Abstract
We hypothesized that the rising levels of inflammatory markers with aging is explained by cardiovascular risk factors and morbidity becoming progressively more prevalent in older persons. Information on inflammatory markers, cardiovascular risk factors, and diseases was collected in 595 men and 748 women sampled from the general population (age, 20-102 years). In both men and women, older age was associated with higher levels of interleukin-6 (IL-6), IL-1 receptor antagonist (IL-1ra), IL-18, C-reactive protein (CRP), and fibrinogen, while soluble IL-6 receptor (sIL-6r) increased significantly with age only in men. Adjusting for cardiovascular risk factors and morbidity, the age regression coefficients became substantially smaller in models predicting IL-6, IL-1ra, IL-18, and fibrinogen and larger in the model predicting sIL6r. Adjustment for cardiovascular morbidity substantially reduced the effect of age on CRP in men but not in women. Findings were confirmed in a subgroup of 51 men and 45 women with low risk profile and no cardiovascular morbidity. Part of the “proinflammatory state” in older persons is related to the high prevalence of cardiovascular risk factor and morbidity.
Introduction
Older persons are often affected by a “low-grade proinflammatory state” characterized by increased levels of cytokines and acutephase proteins (APPs).1,2 Cytokines are intercellular signaling proteins that exert pro- and anti-inflammatory activities through the ligation of specific receptors or stimulating the hepatic production of APPs, such as C-reactive protein (CRP) and fibrinogen.3 Cytokine signaling pathways are important for numerous physiologic systems beyond immunity. For example, interleukin-1 (IL-1), IL-6, and tumor necrosis factor alpha (TNF-α) promote lipolysis, accelerate protein catabolism, decrease insulin sensitivity, are essential factors in hematopoiesis, and may influence bone and muscle remodeling.4-7
It is unclear whether the age-related “proinflammatory state” is caused by a primary impairment of the mechanisms that turn off the inflammatory response or is due to the accumulation of cardiovascular risk factors and diseases with aging.8 In fact, smoking, sedentary state, excessive alcohol consumption, and central obesity are associated with high levels of inflammatory markers.9
Previous studies that screened participants with strict criteria for good health, adequate nutrition, and absence of diseases failed to detect any significant difference in the production of IL-1 and IL-6 between young, middle-aged, and older participants.10 Other studies performed in a limited number of healthy volunteers found that circulating levels of IL-6, CRP, and other biomarkers of inflammation increase with age, although in those studies the definition of healthy status was questionable.11-13
Using data from an epidemiologic study performed on a representative sample of the population, we tested the hypothesis that higher levels of inflammatory markers with age are due, at least in part, to progressively increased burdens of cardiovascular risk factors and morbidity.
Patients and methods
Study population
InCHIANTI (Invecchiare in Chianti; aging in the Chianti area) is a study of factors affecting mobility in late life, conducted in the Tuscany region (Italy).14 In 1998, 1270 persons age 65 years or older were randomly selected from the population. Additionally, 30 men and 30 women randomly sampled from the age strata 20 to 29, 30 to 39, 40 to 49, 50 to 59, and 60 to 69 years were enrolled.
Of the 1714 eligible persons, 640 men and 813 women (84.8%) agreed to participate, 1343 donated a blood sample, and 1327 had complete data for the analysis presented here. Our institutional review board ratified the study protocol. Participants consented to participate and to have their blood samples analyzed for scientific purposes. For those unable to fully consent, surrogate consents were obtained from close relatives.
Measures
Morning, fasting blood samples were collected after a 15-minute resting. Aliquots of serum were stored at -80°C and never thawed before analysis. Serum IL-6, soluble IL-6 receptors (sIL-6r, 80 kDa), IL-1β, IL-1 receptor antagonist (IL-1ra), TNF-α (kits from BIOSOURCE, Camarillo, CA), and IL-18 and TGF-β (kits from Quantikine HS; R&D Systems, Minneapolis, MN) were measured in duplicate by high sensitivity enzyme-linked immunoabsorbent assays (ELISA). CRP was measured in duplicate using an ELISA and colorimetric competitive immunoassay. Fibrinogen, total cholesterol, high-density lipoprotein (HDL) cholesterol, and triglyceride levels were determined by commercial assays (Roche Diagnostics, Mannheim, Germany), and low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula. LDL cholesterol values were grouped as less than 3.36 mM/L (130 mg/dL), 3.36 to 4.11 mM/L (130-159 mg/dL), 4.14 to 4.89 mM/L (160-189 mg/dL), or 4.91 mM/L (190 mg/dL) or higher; HDL cholesterol, as less than 1.03 mM/L (40 mg/dL) versus 1.03 mM/L (40 mg/dL) or higher; and triglycerides, as less than 1.69 mM/L (150 mg/dL), 1.69 to 2.25 mM/L (150-199 mg/dL), or 2.26 mM/L (200 mg/dL) or higher (http://www.nhlbi.nih.gov/guidelines/cholesterol/atp3full.pdf). Body mass index (BMI) was calculated as weight (kg)/height (m2) and dichotomized in the analysis as 27 or higher versus less than 27.15 Waist circumference was dichotomized as greater versus lower than 102 cm in men and 88 cm in women.16 Education was assessed as years in school from self-report. Average daily alcohol intake (< 30 versus ≥ 30 g per day) was estimated by the European Prospective Investigation into Cancer and Nutrition food frequency questionnaire.17 Based on responses to multiple questions, physical activity in the previous year was rated as (a) sedentary: inactive or light-intensity physical activity (ie, walking, light housework) less than 1 hour per week; (b) light physical activity: light-intensity physical activity 2 to 4 hours per week; (c) moderate-high physical activity: light physical activity more than 4 hours/wk or moderate physical activity (ie, brisk walking, playing soccer, gardening) at least 1 to 2 hours/week. Pack-years, a measure of smoking exposure that combines intensity and duration, was calculated as (packs smoked per day) × (years of smoking) based on self-report.
Diseases ascertained according to pre-established criteria that combined information from physician diagnosis, medical records, clinical examination, and blood tests included coronary heart disease ([CHD] angina and myocardial infarction), congestive heart failure (CHF), peripheral artery disease (PAD), stroke, chronic obstructive lung disease ([COPD] including emphysema, chronic bronchitis, and asthma), diabetes, hypertension, osteoporosis, chronic renal failure (CRF), cancer, dementia, and depression.18 Disability was defined as selfreport of needing personal help in performing any basic activity of daily living.19
Subclinical cardiovascular disease was assessed similarly to the Cardiovascular Health Study.20 Carotid atherosclerosis was defined as bilateral atherosclerotic plaques or any stenosis more than 40% assessed by Doppler flow ultrasonography. Ankle-arm index of 0.9 or less was considered a marker of lower extremity arterial obstruction.21 Major electrocardiogram abnormalities were defined as ventricular conduction defects, major Q or QS abnormalities, minor Q or QS with ST-T wave abnormalities, left ventricular hypertrophy, isolated major ST-T wave changes, atrial fibrillation, or presence of severe ventricular arrhythmias.
Statistical analysis
Variables are reported as mean values ± standard deviations (SDs) or percentages. General linear models or Pearson chi-square was used for group comparisons. Log-transformed values for cytokine (except TGF-β) and APP concentrations were used in the analysis, and back-transformed for presentation.
The hypothesis that the apparent effect of age on the circulating level of inflammatory markers was accounted for by the confounding effect of cardiovascular risk factors and diseases in older persons was tested using 4 hierarchical linear regression models: unadjusted, adjusted for cardiovascular risk factors, adjusted also for subclinical cardiovascular diseases, and adjusted also for major chronic diseases. Additional fully adjusted models were fitted to the total population to test for age * sex interaction. Using backward selection, we identified from these models the set of variables that best predicted the variability in the inflammatory markers.
From sex-specific, adjusted models we estimate the mean levels of the inflammatory markers in hypothetical men and women with “low risk” profile (12 years of education; BMI 27 kg/m2; waist circumference < 88 cm in women and < 102 cm in men; moderate physical activity, alcohol consumption < 30 g/d; never smoked, LDL cholesterol < 3.36 mM [130 mg/dL]; HDL cholesterol > 1.03 mM [40 mg/dL], triglycerides < 1.47 mM [130 mg/dL], no major diseases), according to sex and age group. These values were compared with those of 51 male and 45 female InCHIANTI participants with low risk profile and no cardiovascular diseases. All analyses were performed using the SAS statistical package, version 8.2 (SAS Institute, Cary, NC).
Results
The characteristics of the study population are reported in Table 1. In men and women, the levels of IL-6, sIL-6, IL-1ra, IL-18, CRP, and fibrinogen increased significantly with age, while sIL6r increased in men but not in women (Table 2). IL-1β, TNF-α, and TGF-β were similar in men and women and across age groups and, therefore, were not considered in further analyses.
Characteristics of the study population according to sex
. | Men, n = 586 . | Women, n = 741 . | P* . |
|---|---|---|---|
| General characteristics | |||
| Age, y, mean ± SD | 68 ± 16 | 70 ± 16 | < .0001 |
| Years in school, mean ± SD | 7.3 ± 4.4 | 5.8 ± 3.9 | < .0001 |
| Cardiovascular risk factors | |||
| Smoking | |||
| Former smoker, % | 48.4 | 11.2 | < .0001 |
| Current smoker, % | 20.2 | 11.0 | < .0001 |
| Pack-years, mean ± SD | 30.2 ± 22.9 | 14.6 ± 8.8 | < .0001 |
| Body mass index, kg/m2, mean ± SD | 27.0 ± 3.4 | 27.2 ± 4.6 | .62 |
| Waist circumference, men: more than 102 cm, women: more than 88 cm; % | 16.8 | 46.9 | < .0001 |
| Alcohol intake, more than 30 g/day, % | 28.5 | 3.4 | < .0001 |
| Physical activity, % | |||
| Sedentary | 11.5 | 23.8 | |
| Light | 75.5 | 72.2 | |
| Moderate-high | 13.0 | 4.0 | < .0001 |
| LDL cholesterol, % | |||
| Less than 130 mg/dL | 50.1 | 44.5 | |
| 130 to 159 mg/dL | 32.1 | 31.4 | |
| 160 to 189 mg/dL | 14.0 | 17.4 | |
| At least 190 mg/dL | 3.9 | 6.7 | < .02 |
| HDL cholesterol less than 40 mg/dL, % | 19.0 | 7.8 | < .0001 |
| Triglycerides, % | |||
| Less than 150 mg/dL | 72.9 | 77.8 | |
| 150 to 199 mg/dL | 13.3 | 14.0 | |
| 200 to 499 mg/dL | 12.9 | 8.0 | |
| At least 500 mg/dL | 0.8 | 0.1 | < .02 |
| Subclinical cardiovascular disease | |||
| ECG abnormalities | 14.5 | 10.3 | < .005 |
| Ankle brachial index at least 0.90, % | 16.1 | 11.0 | < .001 |
| Carotid stenosis more than 40% and/or bilateral plaques, % | 39.6 | 29.2 | < .0001 |
| Conditions, % | |||
| Coronary heart disease | 7.4 | 3.6 | < .001 |
| Congestive heart failure | 0.8 | 2.1 | .12 |
| Stroke | 2.4 | 1.2 | < .05 |
| Chronic obstructive lung disease | 6.2 | 0.9 | < .0001 |
| Diabetes | 7.4 | 6.4 | .34 |
| Hypertension | 40.4 | 50.0 | < .005 |
| Osteoporosis | 44.4 | 56.3 | < .0005 |
| Chronic renal failure | 1.2 | 2.6 | .4 |
| Cancer | 3.9 | 6.2 | .11 |
| Dementia | 4.4 | 5.1 | .55 |
| Depression | 10.4 | 24.5 | < .0001 |
| Disability in activities of daily living | 6.6 | 8.2 | .86 |
. | Men, n = 586 . | Women, n = 741 . | P* . |
|---|---|---|---|
| General characteristics | |||
| Age, y, mean ± SD | 68 ± 16 | 70 ± 16 | < .0001 |
| Years in school, mean ± SD | 7.3 ± 4.4 | 5.8 ± 3.9 | < .0001 |
| Cardiovascular risk factors | |||
| Smoking | |||
| Former smoker, % | 48.4 | 11.2 | < .0001 |
| Current smoker, % | 20.2 | 11.0 | < .0001 |
| Pack-years, mean ± SD | 30.2 ± 22.9 | 14.6 ± 8.8 | < .0001 |
| Body mass index, kg/m2, mean ± SD | 27.0 ± 3.4 | 27.2 ± 4.6 | .62 |
| Waist circumference, men: more than 102 cm, women: more than 88 cm; % | 16.8 | 46.9 | < .0001 |
| Alcohol intake, more than 30 g/day, % | 28.5 | 3.4 | < .0001 |
| Physical activity, % | |||
| Sedentary | 11.5 | 23.8 | |
| Light | 75.5 | 72.2 | |
| Moderate-high | 13.0 | 4.0 | < .0001 |
| LDL cholesterol, % | |||
| Less than 130 mg/dL | 50.1 | 44.5 | |
| 130 to 159 mg/dL | 32.1 | 31.4 | |
| 160 to 189 mg/dL | 14.0 | 17.4 | |
| At least 190 mg/dL | 3.9 | 6.7 | < .02 |
| HDL cholesterol less than 40 mg/dL, % | 19.0 | 7.8 | < .0001 |
| Triglycerides, % | |||
| Less than 150 mg/dL | 72.9 | 77.8 | |
| 150 to 199 mg/dL | 13.3 | 14.0 | |
| 200 to 499 mg/dL | 12.9 | 8.0 | |
| At least 500 mg/dL | 0.8 | 0.1 | < .02 |
| Subclinical cardiovascular disease | |||
| ECG abnormalities | 14.5 | 10.3 | < .005 |
| Ankle brachial index at least 0.90, % | 16.1 | 11.0 | < .001 |
| Carotid stenosis more than 40% and/or bilateral plaques, % | 39.6 | 29.2 | < .0001 |
| Conditions, % | |||
| Coronary heart disease | 7.4 | 3.6 | < .001 |
| Congestive heart failure | 0.8 | 2.1 | .12 |
| Stroke | 2.4 | 1.2 | < .05 |
| Chronic obstructive lung disease | 6.2 | 0.9 | < .0001 |
| Diabetes | 7.4 | 6.4 | .34 |
| Hypertension | 40.4 | 50.0 | < .005 |
| Osteoporosis | 44.4 | 56.3 | < .0005 |
| Chronic renal failure | 1.2 | 2.6 | .4 |
| Cancer | 3.9 | 6.2 | .11 |
| Dementia | 4.4 | 5.1 | .55 |
| Depression | 10.4 | 24.5 | < .0001 |
| Disability in activities of daily living | 6.6 | 8.2 | .86 |
Age adjusted, from linear or logistic regression models
Levels of inflammatory markers according to sex and age group
. | Age, y . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 20-39 . | 40-49 . | 50-64 . | 65-74 . | 75-84 . | 85 + . | p* . | |||||
| n | ||||||||||||
| Men | 56 | 29 | 48 | 48 | 138 | 48 | NA | |||||
| Women | 61 | 26 | 54 | 301 | 196 | 93 | NA | |||||
| IL-6, pg/mL† | ||||||||||||
| Men | 0.6 (0.4-0.8) | 0.9 (0.7-1.3) | 1.1 (0.9-1.4) | 1.4 (1.2-1.5) | 1.8 (1.6-2.1) | 3.5 (2.6-4.7) | < .0001 | |||||
| Women | 0.6 (0.5-0.8) | 0.8 (0.6-1.1) | 0.8 (0.7-1.0) | 1.1 (1.1-1.2) | 1.5 (1.4-1.7) | 2.1 (1.7-2.6) | < .0001 | |||||
| sIL-6r, ng/mL† | ||||||||||||
| Men | 93 (79-108) | 77 (61-97) | 88 (73-105) | 91 (85-97) | 92 (85-99) | 96 (82-111) | .42 | |||||
| Women | 76 (67-87) | 81 (65-101) | 83 (70-97) | 91 (84-95) | 92 (86-97) | 97 (88-106) | .003 | |||||
| IL-1β, pg/mL† | ||||||||||||
| Men | 0.12 (0.10-0.15) | 0.18 (0.13-0.36) | 0.18 (0.12-0.20) | 0.13 (0.12-0.15) | 0.12 (0.10-0.14) | 0.12 (0.10-0.14) | .26 | |||||
| Women | 0.14 (0.12-0.18) | 0.15 (0.11-0.19) | 0.15 (0.11-0.18) | 0.12 (0.11-0.14) | 0.13 (0.12-0.16) | 0.14 (0.11-0.17) | .64 | |||||
| IL-1ra, pg/mL† | ||||||||||||
| Men | 112 (100-125) | 143 (117-172) | 137 (115-164) | 133 (124-141) | 135 (119-152) | 154 (131-180) | < .05 | |||||
| Women | 114 (96-135) | 125 (97-158) | 117 (100-137) | 125 (117-134) | 144 (133-154) | 145 (130-160) | < .001 | |||||
| TNF-α, pg/mL† | ||||||||||||
| Men | 4.8 (3.8-6.1) | 4.0 (2.8-5.6) | 6.2 (4.8-8.0) | 4.6 (4.1-5.0) | 4.4 (3.8-5.0) | 5.1 (4.0-6.4) | .50 | |||||
| Women | 4.8 (3.6-6.4) | 3.8 (2.7-5.4) | 5.0 (4.1-6.1) | 4.4 (4.0-4.9) | 4.6 (4.1-5.1) | 5.1 (4.3-6.0) | .76 | |||||
| IL-18, pg/mL† | ||||||||||||
| Men | 335 (303-369) | 319 (285-356) | 400 (358-447) | 420 (403-436) | 435 (411-459) | 454 (417-493) | < .0001 | |||||
| Women | 268 (245-292) | 292 (253-336) | 325 (300-352) | 340 (325-353) | 357 (340-373) | 430 (392-470) | < .0001 | |||||
| TGF-β, ng/mL | ||||||||||||
| Men | 14.7 (12.7-16.7) | 10.2 (7.9-12.6) | 12.4 (10.6-14.1) | 11.9 (11.1-12.8) | 11.2 (10.0-12.4) | 13.9 (11.9-15.8) | .12 | |||||
| Women | 12.6 (11.0-14.1) | 12.1 (9.7-14.5) | 11.7 (9.9-13.4) | 11.2 (10.4-11.9) | 13.0 (11.8-14.2) | 12.3 (10.8-13.7) | .69 | |||||
| CRP, g/L† | ||||||||||||
| Men | 1.0 (0.8-1.4) | 1.9 (1.1-3.2) | 1.8 (1.3-2.5) | 2.6 (2.2-2.9) | 3.1 (2.6-3.7) | 5.4 (3.8-7.9) | < .0001 | |||||
| Women | 1.1 (0.8-1.5) | 1.1 (0.6-1.8) | 2.3 (1.8-2.9) | 2.5 (2.2-2.8) | 3.0 (2.6-3.5) | 3.3 (2.6-4.3) | < .0001 | |||||
| Fibrinogen, mg/mL | ||||||||||||
| Men | 269 (254-284) | 321 (290-352) | 315 (296-333) | 345 (335-354) | 345 (331-357) | 396 (372-419) | < .0001 | |||||
| Women | 317 (303-331) | 324 (302-345) | 352 (337-367) | 354 (346-361) | 370 (359-379) | 398 (381-415) | < .0001 | |||||
. | Age, y . | . | . | . | . | . | . | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
. | 20-39 . | 40-49 . | 50-64 . | 65-74 . | 75-84 . | 85 + . | p* . | |||||
| n | ||||||||||||
| Men | 56 | 29 | 48 | 48 | 138 | 48 | NA | |||||
| Women | 61 | 26 | 54 | 301 | 196 | 93 | NA | |||||
| IL-6, pg/mL† | ||||||||||||
| Men | 0.6 (0.4-0.8) | 0.9 (0.7-1.3) | 1.1 (0.9-1.4) | 1.4 (1.2-1.5) | 1.8 (1.6-2.1) | 3.5 (2.6-4.7) | < .0001 | |||||
| Women | 0.6 (0.5-0.8) | 0.8 (0.6-1.1) | 0.8 (0.7-1.0) | 1.1 (1.1-1.2) | 1.5 (1.4-1.7) | 2.1 (1.7-2.6) | < .0001 | |||||
| sIL-6r, ng/mL† | ||||||||||||
| Men | 93 (79-108) | 77 (61-97) | 88 (73-105) | 91 (85-97) | 92 (85-99) | 96 (82-111) | .42 | |||||
| Women | 76 (67-87) | 81 (65-101) | 83 (70-97) | 91 (84-95) | 92 (86-97) | 97 (88-106) | .003 | |||||
| IL-1β, pg/mL† | ||||||||||||
| Men | 0.12 (0.10-0.15) | 0.18 (0.13-0.36) | 0.18 (0.12-0.20) | 0.13 (0.12-0.15) | 0.12 (0.10-0.14) | 0.12 (0.10-0.14) | .26 | |||||
| Women | 0.14 (0.12-0.18) | 0.15 (0.11-0.19) | 0.15 (0.11-0.18) | 0.12 (0.11-0.14) | 0.13 (0.12-0.16) | 0.14 (0.11-0.17) | .64 | |||||
| IL-1ra, pg/mL† | ||||||||||||
| Men | 112 (100-125) | 143 (117-172) | 137 (115-164) | 133 (124-141) | 135 (119-152) | 154 (131-180) | < .05 | |||||
| Women | 114 (96-135) | 125 (97-158) | 117 (100-137) | 125 (117-134) | 144 (133-154) | 145 (130-160) | < .001 | |||||
| TNF-α, pg/mL† | ||||||||||||
| Men | 4.8 (3.8-6.1) | 4.0 (2.8-5.6) | 6.2 (4.8-8.0) | 4.6 (4.1-5.0) | 4.4 (3.8-5.0) | 5.1 (4.0-6.4) | .50 | |||||
| Women | 4.8 (3.6-6.4) | 3.8 (2.7-5.4) | 5.0 (4.1-6.1) | 4.4 (4.0-4.9) | 4.6 (4.1-5.1) | 5.1 (4.3-6.0) | .76 | |||||
| IL-18, pg/mL† | ||||||||||||
| Men | 335 (303-369) | 319 (285-356) | 400 (358-447) | 420 (403-436) | 435 (411-459) | 454 (417-493) | < .0001 | |||||
| Women | 268 (245-292) | 292 (253-336) | 325 (300-352) | 340 (325-353) | 357 (340-373) | 430 (392-470) | < .0001 | |||||
| TGF-β, ng/mL | ||||||||||||
| Men | 14.7 (12.7-16.7) | 10.2 (7.9-12.6) | 12.4 (10.6-14.1) | 11.9 (11.1-12.8) | 11.2 (10.0-12.4) | 13.9 (11.9-15.8) | .12 | |||||
| Women | 12.6 (11.0-14.1) | 12.1 (9.7-14.5) | 11.7 (9.9-13.4) | 11.2 (10.4-11.9) | 13.0 (11.8-14.2) | 12.3 (10.8-13.7) | .69 | |||||
| CRP, g/L† | ||||||||||||
| Men | 1.0 (0.8-1.4) | 1.9 (1.1-3.2) | 1.8 (1.3-2.5) | 2.6 (2.2-2.9) | 3.1 (2.6-3.7) | 5.4 (3.8-7.9) | < .0001 | |||||
| Women | 1.1 (0.8-1.5) | 1.1 (0.6-1.8) | 2.3 (1.8-2.9) | 2.5 (2.2-2.8) | 3.0 (2.6-3.5) | 3.3 (2.6-4.3) | < .0001 | |||||
| Fibrinogen, mg/mL | ||||||||||||
| Men | 269 (254-284) | 321 (290-352) | 315 (296-333) | 345 (335-354) | 345 (331-357) | 396 (372-419) | < .0001 | |||||
| Women | 317 (303-331) | 324 (302-345) | 352 (337-367) | 354 (346-361) | 370 (359-379) | 398 (381-415) | < .0001 | |||||
Values are reported as means and 95% confidence intervals. NA indicates not applicable.
Tests for trend from general linear models
Means and 95% confidence intervals were calculated from log-transformed values that were then back-transformed for data presentation
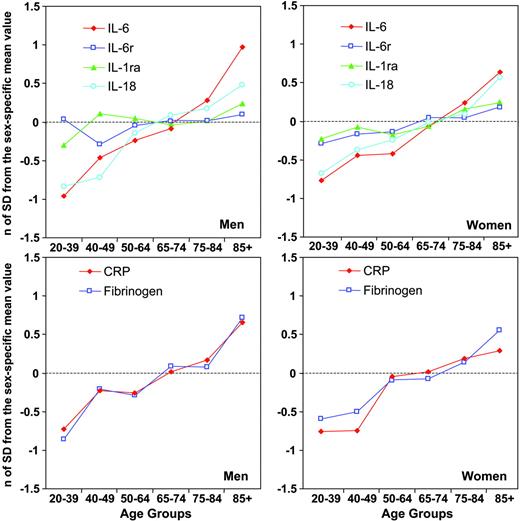
In Figure 1, log-transformed levels of inflammatory markers are expressed as the number of standard deviations from their means and plotted according to sex and age group. The age trajectories for IL-6 and IL-18 and those for CRP and fibrinogen are almost superimposable. The age-related increments of sIL-6r and IL-1ra are also very similar to each other but less steep, especially in men.
Mean values of inflammatory markers according to sex and age group expressed as number of standard deviations from the population mean to make them independent of different units of measure. (Top row)  indicates IL-6; □, IL-6r; ▴, IL-1ra; and ○, IL-18. (Bottom row)
indicates IL-6; □, IL-6r; ▴, IL-1ra; and ○, IL-18. (Bottom row)  indicates CRP; □, fibrinogen.
indicates CRP; □, fibrinogen.
Mean values of inflammatory markers according to sex and age group expressed as number of standard deviations from the population mean to make them independent of different units of measure. (Top row)  indicates IL-6; □, IL-6r; ▴, IL-1ra; and ○, IL-18. (Bottom row)
indicates IL-6; □, IL-6r; ▴, IL-1ra; and ○, IL-18. (Bottom row)  indicates CRP; □, fibrinogen.
indicates CRP; □, fibrinogen.
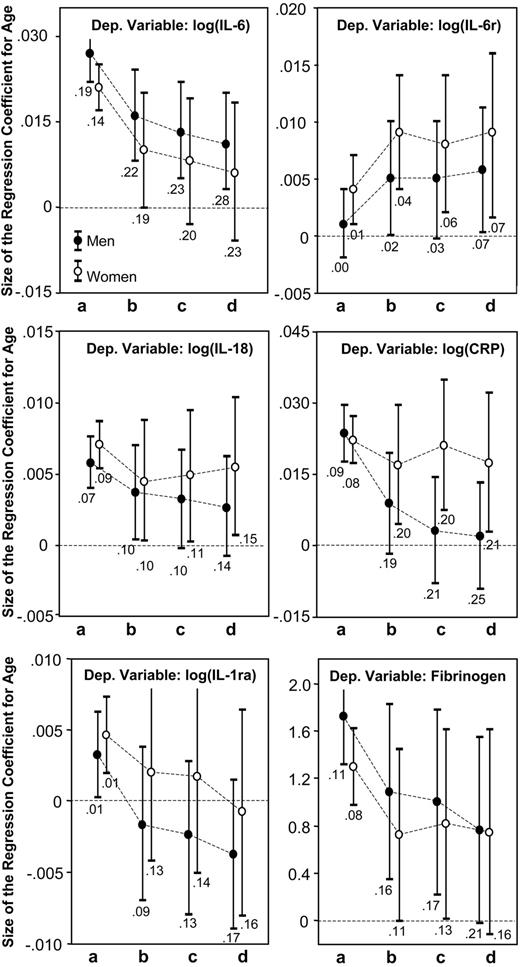
Figure 2 shows the size of the age regression coefficient, estimated from linear models predicting levels of inflammatory markers. Model “a” estimates the crude, unadjusted effect of age on the inflammatory marker; model “b” is adjusted cardiovascular risk factors; model “c” is also adjusted for subclinical cardiovascular diseases; and model “d” is adjusted for CHD, CHF, stroke, PAD, COPD, diabetes, hypertension, osteoporosis, CFR, cancer, dementia, and depression. In both men and women, adjusting for cardiovascular risk factors and morbidity reduced the size of the regression coefficients for age in the models predicting IL-6, IL-1ra, IL-18, and fibrinogen. In men but not in women, adjustment for disease burden completely removed the effect of age on CRP. In the model predicting sIL-6r, the size of the regression coefficients for age increased and became statistically significant after adjustment.
Age regression coefficients and their 95% CIs estimated from linear models predicting level of inflammatory markers. Model “a” estimates the crude affect of age; model “b” is adjusted for cardiovascular risk factors; model “c” is also adjusted for subclinical cardiovascular diseases; and model “d” is adjusted for CHD, CHF, stroke, PAD, COPD, diabetes, hypertension, osteoporosis, CFR, cancer, dementia, and depression. R2 values reported below the confidence interval are for the model used to estimate the age regression coefficients.
Age regression coefficients and their 95% CIs estimated from linear models predicting level of inflammatory markers. Model “a” estimates the crude affect of age; model “b” is adjusted for cardiovascular risk factors; model “c” is also adjusted for subclinical cardiovascular diseases; and model “d” is adjusted for CHD, CHF, stroke, PAD, COPD, diabetes, hypertension, osteoporosis, CFR, cancer, dementia, and depression. R2 values reported below the confidence interval are for the model used to estimate the age regression coefficients.
We also fitted fully adjusted models predicting inflammatory markers in the entire study population. Only for CRP, we found a significant age * sex interaction (P = .004), suggesting that the age-related increase of CRP may have different causes in men and women. Additionally, using backward selection, we identified the variables that best explained the variability of inflammatory markers. Beyond age and sex, the best sets of explanatory variables were low education, sedentary state, high LDL cholesterol, low HDL cholesterol, CHD, and hypertension for IL-6 (r2 = 0.24); high education, low BMI, not smoking, and absence of CHF for sIL-6r (r2 = 0.07); low HDL cholesterol, depression, disability, and CHD for IL-1ra (r2 = 0.16); low HDL cholesterol, diabetes, and CHD for IL-18 (r2 = 0.14); high BMI, low HLD cholesterol, sedentary state, PAD, CHF, and depression for CRP (r2 = 0.24); and sedentary state, high LDL cholesterol, low HDL cholesterol, and CHD and renal failure for fibrinogen (r2 = 0.18).
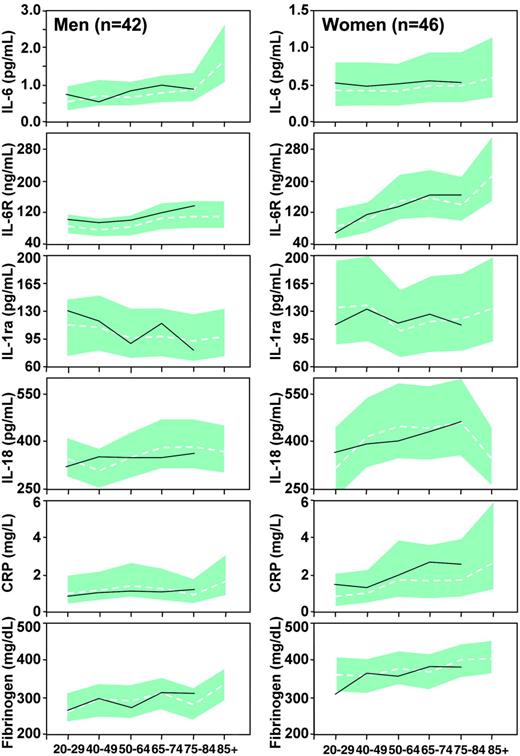
Mean values and 95% confidence intervals (CIs) of inflammatory markers estimated for men and women of different age groups assumed to have low cardiovascular risk profile and no morbidity are plotted in Figure 3, compared with the mean values of inflammatory markers calculated in the subgroup of 51 healthy men and 45 healthy women. Since none of these healthy participants was older than 85 years, values are not reported for the age group 85+. There is a remarkable degree of superimposition between data predicted from the models and those measured in healthy individuals.
Mean values (dashed lines) and 95% CIs (gray shaded areas) of inflammatory markers estimated for men and women of different age groups, under the assumption of low risk profile and no major morbidity. The continuous lines are crude mean values calculated in 51 healthy men and 45 healthy women with low risk profile and no morbidity. None of these healthy participants was older than 85 years.
Mean values (dashed lines) and 95% CIs (gray shaded areas) of inflammatory markers estimated for men and women of different age groups, under the assumption of low risk profile and no major morbidity. The continuous lines are crude mean values calculated in 51 healthy men and 45 healthy women with low risk profile and no morbidity. None of these healthy participants was older than 85 years.
Discussion
In a sample representative of the general population, we found that in both men and women, IL-6, IL-18, IL-1ra, CRP, and fibrinogen increased with age. Serum sIL-6r also increased slightly with age in women but not in men. Adjusting for cardiovascular risk factors and morbidity, the effect of age on IL-6 was substantially reduced. Age remained a significant predictor of higher IL-18 and CRP in women but not in men, although we found a significant age * sex interaction only for CRP. In both men and women, age was no longer a significant correlate of IL-1ra and fibrinogen, while the effect of age on serum sIL-6r increased substantially and became statistically significant. These findings were confirmed in a subset of 51 men and 45 women with a low risk profile and no cardiovascular morbidity.
Previous studies in small samples of healthy volunteers found that the production and release of IL-6, IL-1, and TNF-α into the circulation increased with age.13,22,23 Roubenoff et al2 showed that activated peripheral blood mononuclear cells' production of IL-6 and IL-1ra, but not of IL-1β and TNF-α increased with age. However, other studies that used more stringent definitions for healthy status failed to identify any statistical difference in the production of IL-1 and IL-6 between younger and older individuals.10,24 As none of the previous studies accounted for the confounding effect of cardiovascular risk factors and morbidity, whether age per se causes increased circulating levels of cytokines remained ambiguous. The prevalence of cardiovascular risk factors and morbidity increases with age and is associated with increased levels of proinflammatory markers.25 The successful treatment of risk factors is accompanied by reduced levels of inflammatory markers.26,27 In accordance, our findings suggest that the high prevalence of cardiovascular risk factors and morbidity significantly contributes to the high circulating levels of proinflammatory cytokines often found in older persons. Since increased levels of cytokines and APPs are associated with onset and clinical instability of atherosclerotic diseases,28-30 older persons may be affected by a vicious cycle where cardiovascular disease leads to an inflammatory response and inflammation stimulates the progression of cardiovascular disease.
The confounding effect of cardiovascular morbidity on the relationship between age and CRP was significantly different in men and women, suggesting that similar age trends in CRP serum level in men and women may have different causes. Interestingly, testosterone and estrogens affect cardiovascular risk factors and influence many processes that require an inflammatory response.31-33
The positive relationship between age and serum sIL-6r, which was detected only after adjusting for cardiovascular risk factors, is a novel finding. The IL-6 receptor consists of 2 subunits: a ligand binding 80-kDa low-affinity component (sIL-6r) and a signaltransducing 130-kDa component (soluble glycoprotein 130 [sgp130]), both of which exist in a soluble form. Since large quantities of sgp130 are present in the serum, the sIL-6r concentration is the limiting factor for the formation of the sgp130/IL-6/sIL-6r complex, which blocks the IL-6 activity.34 The increased sIL-6r production with aging may be an attempt to counteract increasing levels of IL-6. Unfortunately, the cardiovascular risk factors that stimulate the production of IL-6 also inhibit the production of sIL-6r.
Adjusting for cardiovascular risk factors and morbidity, we could not fully explain the effect of age on IL-6 in men and on IL-18 and CRP in women. Therefore, we cannot completely exclude the existence of a primary, age-associated dysregulation of cytokine production. However, our findings could also be explained by residual confounding due to the high prevalence of inflammatory, noncardiovascular conditions in older persons, or to age-related differences in severity and duration of cardiovascular risk factors and morbidity that could not be captured by our clinical measures. Additionally, there may be age-cohort effects on the levels of inflammatory markers that could not be detected in this cross-sectional study.
Several lines of research suggest that inflammation, independent of its origin, plays an important role in the pathogenesis of many diseases highly prevalent and frequent causes of disability in the elderly, such as dementia, sarcopenia, and osteoporosis. Thus, treating cardiovascular risk factors may have beneficial effects not only on cardiovascular disease but also on diseases affecting other organ systems. For example, our findings may explain why in prospective studies cardiovascular risk factors and mild inflammation predict Alzheimer disease35,36 and use of statins is associated with lowered risk of fractures.37
Prepublished online as Blood First Edition Paper, November 30, 2004; DOI 10.1182/blood-2004-07-2599.
Supported as a “targeted project” (ICS 110.1\RS97.71) by the Italian Ministry of Health and in part by the US National Institute on Aging (contracts 916413 and 821336).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal