternatively, serum from a patient with high Ab titers against NY-ESO-1 was used. ES121, which induced the highest T-cell interferon γ (IFN-γ) response, was used for NY-ESO-1/IC formation when not specified otherwise. IMX-formulated NY-ESO-1 was generated as described.24 The protein concentration was determined by amino acid analysis.
Generation of NY-ESO-1–specific human CD8+ and CD4+ T-cell lines
A CD8+ T-cell line specific for NY-ESO-1157-165 was established from vaccine-infiltrating lymphocytes of a peptide-vaccinated patient with melanoma. Punch biopsy specimens were obtained from a strong skin reaction site and finely minced with a surgical scalpel. Informed consent was provided according to the Declaration of Helsinki. The cell suspension was sensitized with irradiated autologous peripheral blood mononuclear cells (PBMCs) pulsed with 10 μg/mL NY-ESO-1157-165 peptide. A CD4+ T-cell line specific for the NY-ESO-1 epitope NY-ESO-1157-170, restricted to HLA-DP4+, was established from PBMCs of another patient by limiting dilution. Both T-cell lines were maintained in medium containing 10 IU/mL interleukin 2 (IL-2; Cetus, Emeryville, CA) and restimulated with peptides every 7 to 10 days. The percentage of NY-ESO-1–specific T cells ranged between 10% and 60% for CD8+ T cells and 70% and 95% for CD4+ T cells. Clinical trial protocols were sponsored by the Ludwig Institute for Cancer Research, were approved by the institute's Protocol Review, complied with the guidelines of the National Health and Medical Research Council (NH&MRC), and were approved by the Human Research and Ethics Committee of Austin Health.
Flow cytometric analysis of cell-surface phenotype
The following fluorocrome-conjugated mAbs were purchased from PharMingen (San Diego, CA): CD1c, CD3, CD4, CD8, CD14, CD19, CD20, CD83, CD86, CD123, HLA-DR, HLA-ABC; BDCA-2 and BDCA-4 mAbs were obtained from Miltenyi Biotec, Auburn, CA. The clone BB7.2 (American Type Culture Collection [ATCC], Manassas, VA) was used to screen PBMCs for HLA-A2 expression. Staining was carried out according to standard techniques and flow cytometry analysis was performed with a FACSCalibur (Beckon Dickinson, San Jose, CA).
DC isolation and culture
PBMCs from HLA-A2+ volunteers were prepared by Ficoll-Paque density gradient centrifugation. Monocytes, CD1c+ PBDCs, plasmacytoid DCs, and B cells were isolated by positive selection using magnetic beads against CD14, CD1c, BDCA-4, and CD19, respectively (Miltenyi Biotec). Positive selection was repeated to obtain a purity equal to 96% for each cell type. CD1c+ PBDCs and plasmacytoid DCs were cultured with granulocyte macrophage–colony-stimulating factor (GM-CSF) and IL-3, respectively, as previously described.8 MoDCs were generated by culturing CD14+ cells with GM-CSF and IL-4 for 6 to 7 days. Cell cultures were maintained in RPMI 1640 supplemented with 20 mM HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid), 60 mg/L penicillin G, 12.6 mg/L streptomycin, 2 mM l-glutamine, 1% nonessential amino acids, and 10% heat-inactivated fetal calf serum (FCS).
Antigen pulsing and DC maturation
DCs (5 × 105/mL) were incubated with NY-ESO-1 protein, NY-ESO-1/IC, or NY-ESO-1/IMX for 2 hours at 37°C, washed, and cultured overnight in the absence or presence of specific stimuli: soluble CD40L trimer (1 μg/mL, kindly provided by Immunex, Seattle, WA), E coli–derived lipopolysaccharide [LPS] (100 ng/mL; Sigma, St Louis, MO), CpG oligodeoxynucleotide (ODN) 2006 or ODN2216 (6 μg/mL), R-848 (1 μg/mL; InvivoGen, San Diego, CA), or a combination of tumor necrosis factor α (TNF-α), IL-1β, IL-6 (10 ng/mL each; PeproTech, Rocky, NJ), and prostaglandin E2 (PGE2; 1 μg/mL; Sigma). DCs pulsed with the peptides NY-ESO-1157-165 (SLLMWITQC) or NY-ESO-1157-170 (SLLMWITQCFLPVF) (Auspep, Parkville, Victoria, Australia) served as positive controls. The NY-ESO-1157-165 peptide was treated with 500 μM of Tris (2-carboxyethyl)-phosphine hydrochloride (TCEP; Pierce Endogen, Rockford, IL) for 60 minutes before use to prevent dimerization of the cysteine residues.
Antigen presentation kinetic studies
For assessing MHC I and II presentation kinetics, MoDCs were pulsed with NY-ESO-1 protein, NY-ESO-1/IMX, NY-ESO-1/IC (each 10 μg/mL), or preformed peptide, washed, and subsequently matured with CD40L. These MoDCs were cultured for various periods before assessing their capacity to stimulate CD4+ and CD8+ T cells. T-cell assays were performed in the presence of brefeldin A, which abrogated further transport of peptide/MHC complexes to the cell surface during the 4-hour assay period.
Inhibition of antigen processing
Where indicated, DCs were incubated with the following inhibitors 45 minutes prior to antigen pulsing: lactacystin (clasto-lactacystin β-lactone), epoxomicin, and brefeldin A (all from Sigma); concanamycin B (gift from Dr J. Villadangos, Walter and Eliza Hall Institute, Parkville, Victoria, Australia); Vaccinia virus encoding OVA (Vac-OVA) or ICP-47 (Vac-ICP-47) (gift from Drs. J. Yewdell and J. Bennink, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD). DCs were incubated with vaccinia virus at a multiplicity of infection (MOI) of 3 and 6 at 37°C for 1 hour in RPMI without serum. Infected DCs were washed 3 times in RPMI/10% FCS and pulsed with NY-ESO-1 formulations 3 hours later.
Antigen presentation assays
IFN-γ production by CD4+ or CD8+ T cells was assessed by either intracellular cytokine staining (ICS) or by enzyme-linked immunospot (ELISpot) assay. For ICS, 1 × 105 DCs and 1 × 104 to 2 × 104 T cells were incubated for 4 hours in 200 μL RPMI/10% FCS in 96-well round-bottom plates in the presence of 10 μg/mL brefeldin A. Cells were washed and stained with anti-CD4 or anti-CD8 mAbs for 20 minutes at 4°C, fixed with 1% paraformaldehyde, and stained with anti–IFN-γ mAb (Beckon Dickinson) in a 0.25% saponin buffer. Samples were analyzed by flow cytometry and data were analyzed using FloJo software (version 3.4; Tree Star, San Carlos, CA). For ELISpot assays, ELISpot plates (MAHA S45; Millipore Multiscreen, Bedford, MA) were coated with 5 μg/mL anti–IFN-γ mAb (CSL Limited) overnight at 4°C and blocked with PBS/10% FCS. DCs were cocultured with CD8+ T cells for 16 to 18 hours. Subsequently, cells were removed and plates washed with water and incubated with anti–IFN-γ rabbit polyclonal Ab (CSL Limited). After washing with PBS/0.05% Tween 20, AEC substrates (Sigma) were added and color development was stopped after 6 to 8 minutes.
Results
Delivering NY-ESO-1 as an IC or with IMX induces efficient cross-presentation and MHC II presentation by MoDCs
MoDCs have previously been shown to cross-present NY-ESO-1 immune complexes but not protein alone to CD8+ T cells.20 To determine the influence of different protein formulations on cross-presentation efficiency, we pulsed MoDCs from an HLA-A2+ cancer patient with NY-ESO-1 protein alone, NY-ESO-1 immune complexes (NY-ESO-1/IC), or IMX-formulated NY-ESO-1 protein (NY-ESO-1/IMX), each of those containing identical amounts of antigen. These DCs were examined for their ability to stimulate IFN-γ production (ELISpot) by an autologous CD8+ T-cell line, which recognizes the epitope NY-ESO-1157-165 in the context of HLA-A2 (Figure 1A). Cross-presentation by MoDCs pulsed with NY-ESO-1 protein alone was inefficient. In contrast, formulating NY-ESO-1 as an IC enhanced cross-presentation efficiency. Formulating NY-ESO-1 protein with IMX was also a very effective strategy for inducing cross-presentation by MoDCs. Similar results were obtained with MoDCs from an HLA-A2+-matched healthy donor using an intracellular cytokine staining assay for IFN-γ (Figure 1B). In order to optimize immune complex formation, we compared 2 anti–NY-ESO-1 mAbs, ES121 and E978, with serum from a clinical trial participant who had a high antibody titer against NY-ESO-1. All 3 NY-ESO-1/IC formulations were cross-presented to CD8+ T cells (Figure 1C). ES121, which induced the highest IFN-γ response, was used in subsequent experiments.
Cross-presentation of NY-ESO-1 formulations by MoDCs. (A) MoDCs of an HLA-A2+ patient with melanoma were pulsed with 10 μg/mL of either NY-ESO-1 protein, NY-ESO-1/IC (ES121), NY-ESO-1/IMX, or IMX alone and cultured overnight before coculture with autologous NY-ESO-1157-165–specific CD8+ T cells. IFN-γ–producing T cells were quantified by ELISpot assay. Peptide-pulsed MoDCs served as a positive control. (B) MoDCs from an HLA-A2+ healthy donor were pulsed with NY-ESO-1 formulations as described for panel A. Induction of IFN-γ production by NY-ESO-1–specific CD8+ T cells was quantified by intracellular cytokine staining (ICS). Data are representative of 6 experiments. (C) Comparison of 3 different NY-ESO-1/IC formulations generated by incubating NY-ESO-1 protein with anti–NY-ESO-1 mAbs, ES121 or E978, or with serum from a patient with high serum titers of anti–NY-ESO-1 antibodies. Induction of IFN-γ production by NY-ESO-1–specific CD8+ T cells was quantified by ICS. Data are representative of 3 experiments.
Cross-presentation of NY-ESO-1 formulations by MoDCs. (A) MoDCs of an HLA-A2+ patient with melanoma were pulsed with 10 μg/mL of either NY-ESO-1 protein, NY-ESO-1/IC (ES121), NY-ESO-1/IMX, or IMX alone and cultured overnight before coculture with autologous NY-ESO-1157-165–specific CD8+ T cells. IFN-γ–producing T cells were quantified by ELISpot assay. Peptide-pulsed MoDCs served as a positive control. (B) MoDCs from an HLA-A2+ healthy donor were pulsed with NY-ESO-1 formulations as described for panel A. Induction of IFN-γ production by NY-ESO-1–specific CD8+ T cells was quantified by intracellular cytokine staining (ICS). Data are representative of 6 experiments. (C) Comparison of 3 different NY-ESO-1/IC formulations generated by incubating NY-ESO-1 protein with anti–NY-ESO-1 mAbs, ES121 or E978, or with serum from a patient with high serum titers of anti–NY-ESO-1 antibodies. Induction of IFN-γ production by NY-ESO-1–specific CD8+ T cells was quantified by ICS. Data are representative of 3 experiments.
Next, we assessed whether these NY-ESO-1 formulations also induce MHC II presentation. Antigen-pulsed MoDCs were cocultured with a CD4+ T-cell line, which recognizes the epitope NY-ESO-1157-170 in the context of HLA-DP4 (Figure 2C). In contrast to cross-presentation, MHC II presentation was highly efficient for NY-ESO-1 protein alone and NY-ESO-1/IMX did not confer an advantage over protein alone. Most efficient CD4+ T-cell activation was induced with NY-ESO-1/IC.
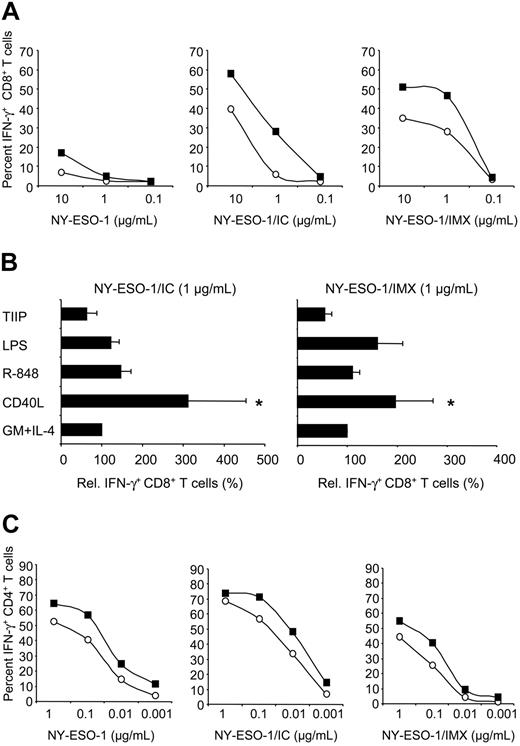
Mode of antigen delivery and DC activation influence cross-presentation and MHC II presentation efficiency. MoDCs from an HLA-A2+ donor were pulsed with NY-ESO-1 protein, NY-ESO-1/IC (mAb ES121), or NY-ESO-1/IMX at the indicated concentrations (referring to antigen content) and cultured overnight in media containing GM-CSF and IL-4 in the absence or presence of CD40L, LPS, or R-848, or a combination of TNF-α, IL-1β, IL-6, and PGE2 (TIIP). Antigen-pulsed MoDCs were cocultured with NY-ESO-1–specific T cells and induction of IFN-γ production by CD8+ T cells (A-B) or CD4+ T cells (C) was quantified by intracellular cytokine staining assay (ICS). (A,C) ○ indicates GM-CSF + IL-4 only; ▪, CD40L. Data in panels A and C are representative of 9 experiments using MoDCs from different donors. *P < .01. In panel B, CD8+ T-cell IFN-γ induced by MoDCs cultured with GM-CSF and IL-4 only was normalized to 100%. Data are mean plus or minus standard deviation (SD) of 6 to 10 experiments.
Mode of antigen delivery and DC activation influence cross-presentation and MHC II presentation efficiency. MoDCs from an HLA-A2+ donor were pulsed with NY-ESO-1 protein, NY-ESO-1/IC (mAb ES121), or NY-ESO-1/IMX at the indicated concentrations (referring to antigen content) and cultured overnight in media containing GM-CSF and IL-4 in the absence or presence of CD40L, LPS, or R-848, or a combination of TNF-α, IL-1β, IL-6, and PGE2 (TIIP). Antigen-pulsed MoDCs were cocultured with NY-ESO-1–specific T cells and induction of IFN-γ production by CD8+ T cells (A-B) or CD4+ T cells (C) was quantified by intracellular cytokine staining assay (ICS). (A,C) ○ indicates GM-CSF + IL-4 only; ▪, CD40L. Data in panels A and C are representative of 9 experiments using MoDCs from different donors. *P < .01. In panel B, CD8+ T-cell IFN-γ induced by MoDCs cultured with GM-CSF and IL-4 only was normalized to 100%. Data are mean plus or minus standard deviation (SD) of 6 to 10 experiments.
Effect of DC maturation on cross-presentation and MHC II presentation efficiency
Upon activation, DCs undergo a program termed maturation, transforming them from cells specialized in antigen uptake into cells that migrate into regional lymph nodes to stimulate T cells. Several classes of stimuli inducing DC activation have been identified, including microbial products (eg, TLR ligands), proinflammatory mediators, and membrane-bound molecules of the TNF family expressed by activated T cells (eg, CD40L). To assess the influence of DC maturation on cross-presentation efficiency, we activated antigen-pulsed MoDCs with CD40L, or LPS (TLR4 ligand), or R-848 (TLR7/TLR8 ligand), or proinflammatory cytokines (a combination of TNF-α, IL-1β, IL-6, and PGE2). Despite the induction of similar surface expression levels of the activation markers CD83, CD86, and HLA-DR by all of these stimuli (data not shown), only CD40L significantly enhanced cross-presentation efficiency (9 of 9 donors for 1 μg/mL NY-ESO-1/IC, *P < .01; Figure 2A-B). Thus, cross-presenting function is not up-regulated by DC maturation per se, but rather is dependent on the class of the maturation stimulus. Activation of MoDCs with CD40L also enhanced MHC II presentation efficiency, but this effect was less pronounced than for cross-presentation (Figure 2C).
Influence of antigen formulation on antigen-presentation kinetics
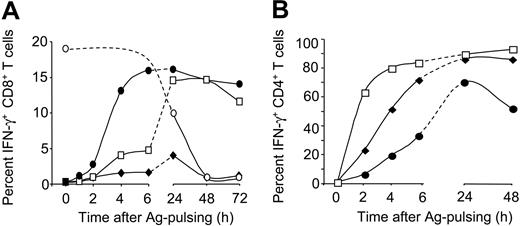
To assess the kinetics of cross-presentation and MHC II presentation for the different NY-ESO-1 formulations, we performed T-cell assays 1 to 72 hours after antigen pulsing of DCs (Figure 3A). Cross-presentation of protein alone was inefficient throughout the time course. Striking differences between NY-ESO-1/IC and NY-ESO-1/IMX were observed at early time points. DCs cross-presented NY-ESO-1/IMX very rapidly, inducing CTL activation as early as 2 to 4 hours after antigen exposure. In contrast, cross-presentation of NY-ESO-1/IC was inefficient within the first 6 hours, but reached levels similar to NY-ESO-1/IMX by 24 hours. For both NY-ESO-1 formulations, cross-presenting capacity was maintained for 3 days. This was significantly longer than for peptide-pulsed MoDCs, where, due to the “off-rate” of peptide binding, CTL activation declined during the first 24 hours and was lost thereafter. MHC II presentation by MoDCs pulsed with the different NY-ESO-1 protein formulations was also maintained for several days (Figure 3B). Interestingly, the kinetic profile elicited by the NY-ESO-1 formulations was different from that observed for cross-presentation in that CD4+ T-cell activation occurred most rapidly for NY-ESO-1/IC, followed by NY-ESO-1 protein and finally by NY-ESO-1/IMX. Thus, NY-ESO-1/IMX appeared to selectively favor cross-presentation, which led us to speculate that the 2 antigen formulations target different processing pathways.
NY-ESO-1 formulations induce distinct antigen-presentation kinetics. MoDCs were pulsed with NY-ESO-1 protein ( ), NY-ESO-1/IC (□), or NY-ESO-1/IMX (•; 10 μg/mL for MHC I and 1 μg/mL for MHC II assays) or peptide (○) for 1 hour, washed, and activated with CD40L. After various time points, DCs were cocultured with NY-ESO-1–specific CD8+ T cells (A) or CD4+ T cells (B). T-cell IFN-γ production was quantified by ICS. Data are representative of 4 experiments.
), NY-ESO-1/IC (□), or NY-ESO-1/IMX (•; 10 μg/mL for MHC I and 1 μg/mL for MHC II assays) or peptide (○) for 1 hour, washed, and activated with CD40L. After various time points, DCs were cocultured with NY-ESO-1–specific CD8+ T cells (A) or CD4+ T cells (B). T-cell IFN-γ production was quantified by ICS. Data are representative of 4 experiments.
NY-ESO-1 formulations induce distinct antigen-presentation kinetics. MoDCs were pulsed with NY-ESO-1 protein ( ), NY-ESO-1/IC (□), or NY-ESO-1/IMX (•; 10 μg/mL for MHC I and 1 μg/mL for MHC II assays) or peptide (○) for 1 hour, washed, and activated with CD40L. After various time points, DCs were cocultured with NY-ESO-1–specific CD8+ T cells (A) or CD4+ T cells (B). T-cell IFN-γ production was quantified by ICS. Data are representative of 4 experiments.
), NY-ESO-1/IC (□), or NY-ESO-1/IMX (•; 10 μg/mL for MHC I and 1 μg/mL for MHC II assays) or peptide (○) for 1 hour, washed, and activated with CD40L. After various time points, DCs were cocultured with NY-ESO-1–specific CD8+ T cells (A) or CD4+ T cells (B). T-cell IFN-γ production was quantified by ICS. Data are representative of 4 experiments.
Proteasome-dependent and proteasome-independent cross-presentation of NY-ESO-1
Cross-presentation is thought to occur via the “phagosome-to-cytosol” pathway, which requires translocation of the antigen from the phagosome into the cytosol for proteolytic cleavage into MHC I epitopes by the proteasome.17,25 These epitopes are subsequently transported via TAP (transporter associated with antigen-presentation) into the endoplasmic reticulum (ER) or back into the phagosome for MHC loading.26,27 Alternatively, MHC I epitopes may be generated within the phagosomes via the putative “vacuolar” pathway.28,29 In an attempt to identify whether qualitative differences in antigen processing were responsible for the different cross-presentation efficiencies for NY-ESO-1/IC and NY-ESO-1/IMX, we subjected MoDCs to various inhibitors of antigen processing.
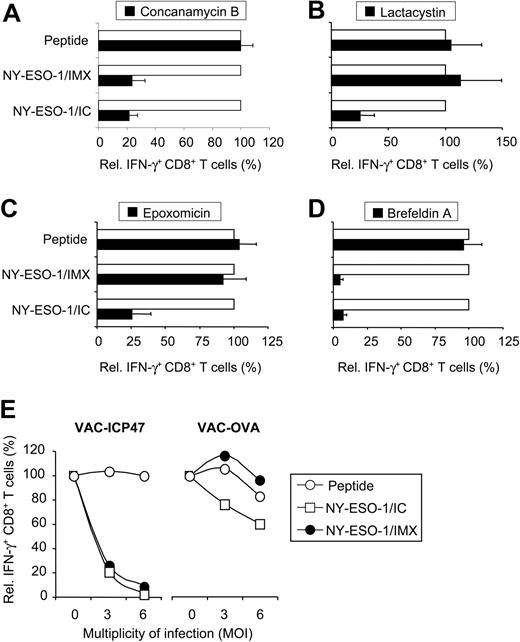
To assess whether internalized NY-ESO-1 protein required processing in acidic compartments, DCs were treated with the proton pump inhibitor concanamycin B. Raising lysosomal pH effectively inhibited cross-presentation of both NY-ESO-1 formulations (Figure 4A). This indicates that DCs preprocess NY-ESO-1 protein in acidic organelles in order to generate MHC I epitopes. Next, we addressed whether MHC I epitope generation requires cytosolic processing by the proteasome, using 2 different proteasome inhibitors, lactacystin (Figure 4B) and expoxomicin (Figure 4C). Both proteasome inhibitors effectively blocked cross-presentation of NY-ESO-1/IC. Interestingly, cross-presentation of NY-ESO-1/IMX was not influenced by proteasome inhibitors, indicating that the IMX-formulation targeted NY-ESO-1 to a proteasome-independent processing pathway. To examine whether peptides generated by either pathway required the TAP peptide transporter for translocation into the lumen of the ER (or ER-phagosome) for MHC loading, we infected MoDCs with vaccinia virus encoding either OVA (as a control) or ICP-47 (blocks TAP function).30 Cross-presentation was effectively blocked by ICP-47–encoding vaccinia, indicating the requirement of TAP for both NY-ESO-1 formulations (Figure 4E). In addition, blocking the transport of peptide/MHC I complexes from the ER to the cell surface with brefeldin A completely inhibited cross-presentation (Figure 4D). These findings indicate that, depending on the NY-ESO-1 formulation, DCs can generate the MHC I epitope via 2 distinct cytosolic pathways.
Antigen processing of NY-ESO-1/IC and NY-ESO-1/IMX occurs via 2 distinct cytosolic pathways. MoDCs were incubated in the absence (□) or presence (▪) of inhibitors 30 to 45 minutes before pulsing with NY-ESO-1/IC or NY-ESO-1/IXM (each 10 μg/mL): (A) concanamycin B (20 nM); (B) lactacystin (10 μM); (C) epoxomicin (5 μM); and (D) brefeldin A (1 μM). After overnight culture, MoDCs were assessed for their ability to induce IFN-γ by CD8+ T cells. To control for unspecific effects, MoDCs were pulsed with peptide (0.3 μg/mL) for each inhibitor condition. CD8+ T-cell IFN-γ induced by DCs in the absence of inhibitors was normalized to 100%. Data are mean plus or minus SD of 4 to 6 experiments. (E) MoDCs were incubated in the absence or presence of vaccinia virus encoding either ICP-47 or OVA at 3 or 6 MOI before pulsing with NY-ESO-1 formulations (□, NY-ESO-1/IC; •, NY-ESO-1/IMX) or peptide (○). CD8+ T-cell IFN-γ induced by MoDCs in the absence of virus was normalized to 100%. Data are mean plus or minus SD of 2 experiments.
Antigen processing of NY-ESO-1/IC and NY-ESO-1/IMX occurs via 2 distinct cytosolic pathways. MoDCs were incubated in the absence (□) or presence (▪) of inhibitors 30 to 45 minutes before pulsing with NY-ESO-1/IC or NY-ESO-1/IXM (each 10 μg/mL): (A) concanamycin B (20 nM); (B) lactacystin (10 μM); (C) epoxomicin (5 μM); and (D) brefeldin A (1 μM). After overnight culture, MoDCs were assessed for their ability to induce IFN-γ by CD8+ T cells. To control for unspecific effects, MoDCs were pulsed with peptide (0.3 μg/mL) for each inhibitor condition. CD8+ T-cell IFN-γ induced by DCs in the absence of inhibitors was normalized to 100%. Data are mean plus or minus SD of 4 to 6 experiments. (E) MoDCs were incubated in the absence or presence of vaccinia virus encoding either ICP-47 or OVA at 3 or 6 MOI before pulsing with NY-ESO-1 formulations (□, NY-ESO-1/IC; •, NY-ESO-1/IMX) or peptide (○). CD8+ T-cell IFN-γ induced by MoDCs in the absence of virus was normalized to 100%. Data are mean plus or minus SD of 2 experiments.
Human DC populations differ in their antigen-presenting abilities
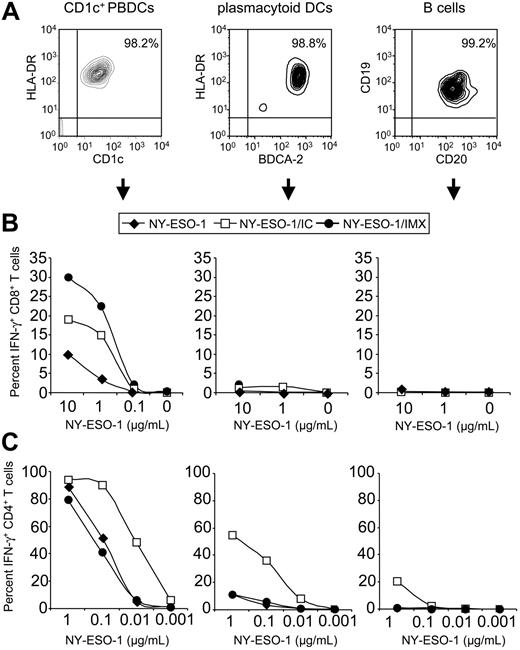
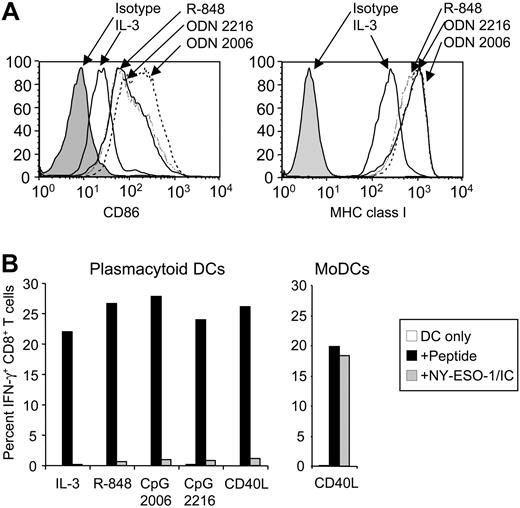
Despite the clinical use of different human DC populations for cancer immunotherapy, little is known about their capacity for presenting protein antigens on MHC I and II. We therefore examined whether primary DC populations were functionally equivalent to in vitro–derived MoDCs. Two DC populations are found in human blood, CD1c+ PBDCs and plasmacytoid DCs, which together comprise less than 1% of total PBMCs. To compare their antigen-presenting capacities, we isolated CD1c+ PBDCs, plasmacytoid DCs, and B cells to high purity (95%-99%) from PBMCs of HLA-A2+ healthy volunteers (Figure 5A). These primary antigen-presenting cells differed strikingly in their ability to cross-present. Like MoDCs, CD1c+ PBDCs cross-presented NY-ESO-1/IC and, most efficiently, NY-ESO-1/IMX (Figure 5B). Culture of CD1c+ PBDCs with GM-CSF was sufficient to induce cross-presenting function, and activation with CD40L or R-848 conferred no benefit (data not shown). In contrast, neither plasmacytoid DCs nor B cells could cross-present (Figure 5B). To rule out the possibility that this lack of function was due to insufficient cell activation, we cultured plasmacytoid DCs in the presence of CD40L, R-848, or 2 different types of CpG oligodeoxynucleotides (ODN2216 and ODN2006). Despite the induction of maturation by these stimuli, as assessed by up-regulation of CD86 and MHC I, plasmacytoid DCs were still unable to cross-present (Figure 6A-B). In addition, no cross-presentation was observed for monocytes and macrophages (data not shown).
Primary DC types differ in their antigen-presenting capacities. (A) FACS analysis of CD1c+ PBDCs, plasmacytoid DCs, and B cells directly after isolation from a healthy donor. Primary antigen-presenting cells were pulsed with NY-ESO-1 protein, NY-ESO-1/IC, or NY-ESO-1/IMX at the indicated concentrations, cultured overnight in medium containing GM-CSF for CD1c+ PBDCs or IL-3 for plasmacytoid DCs, and cocultured with NY-ESO-1–specific T cells. IFN-γ production by CD8+ T cells (B) and CD4+ T cells (C) was quantified by ICS.  indicates NY-ESO-1; □, NY-ESO-1/IC; •, NY-ESO-1/IMX. Data are representative of 5 experiments.
indicates NY-ESO-1; □, NY-ESO-1/IC; •, NY-ESO-1/IMX. Data are representative of 5 experiments.
Primary DC types differ in their antigen-presenting capacities. (A) FACS analysis of CD1c+ PBDCs, plasmacytoid DCs, and B cells directly after isolation from a healthy donor. Primary antigen-presenting cells were pulsed with NY-ESO-1 protein, NY-ESO-1/IC, or NY-ESO-1/IMX at the indicated concentrations, cultured overnight in medium containing GM-CSF for CD1c+ PBDCs or IL-3 for plasmacytoid DCs, and cocultured with NY-ESO-1–specific T cells. IFN-γ production by CD8+ T cells (B) and CD4+ T cells (C) was quantified by ICS.  indicates NY-ESO-1; □, NY-ESO-1/IC; •, NY-ESO-1/IMX. Data are representative of 5 experiments.
indicates NY-ESO-1; □, NY-ESO-1/IC; •, NY-ESO-1/IMX. Data are representative of 5 experiments.
Lack of cross-presenting function of plasmacytoid DCs persists after activation with maturation-inducing stimuli. (A) Surface expression of CD86 and MHC I by plasmacytoid DCs following culture with IL-3 in the absence or presence of CpG ODN2006, ODN2216, or R-848. (B) Freshly isolated plasmacytoid DCs were pulsed with NY-ESO-1/IC (10 μg/mL), matured with the indicated stimuli, and cocultured with NY-ESO-1–specific CD8+ T cells (left graph). NY-ESO-1/IC–pulsed MoDCs served as a positive control (right graph). IFN-γ production by CD8+ T cells was quantified by ICS. □ indicates DCs only; ▪, + peptide; ▦, + NY-ESO-1/IC. Data are representative of 5 experiments.
Lack of cross-presenting function of plasmacytoid DCs persists after activation with maturation-inducing stimuli. (A) Surface expression of CD86 and MHC I by plasmacytoid DCs following culture with IL-3 in the absence or presence of CpG ODN2006, ODN2216, or R-848. (B) Freshly isolated plasmacytoid DCs were pulsed with NY-ESO-1/IC (10 μg/mL), matured with the indicated stimuli, and cocultured with NY-ESO-1–specific CD8+ T cells (left graph). NY-ESO-1/IC–pulsed MoDCs served as a positive control (right graph). IFN-γ production by CD8+ T cells was quantified by ICS. □ indicates DCs only; ▪, + peptide; ▦, + NY-ESO-1/IC. Data are representative of 5 experiments.
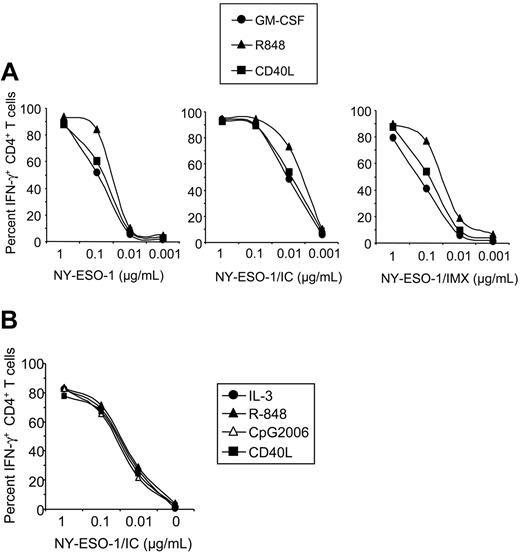
These primary antigen-presenting cell types also differed in their ability to present NY-ESO-1 on MHC II. CD1c+ PBDCs were highly efficient in MHC II presentation, which was optimal for NY-ESO-1/IC (Figure 5C). Figure 7 shows that activation of different DC types with commonly used TLR ligands or CD40L does not enhance MHC II presentation efficacy. Thus, further activation does not appear to be required to achieve potent CD4+ T-cell activation in response to protein formulations presented by DCs. Activating CD1c+ PBDCs with CD40L or R-848 did not significantly enhance MHC II presenting function (Figure 7A). In comparison, plasmacytoid DCs expressed little MHC II presenting capacity for NY-ESO-1 protein or NY-ESO-1/IMX. Interestingly, targeting the antigen to Fcγ receptors with NY-ESO-1/IC significantly improved their ability to stimulate antigen-specific CD4+ T cells (Figure 5C). In this respect, plasmacytoid DCs were 1 to 2 orders of magnitude more potent than B cells, but still one order of magnitude less efficient than CD1c+ PBDCs. Finally, MHC II presentation of NY-ESO-1/IC for IL-3–cultured plasmacytoid DCs was equally effective as for cells activated with CD40L, R-848, or CpG ODN2006, indicating that plasmacytoid DCs do not require further activation to express this function (Figure 7B). As a general rule, presentation on MHC II is highly efficient as compared with cross-presentation on MHC I, the latter requiring higher doses of antigen and ideally subsequent maturation of DCs with CD40L (Figure 2).
Activation of primary DC types does not enhance MHC class II presentation of NY-ESO-1. (A) CD1c+ PBDCs were pulsed with NY-ESO-1 protein, NY-ESO-1/IC, or NY-ESO-1/IMX at the indicated concentrations and cultured overnight with GM-CSF in the absence (•) or presence of R-848 (▴) or CD40L (▪) before coculture with NY-ESO-1–specific CD4+ T cells. IFN-γ production by CD4+ T cells was quantified by ICS. Data are representative of 4 experiments. (B) Plasmacytoid DCs were pulsed with NY-ESO-1/IC and cultured overnight with IL-3 in the absence (•) or presence of CD40L (▪), R-848 (▴), or CpG ODN2006 (▵) before coculture with NY-ESO-1–specific CD4+ T cells. Data are representative of 2 experiments.
Activation of primary DC types does not enhance MHC class II presentation of NY-ESO-1. (A) CD1c+ PBDCs were pulsed with NY-ESO-1 protein, NY-ESO-1/IC, or NY-ESO-1/IMX at the indicated concentrations and cultured overnight with GM-CSF in the absence (•) or presence of R-848 (▴) or CD40L (▪) before coculture with NY-ESO-1–specific CD4+ T cells. IFN-γ production by CD4+ T cells was quantified by ICS. Data are representative of 4 experiments. (B) Plasmacytoid DCs were pulsed with NY-ESO-1/IC and cultured overnight with IL-3 in the absence (•) or presence of CD40L (▪), R-848 (▴), or CpG ODN2006 (▵) before coculture with NY-ESO-1–specific CD4+ T cells. Data are representative of 2 experiments.
Discussion
We undertook this study to explore strategies for optimizing MHC I and II presentation of full-length tumor antigen by human DCs. NY-ESO-1 was chosen as a model tumor antigen because it contains both CD8+ and CD4+ T-cell epitopes. We found that primary and in vitro–generated human DC types differ strikingly in their antigen-presenting abilities. Only CD1c+ PBDCs and MoDCs were capable of cross-presenting NY-ESO-1 to CD8+ T cells, whereas plasmacytoid DCs (as well as B cells, monocytes, and macrophages) lacked this function, irrespective of their maturational stage. These findings are in agreement with a recent study demonstrating that plasmacytoid DCs can only present MHC I epitopes derived from intact virus (live or heat inactivated), but not from boiled virus.31 Nagata et al20 reported that DCs generated in vitro from CD34+ progenitors with TGF-β, which directs DC development toward Langerhans cells, also lacked cross-presenting function.20 Thus, not all DC types are equally suitable for cancer vaccines using proteins as tumor antigen source, where cross-presenting function is essential.
Human DC types also differed in their MHC II presentation abilities. Like MoDCs, CD1c+ PBDCs pulsed with NY-ESO-1 protein were potent stimulators of CD4+ T cells, whereas plasmacytoid DCs (and B cells) were far less efficient in this respect. This raises the question of whether plasmacytoid DCs can be regarded as antigen-presenting cells that play a role in adaptive immunity in situations other than viral infections. Arguing in favor is our observation that targeting the tumor antigen to Fcγ receptors with NY-ESO-1/IC strongly enhanced MHC II presentation by plasmacytoid DCs. This is the first demonstration that plasmacytoid DCs might play a direct role in tumor-directed CD4+ T-cell responses. Whether MHC II presentation by plasmacytoid DCs enhances or suppresses tumor-directed T-cell responses requires further investigation since their regulatory abilities can promote or suppress T-cell function.32 This is especially important given the recent demonstration that plasmacytoid DCs reside within tumors, where they may play a role in mediating tumor-induced T-cell anergy.33-35
It has been shown that effective T-cell activation is dependent on the maturational stage of DCs.36-38 Several classes of maturation-inducing stimuli have been identified, but their impact on cross-presentation remains unclear. A recent mouse study showed that cross-presentation efficiency depends on the type of stimulus.39 Disruption of spontaneously forming cell clusters and CD40L promoted cross-presentation, but TLR ligands did not. Likewise, in our study CD40L enhanced cross-presentation by human MoDCs, whereas TLR ligands as well as proinflammatory cytokines were ineffective in this respect. However, none of these maturation-inducing stimuli enhanced cross-presentation by CD1c+ PBDCs. Exogenous stimuli may not be as critical for this DC type given that they mature spontaneously during in vitro culture. We have previously shown that CD1c+ PBDCs also differ from MoDCs by rapidly and spontaneously acquiring migratory function to the lymph-node–directing chemokines CCL19 and CCL21.7-9 This acquisition of migratory and cross-presenting function without need for further ex vivo manipulation makes CD1c+ PBDCs particularly attractive for DC-based immunotherapy.
Our study further highlights the critical impact of antigen formulation on cross-presentation efficiency. Pulsing DCs with NY-ESO-1 protein resulted only in presentation on MHC II. Cross-presentation to MHC I was significantly enhanced when NY-ESO-1 was formulated as an IC or with IMX. In addition to inducing activation of both CD8+ and CD4+ T cells, these 2 NY-ESO-1 formulations conferred the striking advantage of prolonging antigen presentation by DCs. Most clinical trials are conducted with the assumption that the injected DCs will migrate to regional lymph nodes to stimulate T cells, a process which may take 1 to 2 days.40,41 Thus, long-lived peptide/MHC display by DCs is critical for successful vaccination. In this respect, the efficacy of DCs pulsed with exogenous peptide is limited by the “off-rate” of peptide binding. The use of antigen requiring processing by DCs can lead to more favorable presentation kinetics.42,43 We found that DCs pulsed with NY-ESO-1/IC or NY-ESO-1/IMX efficiently stimulated both CD4+ and CD8+ T cells for up to 3 days, when peptide-pulsed DCs had already lost this ability. Therefore, these tumor antigen formulations can significantly prolong the “window” for productive DC interaction with rare tumor antigen–specific T cells residing in lymph nodes.
In addition, our study provides evidence that the mode of antigen delivery can have a decisive impact on the antigen processing by DCs. Differences between NY-ESO-1/IC and NY-ESOI-1/IMX processing became apparent in kinetic assays. Rapid and highly efficient cross-presentation, but delayed MHC II presentation, was characteristic for NY-ESO-1/IMX. In contrast, for NY-ESO-1/IC, cross-presentation was slow, but MHC II presentation was rapid and highly efficient. These findings could reflect differences in antigen handling, with antigen formulations accessing preferentially MHC I or II processing pathways. However, inhibitor studies revealed that the generation of the MHC I epitope occurred via 2 distinct cross-presentation pathways expressed by DCs. After initial lysosomal antigen processing (inhibited by concanamycin B) and translocation into the cytosol (indicated by TAP-dependency), cross-presentation of NY-ESO-1/IC required proteolysis by the proteasome (inhibited by epoxomicin or lactacystin). In contrast, for NY-ESO-1/IMX the MHC class I epitope was generated via an alternative, proteasome-independent fashion. A similar observation has recently been described for murine DCs, which cross-presented ISCOM-formulated OVA in a proteasome-independent fashion.44
We found that both processing pathways were specific for DCs, as other antigen-presenting cell types were incapable of cross-presenting either antigen formulation. It remains open whether IMX-formulation impeded the access of the antigen to the proteasome or whether it resulted in the generation of a different intermediate peptide precursor that was a substrate for another, yet unidentified cytosolic protease. Possible candidates are leucin aminopeptidase, bleomycin hydrolase, puromycin-sensitive aminopeptidase, thimet oligopeptidase, and tripeptidyl peptidase II (TPP II) (reviewed in Kloetzel45 ). The nature of this alternative processing pathway is the focus of ongoing investigations. An exciting area for further research will be whether IMX formulation influences the variety of epitopes that can be displayed by DCs. Possibly, targeting antigen to both pathways generates a broader tumor-specific CTL repertoire; for example, against epitopes that cannot be generated by the immunoproteasome.46 A recently completed clinical trial at our institute demonstrated that a NY-ESO-1/IMX vaccine can induce CD8+ T-cell responses against a broad range of MHC I epitopes, including epitopes that were previously unknown.23 Whether a NY-ESO-1/IMX–based DC vaccine has the potential to induce effective immune responses against tumors warrants further investigation.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-08-3105.
Supported by a program grant from the Australian National Health and Medical Research Council (NH&MRC) and the Ludwig Institute for Cancer Research, the Mildred Scheel Stiftung (M.S.), the Boehringer Ingelheim Stiftung (C.J.), and by an NH&MRC Career Development Award (I.D.D.). E.M. is an Honorary Senior Research Fellow of the Ludwig Institute for Cancer Research.
E.M. and J.C. contributed equally to this work.
Several authors (D.D., S.G., L.M., and E.M.) are employed by CSL Limited, whose potential product was studied in the present work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Immunex, an Amgen company, for soluble CD40L trimer; J. Yewdell for ICP-47 encoding vaccinia virus; and C. Keech for virus preparation.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal