Abstract
Aspergillus fumigatus (AF) is a ubiquitous mold and is the most common cause of invasive aspergillosis, an important source of morbidity and mortality in immunocompromised hosts. Using cytokine flow cytometry, we assessed the magnitude of functional CD4+ and CD8+ T-cell responses following stimulation with Aspergillus antigens. Relative to those seen with cytomegalovirus (CMV) or superantigen stimulation, responses to Aspergillus antigens were near background levels. Subsequently, we confirmed that gliotoxin, the most abundant mycotoxin produced by AF, was able to suppress functional T-cell responses following CMV or staphylococcal enterotoxin B (SEB) stimulation. Additional studies demonstrated that crude AF filtrates and purified gliotoxin inhibited antigen-presenting cell function and induced the preferential death of monocytes, leading to a marked decrease in the monocyte-lymphocyte ratio. Analysis of caspase-3 activation confirmed that gliotoxin preferentially induced apoptosis of monocytes; similar effects were observed in CD83+ monocyte-derived dendritic cells. Importantly, the physiologic effects of gliotoxin in vitro were observed below concentrations recently observed in the serum of patients with invasive aspergillosis. These studies suggest that the production of gliotoxin by AF may constitute an important immunoevasive mechanism that is mediated by direct effects on antigen-presenting cells and both direct and indirect effects on T cells.
Introduction
Aspergillus fumigatus (AF) is the most common cause of invasive aspergillosis (IA) and a major source of infection-related mortality in immunocompromised patients, such as allogeneic stem cell transplant (SCT) recipients.1,2 In these patients prophylactic antifungal therapy has been found to have little effect on disease incidence.1,3-5 Despite advances in early diagnosis and new antifungal agents,3,6,7 IA remains a leading cause of death in this patient population, with an attributable mortality rate ranging from 30% to 80%.8 AF is among the most ubiquitous of those fungi with airborne conidia (spores) and is commonly found in human domiciles. Pulmonary infection by AF, the predominant type of IA, is acquired through the inhalation of Aspergillus conidia, while the invasion stage of the disease is characterized by hyphal destruction of pulmonary tissue.1 The mediocre efficacy of antifungals in the setting of profound immunosuppression contributes to the poor prognosis of this opportunistic infection. The development of effective strategies to improve AF-specific immune reconstitution should greatly influence the natural history of IA.
Historically, there was a biphasic distribution of IA following bone marrow transplantation (BMT); IA was most common in the pre-engraftment period associated with neutropenia, with a second peak in incidence associated with acute and/or chronic graft-versus-host disease (GVHD).5 However, recent reports have indicated late-onset IA predominates after allogeneic SCT, often in concomitance with the occurrence of GVHD and corticosteroid administration,9,10 when patients are not neutropenic.3,6,7 It has been shown that lymphopenia also may function as a risk factor during the late posttransplant period. The protective role for T cells is supported by increased risk of IA associated with T-cell depletion or CD34 selection.6 These studies provided evidence that the adoptive transfer of T cells may be important in protection from disease in recipients.11
Because AF is a ubiquitous environmental fungus, it seems likely that healthy subjects would have detectable AF-specific T cells within peripheral blood mononuclear cells (PBMCs). Recently, it has been reported by Hebart et al12 that it is possible to detect AF-specific T-cell activation in healthy individuals in response to AF cellular extracts with a predominant release of interferon γ (IFNγ)in culture supernatants, indicating a TH1 response. Patients with IA showed a favorable response to antifungal therapy in the presence of a higher IFNγ–interleukin 10 ratio, suggesting the role of a TH1 response for the control of invasive disease.13,14
As is the case with other fungi, AF produces a number of toxins. The most abundant mycotoxin produced by AF is gliotoxin (GT), a hydrophobic metabolite that belongs to the class of epipolythiodioxopiperazine (ETP) compounds, characterized by the presence of a quinoid moiety and disulfide bridge across the piperazine ring.5,15 GT exhibits diverse biologic activities on the immune system. In murine models of IA, GT was shown to inhibit macrophage and polymorphonuclear cell function, including phagocytosis and bactericidal activity.16-18 Further, GT was shown to block the generation of alloreactive cytotoxic T lymphocytes.19 It has been shown that GT specifically inhibits the nuclear transcription factor NF-κB, a central regulator of the immune response, preventing the NF-κB–induced transcription of various inflammatory cytokines, hematopoietic growth factors, growth factor receptors, and cell adhesion molecules.20
The immunosuppressive properties of GT, in conjunction with the fact that the mycotoxin is released only by the hyphal form of AF,21 suggest that the in situ production of GT by AF might play an important role in the pathogenesis of IA. Because of its known effects on lymphocytes, GT has been considered for immunosuppressive therapy in bone marrow22 and solid organ transplantation.23 Recently, we demonstrated that high levels of circulating GT (eg, > 100 ng/mL) were detectable in serum specimens obtained from most patients with documented IA and only occasionally detectable in similar patients at risk for IA but without evidence of disease.24
In this study we show that CD4+ and CD8+ T cells in healthy donors demonstrate only weak functional responses to AF crude filtrates by cytokine flow cytometry (CFC) and that AF may actively suppress the T-cell response to viral pathogens and mitogens via production of GT. In addition, we sought to determine the mechanism by which GT inhibits T-cell responses. We demonstrated that GT acts primarily by targeting and killing antigen-presenting cells (eg, monocytes and dendritic cells), resulting in impaired antigen presentation. We also found, in vitro, that this effect on peripheral blood antigen-presenting cells is reached with doses of GT significantly below those demonstrated in serum in patients with documented IA.
Materials and methods
Peripheral blood mononuclear cell isolation
Healthy donor samples were obtained under protocols approved by the M.D. Anderson Cancer Center Institutional Review Board following informed consent conforming to principles consistent with those outlined in the Declaration of Helsinki. PBMCs were collected from healthy donors, separated by use of Ficoll-Hypaque density gradient centrifugation (Histopaque-1077, Sigma, St Louis, MO), washed twice in phosphate-buffered saline (PBS), and resuspended with RPMI-1640 medium supplemented with 10% AB serum (Sigma), penicillin, and streptomycin. All cell preparations were more than 95% viable as judged by trypan blue exclusion.
Preparation of Aspergillus fumigatus culture filtrate
Water-soluble extracts of AF antigens were prepared using a modification of methods reported by Hebart et al.12 Conidia of A fumigatus strain AF293, which is used in the A fumigatus sequencing project (Fungal Research Trust, Macclesfield, United Kingdom) were kindly provided by Dr David Denning (University of Manchester, United Kingdom) and were inoculated into 250 mL Erlenmeyer flasks containing Sabouraud liquid medium. Cultures were incubated at 37°C for 36 hours in a shaking incubator. The mycelial mat recovered by filtration was extensively washed with sterile water. Mycelium was then disrupted in a glass bead cell homogenizer in 50 mM Tris (tris(hydroxymethyl)aminomethane)–HCl buffer, pH 7.5, and the water-soluble cellular extracts were recovered after centrifugation. Protein content of the extracts was measured by the Bio-Rad protein assay according to the manufacturer's instructions (Bio-Rad, Hercules, CA) and standardized for use in PBMC stimulation.
Assessment of T-cell function by cytokine flow cytometry (CFC)
Functional T-cell responses were assessed by CFC as previously described.25,26 PBMCs were stimulated for 6 hours with staphylococcal enterotoxin B (SEB), cytomegalovirus preparations (CMV lysate, an overlapping peptide mixture spanning the antigenic pp65 protein, or an HLA A2–restricted pp65 peptide epitope), or AF crude filtrates. After 1 hour, brefeldin A (Sigma) was added to block the cellular release of cytokines. GT-induced T-cell suppression was assessed by incubating PBMCs in the presence of GT (Sigma) at defined concentrations outlined in the “Results” section. Functional activation in the CFC assay was assessed in the presence and absence of GT. Following stimulation, PBMCs were permeabilized and stained with fluorescein isothiocyanate (FITC)–conjugated CD4 or CD8, phycoerythrin (PE)–conjugated interferon γ (IFNγ) or tumor necrosis factor α (TNFα), and PerCP-conjugated CD69. They were then analyzed by sequential gating on lymphocytes by scatter, T cells expressing CD4 or CD8, and finally on cells co-expressing the activation marker CD69 and producing intracellular cytokines. FACScan analysis was carried out using 106 PBMCs per sample. Flow cytometric data were illustrated using FlowJo software (Tree Star, Ashland, OR).
Assessment of apoptosis using a caspase-3 induction assay
Apoptosis was measured using a flow cytometric assay system optimized for the detection of activated caspase-3 (BD Biosciences, San Jose, CA). PBMCs were permeabilized and stained with antibodies specific for the following: FITC-conjugated CD4, PE-conjugated active caspase-3, and PerCP-conjugated CD14; or with FITC-conjugated CD4, PE-conjugated active caspase-3, and PerCP-conjugated CD8. PBMCs were incubated in the presence or absence of GT at defined concentrations. The apoptotic fraction was assessed by flow cytometry by sequential gating on the monocyte fraction by scatter and on CD4+dim/CD14+ cells, and then on active caspase-3+ cells. Apoptosis in lymphoid subpopulations was assessed by sequential gating on lymphocytes by scatter, CD4+ or CD8+ or CD19+ cells, and then active caspase-3+ cells.
Assessment of apoptosis by annexin V staining
Cells used for assessment of apoptosis by annexin V staining were washed twice in PBS and resuspended in PBS + 2 nM ethylenediaminetetraacetic acid (EDTA) + 0.5% bovine serum albumin (BSA) after Ficoll separation. For isolation of CD14+ monocytes and CD14- lymphocytes, PBMCs were labeled with magnetic cell selection (MACS) CD14 microbeads and separated by the use of MS+ columns and the miniMACS separation system (Miltenyi Biotec, Bergisch, Germany). The purity of each cell population was 95% to 99% when confirmed by flow cytometry.
The apoptotic percentage in the monocyte and lymphocyte separated fractions was determined by analyzing phospatidylserine exposure and membrane integrity by double-staining with an FITC-conjugated anti–annexin V antibody (BD Biosciences, San Jose, CA) and propidium iodide (PI) (Sigma) and flow cytometric analysis (FACSCalibur and Cell Quest Pro software, BD Biosciences).27 Cells (106) were washed with PBS, resuspended in 50 μL of annexin binding buffer (BD Biosciences), and then incubated for 10 minutes at room temperature in the dark with an FITC-conjugated anti–annexin V antibody and PI (final concentration of 1 μg/mL). The cells were then washed and resuspended in binding buffer, and flow cytometric analysis was performed within 30 minutes of staining. The extent of apoptosis observed was reported as a percentage using the following formula: apoptotic fraction = (annexinV + PI– events)/[(annexinV + PI– events) + (annexinV–PI– events)].
Flow cytometric analysis of caspase-3 activation in mature dendritic cells
Monocytes were isolated from healthy donor PBMCs via adherence. Dendritic cell (DC) differentiation was induced using granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4). Subsequently, mature dendritic cells (mDCs) were obtained by culturing adherent PBMCs for 5 days with IL-4 and GM-CSF in a T-75 cm2 culture tissue flask and for the next 2 days with TNFα, IL-6, IL-1β, prostaglandin E2, and poly I/C in a T-25 cm2 tissue culture flask.
Mature DCs (mDCs) were permeabilized and stained with FITC-conjugated CD83 and PE-conjugated active caspase-3. mDCs were then analyzed by flow cytometry by sequential gating on DCs, using scatter and CD83 expression, and then on active caspase-3+ cells.
Statistical methods
Statistical analyses were performed using Prism statistical software (Graph-Pad Software, San Diego, CA). Intergroup comparisons were performed using the Mann-Whitney U test (for univariate nonparametric group analyses). All P values were 2-tailed and considered significant if less than .05. Results were presented using Prism (GraphPad) and Illustrator (Adobe, Seattle, WA) software on Macintosh computers (Apple Computer, Cupertino, CA).
Results
AF crude filtrates do not significantly activate human lymphocytes
Eight healthy donors were studied by CFC to detect AF-specific T cells within peripheral blood. Despite the use of different AF preparations (lysates, conidia, water-soluble cellular extracts) and escalating doses of AF antigens (ranging from 0.1 μLto 32 μL), we observed only occasional and relatively weak activation of T cells in CFC assays; for instance, the mean percentages in the AF-stimulated cells (0.19%) were comparable to those in the unstimulated cells (≤ 0.16%) (P = .65). Results from 8 representative CMV-seropositive donors, assessed following stimulation with AF, CMV, and the superantigen staphylococcal enterotoxin B (SEB), are shown in Figure 1. Frequencies of activated aspergillus-specific T cells significantly above background levels were not detected following stimulation using increasing concentrations of an AF lysate (unstimulated vs AF amounts in a 200-μL stimulation volume: 0.1 μL, 0.5 μL, 1 μL, 2 μL, 4 μL, 16 μL, 32 μL, P > .06 for all comparisons, data not shown). In contrast, the responses to control antigens such as SEB and CMV were in the expected ranges (with mean frequencies of 12.7% and 1.82%, respectively, for CD4 + TNFα + cells stimulated with SEB and CMV). Similarly, we failed to detect cytokine production in AF-stimulated CD8+ T cells (not shown). Given the ubiquity of AF as an environmental pathogen, these data suggested that human subjects were unable to sustain frequencies of AF-specific T cells similar to those recognizing endogenous herpes viruses. Based on the potential immunosuppressive properties of gliotoxin,17,18 we hypothesized that mycotoxin-mediated immune suppression might have contributed to our observed results.
Lack of significant AF-specific T-cell responses by CFC in healthy donors. PBMCs from healthy CMV-seropositive donors were stimulated with SEB, CMV lysate, and AF lysate or left unstimulated (US) and analyzed by CFC by sequential gating of lymphocytes, CD4+ T cells, and TNFα CD69+ cells. Stimulation with SEB and CMV lysate resulted in the expected fraction of functional cells with an average of 12.7% and 1.82%, respectively (US vs SEB, P = .0002; US vs CMV, P = .0002). Incubation of cells with Aspergillus fumigatus lysates resulted in frequencies of activated cells comparable to that seen in the unstimulated control sample (mean of 0.19% vs 0.16% in the US; US vs AF, P = .64). Similarly, we failed to detect cytokine production in AF-stimulated CD8+ T cells (not shown). Horizontal bars indicate means.
Lack of significant AF-specific T-cell responses by CFC in healthy donors. PBMCs from healthy CMV-seropositive donors were stimulated with SEB, CMV lysate, and AF lysate or left unstimulated (US) and analyzed by CFC by sequential gating of lymphocytes, CD4+ T cells, and TNFα CD69+ cells. Stimulation with SEB and CMV lysate resulted in the expected fraction of functional cells with an average of 12.7% and 1.82%, respectively (US vs SEB, P = .0002; US vs CMV, P = .0002). Incubation of cells with Aspergillus fumigatus lysates resulted in frequencies of activated cells comparable to that seen in the unstimulated control sample (mean of 0.19% vs 0.16% in the US; US vs AF, P = .64). Similarly, we failed to detect cytokine production in AF-stimulated CD8+ T cells (not shown). Horizontal bars indicate means.
GT inhibits human T-cell function in a dose-dependent manner
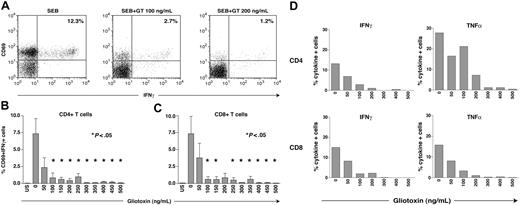
We tested the capacity of GT to suppress functional CD4+ and CD8+ T-cell responses to known antigens, including SEB and CMV preparations. We found a dose-dependent reduction in the frequency of cytokine-producing SEB- and CMV-stimulated T cells when GT was added to the culture. As evidenced by a typical subject (Figure 2A), the SEB response in the absence of GT was 12.3%, while the addition of GT, even at a low concentration of 100 ng/mL, resulted in a diminished response of only 2.7% in the CD4+ cells. The response decreased further to 1.2% when the concentration of GT was increased to 200 ng/mL. Figures 2B-C display the composite results from 5 healthy donors. Cells were stimulated with SEB and then incubated with GT at the specified concentrations. At minimal concentrations (50 ng/mL) the responses declined by approximately 50% in both the CD4+ and CD8+ T-cell compartments. This decline continued in both populations until a near-complete disappearance of specific responses was noted with doses equal to 300 ng/mL for CD4+ T cells and equal to 400 ng/mL for CD8+ T cells. These concentrations are below those typically found in crude AF lysates and approximate to those found in serum in patients with IA.24 Figure 2D depicts concordant suppression of T-cell activation in both CD4+ and CD8+ T-cell subsets in a representative experiment, irrespective of whether IFNγ or TNFα production was assessed as an end point in the CFC assay. Comparable suppression was seen following stimulation with CMV antigens in CD4+ and CD8+ T cells, in experiments assessing both IFNγ and TNFα production (data not shown).
GT inhibits functional CD4+ and CD8+ T-cell responses. PBMCs from 5 healthy donors were stimulated with SEB, CMV lysates (to stimulate CD4+ T cells), or a CMV pp65 peptide mixture (to stimulate CD8+ T cells). Escalating doses of GT were added ranging from 50 ng/mL to 500 ng/mL. Functional T-cell responses following SEB and CMV stimulation were assessed by cytokine flow cytometry by gating on CD4+ or CD8+ T cells co-expressing CD69 and intracellular IFNγ. Panel A shows a typical example of CFC FACS plots after stimulation with SEB and addition of GT. Panel B displays the effects of GT on the activation of CD4+ cells. P values (Mann-Whitney) are shown versus the no-GT sample. Panel C displays the effects on activation of CD8+ T cells. A decrease in the frequency of cytokine-producing SEB-activated T cells was found when GT was added to the culture in increasing concentrations. P values (Mann-Whitney) are shown versus the no-GT sample. GT-induced suppression was most pronounced at concentrations equal to 300 ng/mL for CD4+ and equal to 400 ng/mL for CD8+ T cells; at these concentrations functional activation was minimal. Similar results (data not shown) were seen following CMV antigen stimulation and when TNFα production was used as an analysis end point. Panel D illustrates a representative experiment illustrating gliotoxin-induced suppression of cytokine production (IFNγ, left panels; and TNFα, right panels) in both CD4+ and CD8+ T cells following SEB stimulation. Error bars indicate SEM; *P < .05.
GT inhibits functional CD4+ and CD8+ T-cell responses. PBMCs from 5 healthy donors were stimulated with SEB, CMV lysates (to stimulate CD4+ T cells), or a CMV pp65 peptide mixture (to stimulate CD8+ T cells). Escalating doses of GT were added ranging from 50 ng/mL to 500 ng/mL. Functional T-cell responses following SEB and CMV stimulation were assessed by cytokine flow cytometry by gating on CD4+ or CD8+ T cells co-expressing CD69 and intracellular IFNγ. Panel A shows a typical example of CFC FACS plots after stimulation with SEB and addition of GT. Panel B displays the effects of GT on the activation of CD4+ cells. P values (Mann-Whitney) are shown versus the no-GT sample. Panel C displays the effects on activation of CD8+ T cells. A decrease in the frequency of cytokine-producing SEB-activated T cells was found when GT was added to the culture in increasing concentrations. P values (Mann-Whitney) are shown versus the no-GT sample. GT-induced suppression was most pronounced at concentrations equal to 300 ng/mL for CD4+ and equal to 400 ng/mL for CD8+ T cells; at these concentrations functional activation was minimal. Similar results (data not shown) were seen following CMV antigen stimulation and when TNFα production was used as an analysis end point. Panel D illustrates a representative experiment illustrating gliotoxin-induced suppression of cytokine production (IFNγ, left panels; and TNFα, right panels) in both CD4+ and CD8+ T cells following SEB stimulation. Error bars indicate SEM; *P < .05.
GT induces preferential death of monocytes
Additional studies demonstrated that incubation with GT resulted in a substantial decrease in the frequency of viable monocytes during the stimulation period but no significant decrease in the frequency of lymphocytes (Figure 3). Following incubation with GT, the percentage of cells within the monocytoid gate was just 1.24%, while those within the lymphoid gate represented 47.4% of the total cell population (Figure 3A). In contrast, in the absence of GT, approximately 16.2% of cells fell within a monocyte gate, while 49.8% of cells had scatter properties consistent with lymphocytes (Figure 3B). Incubation of PBMCs with crude filtrates of AF hyphae also caused a considerable decrease in the number of viable monocytes, assessed by scatter, just as seen with incubation with GT, though the presence of GT could not be confirmed within the crude filtrates. While the lymphocyte fraction was reduced slightly from 28.6% to 20.3% after incubation with an AF lysate, the monocyte fraction was reduced substantially from 11.4% to 2.4% (Figure 3C). This reduction in the monocyte fraction led to a skewing in the number of lymphocytes compared to monocytes with the ratio (lymphocytes-monocytes) becoming greater with increasing doses of GT (Figure 3E). PBMCs cultured without GT contained a ratio of lymphocytes to monocytes of 2.8:1; in PBMCs incubated with GT at 150 ng/mL, this ratio increased to 13:1, while incubation with GT at 300 ng/mL resulted in a ratio of 42:1. Linear regression analysis (not shown) revealed a positive correlation between increasing GT concentration and higher lymphocyte-monocyte ratio (r2 = 0.91, P < .001). Taken together, these results suggest that GT induces preferential death of monocytes in vitro, leading to marked decreases in the ratio of monocytes to lymphocytes.
GT induces preferential death of monocytes. Incubation of PBMCs with GT resulted in a substantial decrease in the frequency of viable monocytes during the stimulation period and an increase in the lymphocyte-monocyte ratio. Panel A demonstrates GT-induced loss of monocytes by scatter. In the example shown, after 6 hours of incubation with 35 ng/mL GT, the lymphocyte fraction was 47.4%, while the monocyte fraction was 1.24% of the gated PBMC population. Panel B represents the control sample, in which monocytes amount to 16.2% of gated events, while the lymphocyte fraction was 49.8%. Panel C displays a similar loss of monocytes following incubation with an AF lysate. Following incubation for 6 hours with 32 μLAF lysate, the lymphocyte fraction was 20.3%, while the monocyte fraction was 2.4%. Panel D represents the control sample. Unstimulated cells show typical lymphocyte and monocyte fractions within the PBMC population, 28.6% and 11.4%, respectively. The ratio of lymphocytes to monocytes increased with escalating doses of GT. Panel E illustrates that the preferential loss of monocytes leads to an increasing lymphocyte-monocyte ratio in the presence of GT. Unstimulated PBMCs displayed a lymphocyte (□)–monocyte (▪) ratio of 2.8:1. This ratio increased to 13:1 for cells incubated with GT 150 ng/mL and 42:1 when PBMCs were incubated with GT 300 ng/mL. By linear regression (not shown) a strong positive correlation was seen between increasing GT concentration and lymphocyte-monocyte ratio (r2 = 0.91, P < .001). Arrows indicate location of monocytes by scatter. Error bars indicate SEM.
GT induces preferential death of monocytes. Incubation of PBMCs with GT resulted in a substantial decrease in the frequency of viable monocytes during the stimulation period and an increase in the lymphocyte-monocyte ratio. Panel A demonstrates GT-induced loss of monocytes by scatter. In the example shown, after 6 hours of incubation with 35 ng/mL GT, the lymphocyte fraction was 47.4%, while the monocyte fraction was 1.24% of the gated PBMC population. Panel B represents the control sample, in which monocytes amount to 16.2% of gated events, while the lymphocyte fraction was 49.8%. Panel C displays a similar loss of monocytes following incubation with an AF lysate. Following incubation for 6 hours with 32 μLAF lysate, the lymphocyte fraction was 20.3%, while the monocyte fraction was 2.4%. Panel D represents the control sample. Unstimulated cells show typical lymphocyte and monocyte fractions within the PBMC population, 28.6% and 11.4%, respectively. The ratio of lymphocytes to monocytes increased with escalating doses of GT. Panel E illustrates that the preferential loss of monocytes leads to an increasing lymphocyte-monocyte ratio in the presence of GT. Unstimulated PBMCs displayed a lymphocyte (□)–monocyte (▪) ratio of 2.8:1. This ratio increased to 13:1 for cells incubated with GT 150 ng/mL and 42:1 when PBMCs were incubated with GT 300 ng/mL. By linear regression (not shown) a strong positive correlation was seen between increasing GT concentration and lymphocyte-monocyte ratio (r2 = 0.91, P < .001). Arrows indicate location of monocytes by scatter. Error bars indicate SEM.
GT inhibits T-cell activation primarily by inhibiting antigen presentation
Next, we tested the capacity of GT to target the function of antigen-presenting cells (APCs) in vitro. In the typical cytokine flow cytometry assay of T-cell function, PBMCs are stimulated with antigens for 6 hours, with brefeldin A added to block cytokine export for the last 5 hours of the incubation period. The addition of brefeldin A, which is necessary to facilitate the detection of cytokines in activated T cells, is delayed to allow antigen presentation to occur during the first hour of incubation with antigens.
Addition of GT during the first hour of culture, when the majority of antigen-presentation takes place, resulted in greater inhibition of functional T-cell responses than when GT was added during the last 5 hours of the 6 hours of stimulation (Figure 4). Cells were stimulated with SEB or CMV, and either GT was added at time 0 (and washed 1 hour prior to addition of brefeldin A) or after 1 hour of incubation with the antigens. In the experiments in which GT was present during the first hour of stimulation, the frequencies of T cells activated following stimulation with viral antigens or superantigens declined significantly (eg, the frequency of CD4+CD69+TNFα+ T cells declined to 0.64% following SEB stimulation and 0.27% after CMV stimulation, similar to background levels). CD4+ T-cell responses were only moderately suppressed when GT was added after the initial hour of stimulation with SEB and CMV, 10.5% and 0.91%, respectively, compared to 13.9% and 1.86% in the absence of GT. Neither of these represented significant differences between the pairs (P = .19 for SEB and P = .09 for CMV). Responses within the CD8+ cells followed the same pattern. The addition of GT during the first hour resulted in a significantly reduced response to SEB and CMV (0.53% and 0.26%, respectively), while addition of GT after the first hour was associated with near-normal functional responses, 4.2% with SEB and 1.03% with CMV. Thus, the presence of GT in the crucial interval during which antigen presentation primarily occurs led to a marked decrease in T-cell activation. These results support the hypothesis that GT exerts its primary immunosuppressive effects via impairment of antigen presentation, rather than the direct inhibition of lymphocyte function.
GT inhibits T-cell activation via effects on antigen-presenting cells. CFC was performed to assess functional activation in CD4+ and CD8+ T cells after stimulation with SEB or CMV antigens. Cells were either cultured in the absence of GT (□) or with 35 ng/mL GT (▪). Panel A shows the effects of GT on SEB-stimulated CD4+ cells; panel B in CD8+ cells. Panel C shows the effects of GT on CMV-stimulated CD4+ cells; panel D in CD8+ cells. When GT was added at time 0 and washed at 1 hour, we observed a significant abrogation of functional T-cell responses (no GT control percentage always reported first): 14.6% vs 0.64% for CD4+ T-cell responses to SEB (P = .0002), 6.34% vs 0.53% for CD8+ T-cell responses to SEB (P = .0006), 1.96% vs 0.27% for CD4+ T-cell responses to CMV lysate (P = .03), and 0.78% vs 0.26% for CD8+ T-cell responses to an overlapping CMV pp65 peptide mixture (P = .028). When GT was added after the first hour of incubation and present through the remainder of the 6 hours of incubation, the magnitude of functional responses was comparable to that of the cells incubated without GT: 14.0% vs 10.5% (SEB-CD4), 5.84% vs 4.16% (SEB-CD8), 1.86% vs 0.91% (CMV lysate-CD4), and 1.37% vs 1.03% (pp65 mix-CD8). None of these represented a significant difference (P values were all ≥ .09). Error bars indicate SEM.
GT inhibits T-cell activation via effects on antigen-presenting cells. CFC was performed to assess functional activation in CD4+ and CD8+ T cells after stimulation with SEB or CMV antigens. Cells were either cultured in the absence of GT (□) or with 35 ng/mL GT (▪). Panel A shows the effects of GT on SEB-stimulated CD4+ cells; panel B in CD8+ cells. Panel C shows the effects of GT on CMV-stimulated CD4+ cells; panel D in CD8+ cells. When GT was added at time 0 and washed at 1 hour, we observed a significant abrogation of functional T-cell responses (no GT control percentage always reported first): 14.6% vs 0.64% for CD4+ T-cell responses to SEB (P = .0002), 6.34% vs 0.53% for CD8+ T-cell responses to SEB (P = .0006), 1.96% vs 0.27% for CD4+ T-cell responses to CMV lysate (P = .03), and 0.78% vs 0.26% for CD8+ T-cell responses to an overlapping CMV pp65 peptide mixture (P = .028). When GT was added after the first hour of incubation and present through the remainder of the 6 hours of incubation, the magnitude of functional responses was comparable to that of the cells incubated without GT: 14.0% vs 10.5% (SEB-CD4), 5.84% vs 4.16% (SEB-CD8), 1.86% vs 0.91% (CMV lysate-CD4), and 1.37% vs 1.03% (pp65 mix-CD8). None of these represented a significant difference (P values were all ≥ .09). Error bars indicate SEM.
GT induces monocyte death via apoptosis
We sought to determine whether monocyte death induced by GT occurred via apoptosis (Figure 5). To assess apoptosis induction by GT in monocytes, T cells, and B cells, PBMCs either were incubated with or without 35 ng/mL of GT, and the induction of caspase-3, a terminal effector of apoptotic cell death, was assessed using a flow cytometric assay (Figure 5). A typical example of caspase-3 expression in monocytes is shown in Figure 5A. Monocytes express very little active caspase-3 when incubated in media alone; after 3 hours the percentage of caspase-3–positive monocytes was still very low at 1.13%. In those monocytes that were incubated with GT, a relatively high percentage of cells, 6.1%, were caspase-3 positive after just 2 hours, and an even larger proportion, 27.4%, were positive after 3 hours.
GT preferentially induces apoptosis in monocytes. PBMCs from 7 healthy donors were analyzed by flow cytometry for caspase-3 activation at 30 minutes, 1, 2, 3, 4, and 6 hours after addition of GT (35 ng/mL). Panel A shows a typical histogram plot of caspase-3 expression in monocytes. Cells incubated without GT (top row) showed only low levels of caspase-3 expression, while incubation with GT (bottom row) substantially increased caspase-3 induction in a time-dependent fashion. By 2 and 3 hours, respectively, 6.1% and 27.4% of monocytes expressed active caspase-3. Panel B illustrates caspase-3 expression within monocyte and lymphocyte subsets. After 2 hours of incubation with 35 ng/mL GT (bottom row), monocytes (CD4+dimCD14+) a mean of 11.4% of cells expressed active caspase-3. CD4+ T cells, CD8+ T cells, and CD19+ B lymphocytes showed lower frequencies at the same time point (0.64%, 1.13%, and 2.0%, respectively). The frequency of active caspase-3–positive cells increased progressively. By 6 hours, 59.2% of monocytes, 7.1% of CD4+, 25.4% of CD8+, and 18.7% of CD19+ lymphocytes expressed caspase-3. In the absence of GT (top row), caspase-3 activity was detectable only after 4 hours, with relative fractions always below 5%. Error bars indicate SEM.
GT preferentially induces apoptosis in monocytes. PBMCs from 7 healthy donors were analyzed by flow cytometry for caspase-3 activation at 30 minutes, 1, 2, 3, 4, and 6 hours after addition of GT (35 ng/mL). Panel A shows a typical histogram plot of caspase-3 expression in monocytes. Cells incubated without GT (top row) showed only low levels of caspase-3 expression, while incubation with GT (bottom row) substantially increased caspase-3 induction in a time-dependent fashion. By 2 and 3 hours, respectively, 6.1% and 27.4% of monocytes expressed active caspase-3. Panel B illustrates caspase-3 expression within monocyte and lymphocyte subsets. After 2 hours of incubation with 35 ng/mL GT (bottom row), monocytes (CD4+dimCD14+) a mean of 11.4% of cells expressed active caspase-3. CD4+ T cells, CD8+ T cells, and CD19+ B lymphocytes showed lower frequencies at the same time point (0.64%, 1.13%, and 2.0%, respectively). The frequency of active caspase-3–positive cells increased progressively. By 6 hours, 59.2% of monocytes, 7.1% of CD4+, 25.4% of CD8+, and 18.7% of CD19+ lymphocytes expressed caspase-3. In the absence of GT (top row), caspase-3 activity was detectable only after 4 hours, with relative fractions always below 5%. Error bars indicate SEM.
The composite results of 7 experiments are shown in Figure 5B. Caspase-3 induction was assessed in CD14+ monocytes, CD4+ and CD8+ T cells, and in CD19+ B-cell fractions. In the absence of GT, the percentages of caspase-3–positive cells were low in all populations, never reaching 5% even after 6 hours of incubation. In contrast, cells incubated with GT show a marked increase in caspase-3 positivity over the time course. Although this increase occurred to some extent in all cell types, apoptosis was preferentially seen in monocytes. After just 3 hours, the monocyte fraction contains approximately 20% caspase-3–positive cells, and after 6 hours this number rises to almost 60%, and most monocytes in the incubated culture are dead or dying. In the 3 lymphocyte subsets CD4+, CD8+, and CD19+ cells, we observed much lower caspase-3–positive cell fractions of 1.9%, 1.8%, and 6.6% after 3 hours, respectively; and 7.1%, 25.5%, and 18.7% after 6 hours, respectively. These data demonstrated that the preferential destruction of monocytes was due to the induction of apoptosis by GT.
Confirmation of monocyte apoptosis using annexin V staining
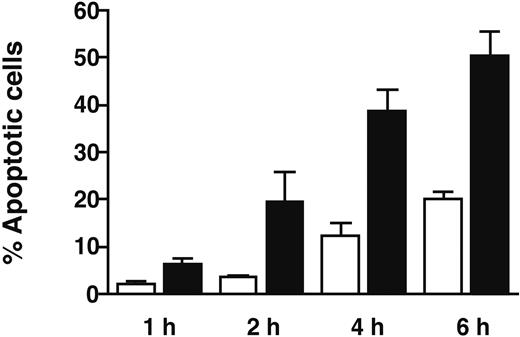
We sought to confirm our finding that GT induces apoptosis preferentially in monocytes in an annexin V assay of apoptosis. To eliminate the possibility of bystander effects, CD14+ and CD14- populations were separated using positive and negative selection using magnetic bead separation, prior to incubation with GT and assessment of annexin V staining by flow cytometry. Annexin V positivity represents a very early stage in the apoptotic pathway. Both CD14+ PBMC subsets (monocytes) and CD14 cells (lymphocytes) were cocultured in the presence of 35 ng/mL GT for 1 to 6 hours. The percentage of annexin-positive apoptotic cells increased during the incubation time in both cell populations, although the predominant effect of the mycotoxin was again seen in monocytes (Figure 6). The percentage of monocytes undergoing apoptosis as judged by annexin V and PI staining was 6.4% after 1 hour and 50.4% after 6 hours. Apoptosis also was induced by GT in lymphocytes, but to a much lesser extent (from an average apoptotic fraction of 2.2% after 1 hour to 20.3% after 6 hours). Apoptosis induction in monocytes was significantly higher than that observed in lymphocytes (P < .003). The controls showed a consistently low level of apoptosis during the time course (≤ 1.15%, data not shown).
Confirmation of GT-induced apoptosis by annexin V staining. Healthy donor PBMCs were separated into CD14+ (monocyte, ▪) and CD14- (lymphocyte, □) fractions using a magnetic bead cell selection system. Separated cells were incubated with 35 ng/mL GT for 1, 2, 4, and 6 hours. The percentage of apoptotic cells within the monocytes increased from 6.4% after 1 hour to 50.4% by the end of a 6-hour incubation. Apoptosis was significantly lower in lymphocytes than in monocytes, consistent with results obtained using caspase-3 induction as a marker of apoptosis. Error bars indicate standard deviation.
Confirmation of GT-induced apoptosis by annexin V staining. Healthy donor PBMCs were separated into CD14+ (monocyte, ▪) and CD14- (lymphocyte, □) fractions using a magnetic bead cell selection system. Separated cells were incubated with 35 ng/mL GT for 1, 2, 4, and 6 hours. The percentage of apoptotic cells within the monocytes increased from 6.4% after 1 hour to 50.4% by the end of a 6-hour incubation. Apoptosis was significantly lower in lymphocytes than in monocytes, consistent with results obtained using caspase-3 induction as a marker of apoptosis. Error bars indicate standard deviation.
GT induces apoptosis in monocyte-derived dendritic cells
Our previous experiments demonstrated that GT induced preferential depletion of peripheral blood monocytes via an apoptotic mechanism. Because DCs are important in primary immune responses and are the sentinel regulators of the T-cell response to Aspergillus infection,28,29 we sought to determine whether monocyte-derived DCs were similarly susceptible to apoptosis induction by GT. To test this hypothesis, we isolated monocytes from PBMCs via adherence and then induced them to differentiate into immature and then mature DCs. We then assessed the induction of apoptosis in monocyte-derived DCs using caspase-3 induction as an end point following sequential gating on DCs by scatter and CD83 expression (Figure 7). Results were similar to those obtained using peripheral blood monocytes, with marked induction of apoptosis in CD83+ DCs in the presence of GT.
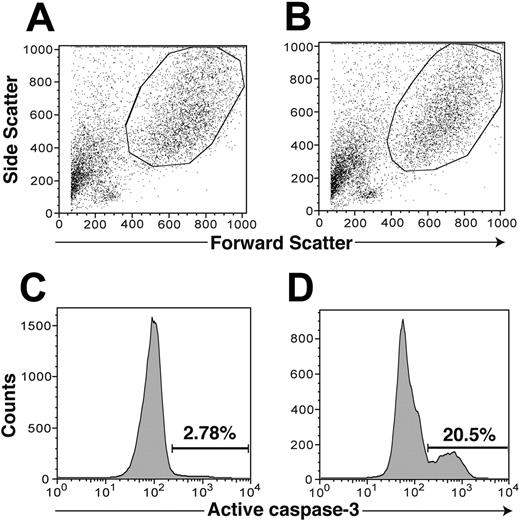
GT induces apoptosis in CD83+ dendritic cells. mDCs were incubated for 3 hours with or without 35 ng/mL GT and analyzed by flow cytometry by sequential gating on DC, CD83-positive cells, and active caspase-3–positive cells. Panel A depicts a control sample with no GT added to the culture. Panel B depicts that same sample with GT added. Panels C and D display the percentage of caspase-3–positive cells within the same cell populations as in panels A and B, respectively. Apoptosis, as evidenced by caspase-3 positivity, in DCs incubated with GT was increased more than 7-fold over the control (20.5% vs 2.8%, respectively).
GT induces apoptosis in CD83+ dendritic cells. mDCs were incubated for 3 hours with or without 35 ng/mL GT and analyzed by flow cytometry by sequential gating on DC, CD83-positive cells, and active caspase-3–positive cells. Panel A depicts a control sample with no GT added to the culture. Panel B depicts that same sample with GT added. Panels C and D display the percentage of caspase-3–positive cells within the same cell populations as in panels A and B, respectively. Apoptosis, as evidenced by caspase-3 positivity, in DCs incubated with GT was increased more than 7-fold over the control (20.5% vs 2.8%, respectively).
Discussion
Aspergillus fumigatus is an important pathogen in the postallogeneic SCT setting. The importance of finding ways to combat this pathogen is evidenced by the fact that invasive aspergillosis occurs in roughly 4% to 10% of SCT recipients, with overall mortality rates ranging from 30% to 80%.2,8 Determining the means by which AF induces pathogenesis could aid in the development of better prophylactic agents as well as better treatment regimens. The results of this study demonstrate that AF-specific T cells were detectable at very low frequencies in the peripheral blood of healthy donors. Importantly, we further demonstrated that concentrations of AF gliotoxin recently demonstrated in human subjects with IA were associated with the suppression of functional T-cell responses in vitro. Furthermore, our results suggest that GT inhibits antigen presentation and induces the preferential death of monocytes via an apoptotic mechanism and that apoptosis was similarly induced in monocyte-derived CD83+ DCs. These results suggest a novel and potentially clinically significant means by which AF may subvert a protective human cellular immune response.
Our results, using a CFC approach to detect AF-specific T-cell responses, differ from those of Hebart et al, who described the presence of detectable proliferative responses using a similar AF-derived antigen preparation in the majority of donors examined. Hebart et al also evaluated cytokine levels in cell-free supernatants after PBMC stimulation, whereas we evaluated cytokine production at the single-cell level by flow cytometry.12 It is possible that the approach we used here is less sensitive, though our prior studies and those of others have validated the use of CFC-based assays as an accurate and clinically meaningful way to detect the frequencies of human T cells recognizing CMV, HIV, and other relevant pathogens.25,26,30-33 It also is conceivable that patient-related variables or differences in the antigenicity of the AF strains used in our respective studies were responsible for the differences observed.
Prior studies have suggested that GT may inhibit T-cell activation via disruption of the NF-κB pathway. Additionally, inhibition of NF-κB activation increases apoptotic death in different cell types.20,34-36 In concordance with this relationship, GT has been found to cause apoptotic death in many cell types, including macrophages, thymocytes, enterocytes, and hepatic stellate cells as well as in a number of cancer cell lines such as HL-60 cells, P815 mastocytoma cells, and L929 fibroblasts in vitro.15,36-41 In vivo experiments have demonstrated the ability of GT to induce apoptosis in thymus, spleen, mesenteric nodes, and liver.42 However, the induction of apoptosis in human monocytes previously has not been suggested as a potential by which AF may suppress and evade the protective human immune response.
As an ETP compound, GT acts as a redox active toxin and has been shown to form mixed disulfides with accessible thiol residues on proteins.43 The inhibition of NF-κB could be caused by the interaction of GT with an essential thiol.20 Reduced GT has been specifically identified in cells following exposure to the toxin.16 The intracellular production of superoxide, hydrogen peroxide, and, finally, hydroxide radical reactive oxygen species during the GT redox cycle could directly damage cellular DNA, leading to a proapoptotic signaling cascade.44 Recently, Bernardo et al45 demonstrated that uptake and the subsequent cell killing by GT is dependent on intracellular glutathione, which is needed to actively concentrate the reduced dithiol form in the cell. Using glutathione depletors such as diethyl maleate and diamide, it was possible to disrupt GT uptake.45 What is not known is whether agents with similar activity might be used to prevent GT uptake in human subjects, perhaps inhibiting the toxic effects of GT production locally.
Our results may have important implications for potential diagnostic and therapeutic strategies aimed at improving outcomes in human IA. The demonstration that GT-induced T-cell suppression and monocyte apoptosis occurs at concentrations below those recently reported in human clinical subjects24 supports the possibility that gliotoxin levels might serve as a useful sentinel marker of disease progression or be associated with poor prognosis in IA. Further clinical studies will be necessary to address this hypothesis and to determine whether the extent of gliotoxin production by a given AF strain might influence pathogenicity and the likelihood of progression to IA in vivo. It also is important to note that other substances secreted by AF, in addition to or instead of gliotoxin, may have mediated the effects we observed due to crude filtrates. Additional studies to fractionate AF lysates could definitively establish what components of crude AF preparations may mediate immune suppression in vitro.
Prior studies have demonstrated a close association between CMV reactivation, CMV disease, and IA.6,7 It is possible that this association is simply due to the poor underlying state of the immune system in susceptible patients and the direct effects of immunosuppressive therapy for GVHD on the reactivation of both infections. However, our results suggest a model wherein early IA may result in the local elaboration of GT, which leads to the demise of APCs and their compromise, with additional direct and indirect effects on T cells known to be important in controlling CMV reactivation.25,26 Thus, GT production during the early stage of IA may directly increase the likelihood of local CMV reactivation, which may potentiate local tissue injury. Additional studies will be required to determine whether GT production at sites of early IA (eg, in the lung) results in an increased propensity to reactivate CMV locally.
Given the importance of IA in the post-SCT setting and the prior success of adoptive cellular therapies for other pathogens, including CMV and Epstein-Barr virus, vaccination and adoptive infusion of T cells has been contemplated as a method of prophylaxis or therapy for human IA. Our experiments suggest that the ability of APCs to present AF antigens is likely to be markedly impaired in the presence of GT. Thus, efforts to expand T cells ex vivo would likely be benefited by the use of AF antigen preparations that contain little or no GT.
In conclusion, we have demonstrated that the production of GT by AF may constitute a novel immunosuppressive mechanism that results in the destruction and functional impairment of the APC arm of the cellular immune response. Further studies will be required to determine the importance of this putative mechanism in the clinical setting. Additional studies may facilitate the development of novel clinical approaches to circumvent or neutralize the effects of GT in vivo. Such studies have the potential to overcome our existing limitations in diagnosing, preventing, and effectively treating AF infections in immunocompromised patients.
Prepublished online as Blood First Edition Paper, November 16, 2004; DOI 10.1182/blood-2004-09-3421.
Supported in part by grants from the Goodwin Family Foundation and the Gillson-Longenbaugh Foundation (K.V.K.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors thank Susan Bryan for expert technical assistance and Elizabeth Shpall for assistance with DC preparation. The authors also thank Jeffrey Molldrem and Eric Wieder for helpful discussions.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal