Comment on Melzner et al, page 2535
Primary mediastinal large B-cell lymphomas have frequent inactivating mutations of SOCS1 that impair JAK2 degradation and in turn maintain a constitutive activation of the JAK2/STAT pathway in these lymphomas.
Primary mediastinal large B-cell lymphoma (PMBL) is an aggressive lymphoid neoplasm thought to arise in thymic B cells that typically presents in young women as a bulky mediastinal mass. PMBL is characterized by a diffuse proliferation of large B cells, often with clear cytoplasm, low or negative immunoglobulin, and HLA class I and II expression, and is associated with a variable degree of sclerosis. These tumors often carry overrepresentation of the chromosomal region 9p24 containing Janus kinase 2 (JAK2).1 JAK tyrosine kinases, activated by cytokines, phosphorylate signal transducers and activators of transcription (STATs), which subsequently dimerize and translocate into the nucleus, promoting gene transcription (see figure). Transcriptional analyses of PMBL have detected a marked overexpression of several elements in the interleukin-4 (IL-4)/IL-13 pathway, including the receptor interleukin-13 receptor alpha-1 (IL-13Rα1), JAK2 and STAT, and several downstream target genes, suggesting that this pathway may play an important role in the pathogenesis of these tumors.2,3 Moreover, a recent study revealed constitutive activation of STAT6 in PMBL associated with JAK2 gene amplification, overexpression, and protein hyperphosphorylation.4
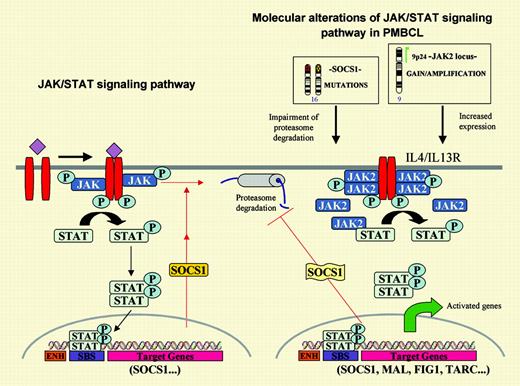
The JAK/STAT signaling pathway and its main oncogenic alterations in primary mediastinal large B-cell lymphoma. On the left, the JAK tyrosine kinases are activated by cytokines and phosphorylate the signal transducers and activators of transcription (STATs), which subsequently dimerize and translocate to the nucleus where they promote the transcription of target genes. One of these genes is the suppressor of cytokine signaling 1 (SOCS1) that inhibits the pathway in an autoregulatory loop by binding to the phosphorylated JAK and promoting its degradation through the proteasome pathway. On the right, PMBL carries 2 putative oncogenic aberrations in the JAK2/STAT pathway.JAK2is amplified and overexpressed and the protein is phosphorylated. Prolonged JAK2 half-life is sustained bySOCS1mutations. Expression profiling of PMBL has detected overexpression of the IL-4/IL-13 receptor, JAK2 and STAT, and several target genes of this pathway.
The JAK/STAT signaling pathway and its main oncogenic alterations in primary mediastinal large B-cell lymphoma. On the left, the JAK tyrosine kinases are activated by cytokines and phosphorylate the signal transducers and activators of transcription (STATs), which subsequently dimerize and translocate to the nucleus where they promote the transcription of target genes. One of these genes is the suppressor of cytokine signaling 1 (SOCS1) that inhibits the pathway in an autoregulatory loop by binding to the phosphorylated JAK and promoting its degradation through the proteasome pathway. On the right, PMBL carries 2 putative oncogenic aberrations in the JAK2/STAT pathway.JAK2is amplified and overexpressed and the protein is phosphorylated. Prolonged JAK2 half-life is sustained bySOCS1mutations. Expression profiling of PMBL has detected overexpression of the IL-4/IL-13 receptor, JAK2 and STAT, and several target genes of this pathway.
In this issue of Blood, Melzner and colleagues provide exciting new insights into JAK2 signaling in PMBL. The authors were intrigued by the fact that the Hodgkin lymphoma cell line L428, also carrying JAK2 gene amplification and overexpression, showed virtually no phosphorylation of JAK2 and STAT proteins, in contrast to the PMBL cell line MedB-1. In addition, JAK2 protein degradation in MedB-1 seemed delayed, whereas JAK2 half-life in the L428 cell line was much shorter and similar to the turnover observed in physiologic conditions. Searching for possible alterations in the mechanisms regulating JAK2 degradation, the authors detected biallelic inactivating mutations of the suppressor of cytokine signaling 1 (SOCS1) in MedB-1 that were not present in the germ line of the original patient. SOCS1 is a transcriptional target of STAT that inhibits JAK2 signaling by mediating its proteasome degradation (see figure). The functional role of these mutations was confirmed by transfection of wild-type SOCS1, which reduced the proliferation rate of the cells and the levels of phosphorylated JAK2 and STAT. In addition, SOCS1-inactivating mutations were also found in 9 of 20 primary PMBL cases, indicating that they represent a frequent molecular aberration in these tumors. Further studies should clarify the downstream genes deregulated by these alterations. Although Melzner et al show the transcriptional modulation of cyclin D1 by JAK2, its role in PMBL is questionable since cyclin D1 mRNA up-regulation does not always translate into protein5 and, in addition, cyclin D1 is not usually expressed in B lymphocytes or B-cell lymphomas other than mantle cell lymphoma.6 It is noteworthy that PMBL appears to carry 2 putative oncogenic alterations targeting the same signaling pathway, namely JAK2 amplification and SOCS1-inactivating mutations (see figure). Intriguingly, these 2 alterations do not seem to be mutually exclusive, since almost all primary tumors with SOCS1 mutations also carry gains of chromosome 9 and, therefore, presumably JAK2 gains or amplifications. These findings may suggest that the alterations have a synergistic effect or, given the role of SOCS1 in maintaining JAK2 activation, 9p gains may be selected for the presence of additional targets such as PDL2, usually coamplified and overexpressed with JAK2 in PMBL.2
I thank Itziar Salaverria and Lluís Hernández for their collaboration in the design of the figure. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal