Abstract
The application of allogeneic stem cell transplantation (alloSCT) is limited by graft-versus-host disease (GVHD). GVHD can be divided into acute and chronic forms that likely have different requirements for initiation and pathogenesis mechanisms. In prior studies we demonstrated that residual host antigen-presenting cells (APCs) were required to initiate acute GVHD (aGVHD) mediated by CD8 T cells. In contrast, here we demonstrate that either donor or host APCs can initiate CD4-mediated GVHD in a model that has features of chronic GVHD (cGVHD). Both donor and host APCs must provide CD80/86-dependent costimulation to elicit maximal cGVHD, and there is no GVHD when both donor and host lack CD80/86. Finally, we were surprised to find that, although either donor or host APCs are sufficient to stimulate skin cGVHD, donor APCs play a dominant role in intestinal cGVHD. Both CD40 and CD80/86 are critical for donor APC function in intestinal cGVHD, but only CD80/86 is required for skin cGVHD. Thus, there are target-tissue–specific differences in APC requirements. These results identify differences in APC requirements between CD8-mediated aGVHD and CD4-mediated cGVHD. They further highlight donor APCs as additional targets for GVHD therapy.
Introduction
Chronic graft-versus-host disease (GVHD) is an increasingly common complication of allogeneic stem cell transplantation (alloSCT), affecting up to 80% of patients in some series. The rise in incidence is likely multifactorial and may be influenced by the greater number of patients at risk because of better supportive care, use of peripheral blood stem cells, delayed leukocyte infusion, nonmyeloablative alloSCT, and early withdrawal of immunosuppression.1-7 Most strategies for preventing and treating GVHD are directed toward depleting or impairing the function of donor T cells. Yet despite the introduction of new anti-GVHD therapies, including those that target tumor necrosis factor-alpha (TNF-α)– and interleukin-2 receptor (IL-2R)–expressing cells, chronic GVHD (cGVHD) continues to be a serious problem. Therefore, a better understanding of the induction and pathogenic mechanisms of cGVHD is needed.
GVHD is initiated when alloreactive T cells are primed by professional antigen-presenting cells (APCs) to undergo clonal expansion and maturation. Our studies have focused on the mechanics of antigen presentation, which are a crucial initial step and a potential therapeutic target. For a period of time following alloSCT, patients are chimeric for host and donor APCs.8-11 Previously, we showed that radiation-resistant host APCs are required for CD8-mediated acute GVHD (aGVHD) across minor histocompatibility antigens (miHAs) and major histocompatibility complex (MHC) mismatches.12,13 Although host APCs are critical in a CD8-dependent aGVHD model, there are reasons to believe that both donor and host APCs may be involved in cGVHD. Because cGVHD occurs (by definition) later than aGVHD, it may be initiated, or at least progress, when hematopoiesis from donor-derived cells predominates.9 However, early T-cell activation could still be important, especially since the immediate posttransplantation period is particularly conducive to T-cell activation when host-derived APCs are still present and functional.
Another potential difference between aGVHD and cGVHD is the relative roles of CD4 and CD8 T cells, which in turn differ in their APC requirements. There is evidence that CD4 cells are particularly important in cGVHD, at least in murine models.14 CD4 cells recognize peptides presented on MHC class II by APCs that primarily (but not exclusively) present exogenously derived peptides. In contrast, CD8 cells preferentially recognize endogenously synthesized peptides presented via the MHC class I processing pathway. In CD4-mediated GVHD, both donor and host APCs should have equal capacity to process and present exogenously acquired host antigens on MHC class II.15-18 However, only host APCs can stimulate donor CD4 cells via endogenously synthesized miHAs presented on MHC class II.19-22
We examined the roles of donor- and host-derived APCs in the MHC-identical, multiple miHA mismatched B6.C or B10.D2 (H-2d) → BALB/c (H-2d) murine model of CD4-dependent cGVHD. This model shares key features of human cGVHD. Its dominant features include skin fibrosis as a result of increased collagen deposition, follicular dropout, loss of subdermal fat, and dermal mononuclear infiltrates. Hepatic disease is characterized by intrahepatic and extrahepatic bile duct mononuclear infiltration followed by fibrous thickening and sclerosis of the bile duct wall.23-25 Pulmonary fibrosis has been observed26 as has inflammation and destruction of salivary and lacrimal glands (B.A., our unpublished observations, February 2003). We have been studying this model in the hopes that the similarities it shows to cGVHD in humans will reveal useful insights into the authentic disease. Using this model, we found that, in contrast to CD8-dependent aGVHD,12 either host or donor APCs were sufficient to induce murine cGVHD. Moreover, the activation of miHA-specific donor T cells by APCs occurred via a CD80/86-dependent mechanism. Interestingly, while host APCs played a more dominant role in skin disease, intact donor APCs were essential for maximal gut GVHD. Thus, APC requirements differ in part depending on the target tissue. These results suggest that strategies that target either donor- or host-derived APCs may mitigate the manifestations of CD4-dependent GVHD and/or cGVHD and provide a strong rationale for targeting both donor and host APCs, rather than just host APCs in such situations.
Materials and methods
Mice
BALB/c mice were purchased from the National Cancer Institute (Frederick, MD). B10.D2.oSN, CD40-/- (on a BALB/c background) and B6.C mice (C57Bl/6 mice onto which the H-2d MHC locus has been backcrossed) were purchased from the Jackson Laboratory (Bar Harbor, ME). CD80/86-/- mice (on a BALB/c background) were backcrossed for more than 10 generations from the original knock-out mice,27 kindly provided by Arlene Sharpe (Brigham and Women's Hospital, and Harvard Medical School, Boston, MA). B6.C, CD80/86-/- (B6.C), and CD40-/- (B6.C) were bred and housed under specific pathogen-free conditions at Yale University School of Medicine. All recipients were 8 to 12 weeks at the time of initial transplantation.
Bone marrow transplantation (BMT)
Donor animals (B10.D2.oSN, B6.C, CD80/86-/- [B6.C], CD40-/- (B6.C]) and recipient animals (BALB/c, CD80/86-/- [BALB/c], CD40-/- [BALB/c]) were all H-2d. Recipient mice received total body irradiation (TBI) from a 137Cs source as either a single dose of 850 cGy or 2 doses of 425 cGy separated by 3 hours. Three to 5 hours following the last irradiation dose all recipients received 0.8 × 107 T-cell–depleted bone marrow (BM) suspended in injection buffer (1 × phosphate-buffered saline, 10 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid], 2.5% acid citrate dextrose anticoagulant, 0.5% penicillin-streptomycin) with or without WT B10.D2 or B6.C donor T cells via tail-vein injection. Total spleen cell dose was 107 cells/recipient; the purified CD4 cell dose was 2 × 106. Animals were given water supplemented with trimethoprim-sulfamethoxazole for 2 weeks following BMT.
Chimeric recipients
(Donor → host) and (host → host) chimeric recipients were prepared by transplanting B10.D2 or BALB/c BM, respectively, into BALB/c mice, as described in “Bone marrow transplantation (BMT).” Recipients were rested for more than 2 months to allow full reconstitution of the hematopoietic system by donor cells. Lymph nodes (LNs) and spleens of (donor → host) chimeras contained less than 2% recipient-type dendritic cells as determined by flow cytometry. Chimeric recipients were then used in a standard GVHD-inducing BMT.
Cell separations
BM cells were isolated and prepared as previously described.28,29 Remaining Thy1.2-positive cells were routinely less than 0.5% of BM cells as determined by flow cytometry.
Splenic CD4 cells were isolated using BioMag beads (QIAGEN, Valencia, CA) as previously described.29 For experiments using CD80/86-/- recipients and pure CD4 cells, CD4 cells were further enriched after BioMag-based purification as follows: BioMag-enriched CD4 cells (70%-80% pure) were incubated with biotinylated anti-CD4 (GK1.5) for 30 minutes on ice. Cells were washed once in magnetic cell sorting (MACS) buffer and then incubated with streptavidin-conjugated microbeads (Miltenyi Biotech, Auburn, CA) for 30 minutes at 4°C. CD4 cells were positively selected using an AutoMACS (Miltenyi Biotech), and resulting cells were more than 98% CD4 as determined by flow cytometry.
Clinical and pathologic scoring
Animals were analyzed for clinical and pathologic cGVHD as previously described.29 The following scoring system was used: healthy appearance = 0; skin lesions with alopecia less than 1 cm2 in area = 1; skin lesions with alopecia 1 to 2 cm2 in area = 2; skin lesions with alopecia more than 2 cm2 in area = 3. Additionally, animals were assigned 0.3 point each for skin disease (lesions or scaling) on ears, tail, and paws. Minimum score = 0, maximum score = 3.9. Incidence and clinical score curves represent all mice with scores 0.6 or higher. Final scores for dead animals were kept in the data set for the remaining time points of the experiment. Slides of skin were scored by a dermatopathologist (J.M.M.; blinded to experimental groups) on the basis of dermal fibrosis, fat loss, inflammation, epidermal interface changes, and follicular dropout (0-2 for each category). Minimum score was 0, and maximum score was 10. Colon slides were scored by a gastrointestinal pathologist (D.J.; blinded to experimental groups) on the basis of inflammation and apoptosis (0-3 for each category). Minimum score was 0, and maximum score was 6.
Statistical methods
The significance of differences in cGVHD incidence was calculated by log-rank Mantel-Cox. The significance of differences between clinical scores and pathology scores were calculated by the Mann-Whitney nonparametric test. Significance of differences of weight changes was calculated by Student t test.
Results
CD80/86 expression on either donor or host APCs is required for cutaneous cGVHD
Our overall goal was to determine the relative contributions of donor- and host-derived APCs in the genesis of cGVHD. Our prior studies in this model determined that cGVHD is initiated by naive donor CD4 cells.29 Because the signals delivered by CD28: CD80/86 interactions are known to be critically important for activation of naive CD4 cells, we chose to use CD80/86-/- donors and/or recipients in our cGVHD experiments. This was the optimal choice for inactivating both donor and host APCs. We could not use MHC class II–deficient donors or recipients, because the H-2d haplotype contains 2 MHC class II β chain genes, and double knockouts are not available. Similarly, invariant chain knockouts and class II transactivator knockouts, in which MHC class II expression has been reported to be reduced, are not suitable because they have substantial MHC class II expression on dendritic cells, especially under inflammatory conditions (Kenty and Bikoff30 and our unpublished data, B.A., March 2002).
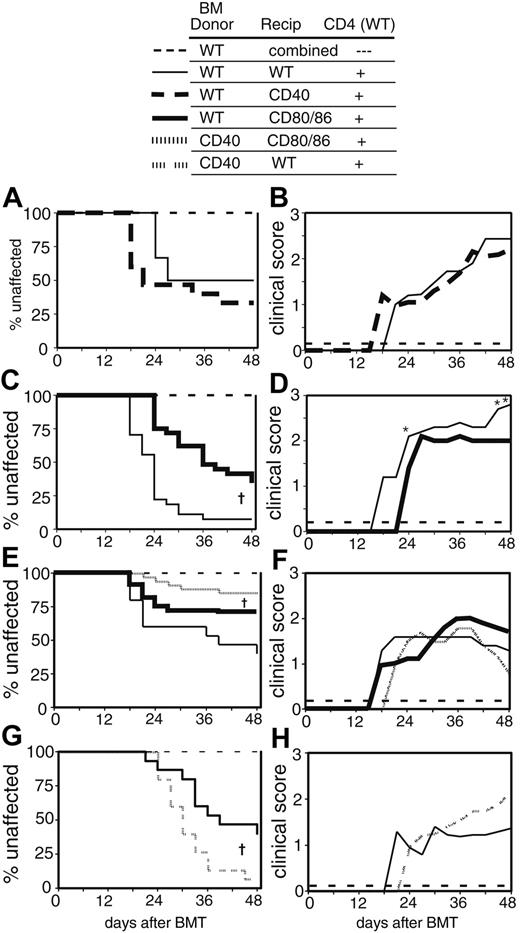
To test the validity of our approach, we first determined whether cGVHD required CD28:CD80/86 interactions. We eliminated CD28:CD80/86 signaling by all APCs (donor and host) by transplanting CD80/86-/- BM and highly purified wild-type (WT) CD4 cells into CD80/86-/- recipients. Strikingly, no clinical cGVHD developed in these mice, in contrast to WT recipients of WT BM and CD4 cells (Figure 1A). Therefore, donor CD4 cells absolutely require signals from CD80/86 to mediate clinical cGVHD of the skin in this model, validating the use of CD80/86 knockouts to identify the roles of donor and host APCs individually.
CD80/86 expression is required for skin cGVHD. Combined data from 3 experiments are shown. On day 0 recipient mice were lethally irradiated and reconstituted with 8 × 106 BM cells WT BM or CD80/86-/- [CD80/86] BM) alone or with 2 × 106 WT CD4 cells (BM + CD4). All BM controls (ctrls): WT or CD80/86-/- recipients of WT or CD80/86-/- BM (n = 20); WT recipients of WT BM + CD4 (n = 17); WT recipients of CD80/86-/- BM + CD4 (n = 8); CD80/86-/- recipients of WT BM + CD4 (n = 28); CD80/86-/- recipients of CD80/86-/- BM + CD4 (n = 16). (A) Incidence of cGVHD. †P < .01 for CD80/86-/- recipients of WT BM + CD4 as compared with all other experimental groups. (B) Average clinical disease score for mice affected with cGVHD (unaffected mice are excluded). †P < .01 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with all other CD4 recipients. BM control mice and CD80/86-/- recipients of CD80/86-/- BM + CD4 did not get cGVHD and are represented on the graph as scoring “0.” (C) Pathology scores for representative mice. Mean score is indicated by a horizontal bar. †P < .01 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with all other CD4 recipients. P = .1715 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with BM control recipients.
CD80/86 expression is required for skin cGVHD. Combined data from 3 experiments are shown. On day 0 recipient mice were lethally irradiated and reconstituted with 8 × 106 BM cells WT BM or CD80/86-/- [CD80/86] BM) alone or with 2 × 106 WT CD4 cells (BM + CD4). All BM controls (ctrls): WT or CD80/86-/- recipients of WT or CD80/86-/- BM (n = 20); WT recipients of WT BM + CD4 (n = 17); WT recipients of CD80/86-/- BM + CD4 (n = 8); CD80/86-/- recipients of WT BM + CD4 (n = 28); CD80/86-/- recipients of CD80/86-/- BM + CD4 (n = 16). (A) Incidence of cGVHD. †P < .01 for CD80/86-/- recipients of WT BM + CD4 as compared with all other experimental groups. (B) Average clinical disease score for mice affected with cGVHD (unaffected mice are excluded). †P < .01 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with all other CD4 recipients. BM control mice and CD80/86-/- recipients of CD80/86-/- BM + CD4 did not get cGVHD and are represented on the graph as scoring “0.” (C) Pathology scores for representative mice. Mean score is indicated by a horizontal bar. †P < .01 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with all other CD4 recipients. P = .1715 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with BM control recipients.
In the next set of experiments we compared cGVHD in CD80/86-/- BM + CD4 T cells → WT versus WT BM + CD4 T cells → CD80/86-/- to debilitate antigen presentation by donor or host APCs, respectively. Cutaneous cGVHD developed in both groups, demonstrating that donor or recipient APCs are sufficient to initiate disease (Figure 1A). However, the incidence of cutaneous cGVHD in CD80/86-/- recipients was less than that in WT recipients (Figure 1A). This suggested that recipient APCs are more important for eliciting cutaneous cGVHD than donor APCs. In support of this, cGVHD incidence was not reduced when CD80/86-/- BM + CD4 T cells were given to WT recipients, suggesting that, when host APCs are intact, reconstitution with defective donor APCs does not affect disease.
Although the incidence of cGVHD was reduced in WT → CD80/86-/- mice, the extent of disease among affected mice as measured by clinical score was indistinguishable from WT → WT or CD80/86-/- → WT cGVHD mice (Figure 1B). Consistent with the clinical score, histologic disease was similar in all affected mice (Figure 1C). Thus, regardless of which APCs were impaired, once cGVHD developed, it was similar to that seen in WT → WT mice.
Host-type APCs are not required to initiate cGVHD
To address whether host-type APCs needed to be resident at the time of transplantation for optimal GVHD induction, we compared cGVHD in (donor → host) and control (host → host) chimeras. cGVHD developed in (donor → host) chimeras (Figure 2), even though more than 98% of APCs were donor-type (flow cytometry, data not shown). The onset and incidence of cGVHD in (donor → host) chimeras was reduced to a slight but statistically significant (P < .01) degree compared with (host → host) chimeras (Figure 2A). Severity and pathology scores were indistinguishable in the 2 groups (Figure 2B-C). Thus, cGVHD can be initiated by donor APCs, but host APCs are required for the maximal penetrance of skin disease, consistent with the data using CD80/86-/- recipients. It was reported in another model that Langerhans cells in skin remained host type unless donor T cells were also transferred. In a fully allogeneic model, persistence of host Langerhans cells correlated with severity of GVHD.31 Although Langerhans cells in our recipients may have remained host-type, GVHD was actually reduced in such mice, indicating a role for host-type APCs other than Langerhans cells.
Donor-type APCs are sufficient for induction of cGVHD. Combined data from 2 experiments are shown. On day 0, chimeric recipient mice (previously prepared) were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (broken line; n = 21); or WT BM plus 107 WT spleen cells (host → host) (thin solid line; n = 32); (donor → host) (bold solid line; n = 36). Data for all BM control recipients were combined. (A) Incidence of cGVHD. †P < .01 for donor → host recipients versus host → host recipients of spleen cells. (B) Average clinical disease score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” (C) Pathology scores for representative mice. Mean score is indicated by a horizontal bar.
Donor-type APCs are sufficient for induction of cGVHD. Combined data from 2 experiments are shown. On day 0, chimeric recipient mice (previously prepared) were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (broken line; n = 21); or WT BM plus 107 WT spleen cells (host → host) (thin solid line; n = 32); (donor → host) (bold solid line; n = 36). Data for all BM control recipients were combined. (A) Incidence of cGVHD. †P < .01 for donor → host recipients versus host → host recipients of spleen cells. (B) Average clinical disease score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” (C) Pathology scores for representative mice. Mean score is indicated by a horizontal bar.
CD80/86 costimulation in cGVHD is independent of CD40
We have so far demonstrated essential roles for CD80/86 in the cGVHD model. Expression of CD80 and CD86 is normally increased as part of dendritic cell (DC) maturation in response to a variety of signals. CD40 could be a critical molecule upstream of CD80/86 up-regulation since, when engaged by CD40L on activated CD4 cells, it induces DC maturation. We thus used CD40-deficient hosts to investigate whether CD40-mediated APC activation and maturation is important for cGVHD. We saw equivalent cGVHD when we transplanted WT BM and total splenocytes into WT and CD40-/- recipients (Figure 3A-B). In contrast, parallel experiments transplanting WT BM and total splenocytes to CD80/86-/- recipients resulted in muted cGVHD (Figure 3C-D), similar to results in prior experiments transplanting purified CD4 cells (Figure 1). Thus, either CD40 is not essential for up-regulating host APC CD80/86 expression or instructive CD80/86 up-regulation is not required at all for cGVHD induction.
Differential roles of CD40 and CD80/86 on donor and host APCs in cGVHD. (A) Incidence of cGVHD in CD40-/- (CD40) recipients. One representative experiment is shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone; both recipient types (n = 9); or WT BM plus 107 WT spleen cells as a source of CD4 cells, WT recipients (n = 15), CD40 recipients (n = 14). (B) Clinical disease in CD40 recipients. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” (C) Incidence of cGVHD in CD80/86-/- recipients. Combined data from 2 experiments are shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone; both recipient types (n = 19); or WT BM plus 107 WT spleen cells as a source of CD4 cells, WT recipients (n = 27), CD80/86-/- recipients (n = 29). †P < .01 for CD80/86-/- recipients versus WT recipients of spleen cells. (D) Clinical disease in CD80/86-/- recipients. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” *P < .05 for CD80/86-/- recipients as compared with WT recipients of spleen cells. (E) Incidence of cGVHD in CD80/86-/- recipients of CD40-/- BM. Combined data from 2 experiments are shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT or CD40-/- BM cells alone; both BM types (n = 22), WT BM plus 2 × 106 purified CD4 cells, WT recipients (n = 15), CD80/86-/- recipients (n = 32); or CD40-/- BM plus 2 × 106 purified CD4 cells, CD80/86-/- recipients (n = 34). †P < .01 for CD80/86-/- recipients of CD40-/- BM + CD4 cells versus WT BM + CD4 cells. (F) Clinical disease in CD80/86-/- recipients of CD40-/- BM. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” (G) Incidence of cGVHD in WT recipients of CD40-/- BM. One representative experiment of 2 is shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT or CD40-/- BM cells alone; both BM types (n = 10), WT BM plus 2 × 106 purified CD4 cells (n = 15), or CD40-/- BM plus 2 × 106 purified CD4 cells (n = 15). †P < .01 for recipients of CD40-/- BM + CD4 cells versus WT BM + CD4 cells. (H) Clinical disease in WT recipients of CD40-/- BM. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.”
Differential roles of CD40 and CD80/86 on donor and host APCs in cGVHD. (A) Incidence of cGVHD in CD40-/- (CD40) recipients. One representative experiment is shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone; both recipient types (n = 9); or WT BM plus 107 WT spleen cells as a source of CD4 cells, WT recipients (n = 15), CD40 recipients (n = 14). (B) Clinical disease in CD40 recipients. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” (C) Incidence of cGVHD in CD80/86-/- recipients. Combined data from 2 experiments are shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone; both recipient types (n = 19); or WT BM plus 107 WT spleen cells as a source of CD4 cells, WT recipients (n = 27), CD80/86-/- recipients (n = 29). †P < .01 for CD80/86-/- recipients versus WT recipients of spleen cells. (D) Clinical disease in CD80/86-/- recipients. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” *P < .05 for CD80/86-/- recipients as compared with WT recipients of spleen cells. (E) Incidence of cGVHD in CD80/86-/- recipients of CD40-/- BM. Combined data from 2 experiments are shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT or CD40-/- BM cells alone; both BM types (n = 22), WT BM plus 2 × 106 purified CD4 cells, WT recipients (n = 15), CD80/86-/- recipients (n = 32); or CD40-/- BM plus 2 × 106 purified CD4 cells, CD80/86-/- recipients (n = 34). †P < .01 for CD80/86-/- recipients of CD40-/- BM + CD4 cells versus WT BM + CD4 cells. (F) Clinical disease in CD80/86-/- recipients of CD40-/- BM. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.” (G) Incidence of cGVHD in WT recipients of CD40-/- BM. One representative experiment of 2 is shown. On day 0, recipient mice were lethally irradiated and reconstituted with 8 × 106 WT or CD40-/- BM cells alone; both BM types (n = 10), WT BM plus 2 × 106 purified CD4 cells (n = 15), or CD40-/- BM plus 2 × 106 purified CD4 cells (n = 15). †P < .01 for recipients of CD40-/- BM + CD4 cells versus WT BM + CD4 cells. (H) Clinical disease in WT recipients of CD40-/- BM. Average clinical score for mice affected with cGVHD (unaffected mice are excluded). BM control mice did not get cGVHD and are represented on the graph as scoring “0.”
CD40 is important but not required on donor APCs when host APCs are inactivated
As noted in “CD80/86 costimulation in cGVHD is independent of CD40,” when donor APCs are intact, CD40 expression on the host also had no effect (Figure 3A-B). Because both host and donor APCs can function to promote cGVHD, it was important to determine whether there was a role for CD40 when only donor APCs can activate alloreactive T cells. Therefore, we infused CD80/86-/- recipients with donor BM that lacked CD40 expression, along with WT donor CD4 T cells. Cutaneous GVHD was reduced but not eliminated in CD80/86-/- recipients of CD40-/- BM compared with recipients of WT BM (Figure 3E-F). This contrasts with the situation when host APCs are intact, as CD40 expression on the donor BM had no detectable role in promoting skin disease in WT recipients (Figure 3G-H). In fact, if anything, when host APCs are intact the absence of CD40 on donor BM leads to increased skin disease incidence (although not increased severity; Figure 3H). This may be because when donor APCs lack CD40 they do not engage counter-regulatory mechanisms such as up-regulation of CD80/86 that can in turn ligate cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) and may also be important for the function of regulatory T cells.32 Similar paradoxical effects have been seen in autoimmunity and transplantation.33 Thus, CD40 has a unique, but not absolutely required, function in promoting GVHD when APCs from the donor are the exclusive means of activating alloreactive CD4 T cells. It is therefore possible that donor APCs taking up exogenous antigens may differ from resident host APCs in their requirements for activation via CD40. In toto, these results may have implications for the mechanism of CD40L-based inhibition of GVHD.34
Gut cGVHD largely depends on intact donor APCs
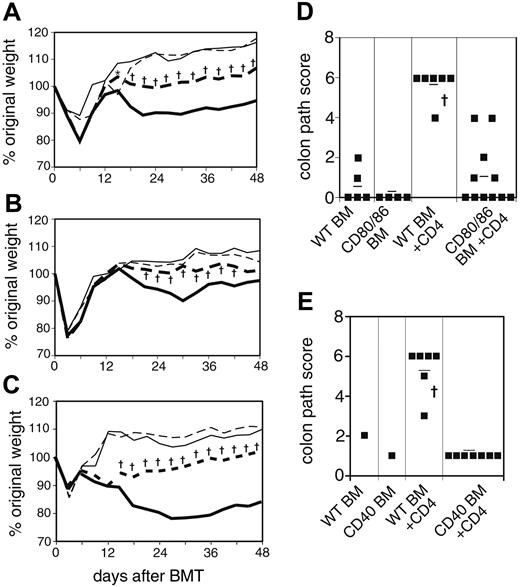
The previously described experiments focused on the prominent target organ, skin. However, we noted the unexpected and consistent findings of increased weight loss (Figure 4A-B) and diarrhea in recipients of WT BM as compared with non-WT BM. Following recovery from irradiation, CD4 recipients of CD80/86-/- BM regained and maintained their original weight, while CD4 recipients of WT BM never returned to their pretransplantation weight (Figure 4A). Mice that received WT or CD80/86-/- BM alone without CD4 cells had equivalent weights at day 18 and later.
Gut GVHD is influenced by donor APCs. (A) Percentage of weight change in WT recipients of CD80/86-/- BM. Combined data from 2 experiments are shown. On day 0, WT recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (thin solid line; n = 7), WT BM plus 2 × 106 WT CD4 cells (bold solid line; n = 17), 8 × 106 CD80/86-/- BM cells alone (thin broken line; n = 6), or CD80/86-/- BM plus 2 × 106 WT CD4 cells (bold broken line; n = 19). *P < .05 or †P < .01 for CD4 recipients of WT BM versus CD80/86-/- BM. (B) Percentage of weight change in WT recipients of CD40-/- BM. Combined data from 2 experiments are shown. On day 0, WT recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (thin solid line; n = 8), WT BM plus 107 WT spleen cells (bold solid line; n = 27), 8 × 106 CD40-/- BM cells alone (thin broken line; n = 10), or CD40-/- BM plus 107 WT spleen cells (bold broken line; n = 28). †P < .01 for spleen cell recipients of WT BM versus CD40-/- BM. (C) Percentage of weight change in CD80/86-/- recipients of CD40-/- BM. On day 0, CD80/86-/- recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (thin solid line; n = 5), WT BM plus 2 × 106 WT CD4 cells (bold solid line; n = 16), 8 × 106 CD40-/- BM cells alone (thin broken line; n = 4), or CD40-/- BM plus 2 × 106 WT CD4 cells (bold broken line; n = 17). †P < .01 for CD4 recipients of WT BM versus CD40-/- BM. (D) Pathology score for representative mice from panel A. Mean score is indicated by a horizontal bar. †P < .01 for WT BM + CD4 cell recipients versus all other experimental groups. P = .19 or .73 for CD80/86-/- BM + CD4 cells versus CD80/86-/- BM control or WT BM control, respectively. (E) Pathology scores for representative mice from panel C. Mean score is indicated by a horizontal bar. †P < .01 for WT BM + CD4 cell recipients versus CD40-/- BM + CD4 cell recipients.
Gut GVHD is influenced by donor APCs. (A) Percentage of weight change in WT recipients of CD80/86-/- BM. Combined data from 2 experiments are shown. On day 0, WT recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (thin solid line; n = 7), WT BM plus 2 × 106 WT CD4 cells (bold solid line; n = 17), 8 × 106 CD80/86-/- BM cells alone (thin broken line; n = 6), or CD80/86-/- BM plus 2 × 106 WT CD4 cells (bold broken line; n = 19). *P < .05 or †P < .01 for CD4 recipients of WT BM versus CD80/86-/- BM. (B) Percentage of weight change in WT recipients of CD40-/- BM. Combined data from 2 experiments are shown. On day 0, WT recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (thin solid line; n = 8), WT BM plus 107 WT spleen cells (bold solid line; n = 27), 8 × 106 CD40-/- BM cells alone (thin broken line; n = 10), or CD40-/- BM plus 107 WT spleen cells (bold broken line; n = 28). †P < .01 for spleen cell recipients of WT BM versus CD40-/- BM. (C) Percentage of weight change in CD80/86-/- recipients of CD40-/- BM. On day 0, CD80/86-/- recipient mice were lethally irradiated and reconstituted with 8 × 106 WT BM cells alone (thin solid line; n = 5), WT BM plus 2 × 106 WT CD4 cells (bold solid line; n = 16), 8 × 106 CD40-/- BM cells alone (thin broken line; n = 4), or CD40-/- BM plus 2 × 106 WT CD4 cells (bold broken line; n = 17). †P < .01 for CD4 recipients of WT BM versus CD40-/- BM. (D) Pathology score for representative mice from panel A. Mean score is indicated by a horizontal bar. †P < .01 for WT BM + CD4 cell recipients versus all other experimental groups. P = .19 or .73 for CD80/86-/- BM + CD4 cells versus CD80/86-/- BM control or WT BM control, respectively. (E) Pathology scores for representative mice from panel C. Mean score is indicated by a horizontal bar. †P < .01 for WT BM + CD4 cell recipients versus CD40-/- BM + CD4 cell recipients.
Similarly, mice that received CD40-/- BM and spleen cells had significantly higher average weight than recipients of WT BM and spleen cells (P < .01) (Figure 4B). In fact, following recovery from irradiation, there was no statistical difference (P > .05) in average weights for CD40-/- BM alone controls as compared with those receiving CD40-/- BM and spleen cells, with the single exception of day 33. In contrast, recipients of WT BM and spleen cells had significantly lower (P < .01) weights than WT BM alone controls for all time points after day 21 (statistics not indicated on graph). As before, weights of WT BM alone versus CD40-/- BM alone recipients were not statistically different. The requirement for CD40 on donor BM to mediate gut disease is similarly present when host APCs are inactivated by the CD80/86 double knockout (Figure 4C). These data additionally show that host APCs need not express CD80/86 for gut GVHD to ensue.
The requirement for intact donor APCs in promoting gut pathology was confirmed histologically in mice that received CD80/86-deficient BM (Figure 4D). Recipients of CD4 T cells along with WT BM had substantially higher gut pathology scores than equivalent mice that received CD80/86-deficient BM (P < .01). Indeed, although a few of the recipients of CD80/86-deficient BM had detectable gut pathology, in aggregate their scores were statistically indistinguishable from recipients of either type of BM without donor CD4 T cells (P = .19 and .73); in other words, without CD80/86 expression on donor BM, there was no statistical evidence that donor T cells caused GVHD compared with BM-alone controls. We also examined gut pathology in the experiment shown in Figure 4C, in which CD80/86-/- recipients received CD40-/- BM. Again, colon pathology was only observed when CD40 was intact on donor BM (Figure 4E), corroborating the weight loss data.
These studies demonstrate that gut GVHD, as indicated by both weight loss and histopathologic disease in this model, is markedly attenuated in recipients of BM deficient in key T-cell–stimulating molecules. This suggests that donor T cells are stimulated to cause gut disease by APCs originating from the donor BM. Host APCs are not necessary as CD80/86-/- recipients that received WT BM and CD4 T cells do get gut GVHD that is comparable to that induced in WT → WT transplantations (data not shown). The finding that donor-derived APCs have a nonredundant function for this form of cGVHD, but not skin cGVHD, points to distinct disease-initiating requirements for different target organs of cGVHD.
Discussion
To understand the initiation of GVHD at a basic level, it is important to determine whether donor, host, or both types of APCs are necessary and sufficient to cause GVHD. We previously showed that in a CD8-mediated miHA-incompatible model of aGVHD, host APCs were necessary for GVHD initiation,12 identifying these as a target for GVHD prevention. In humans, there is ample evidence that both CD4 and CD8 T cells can mediate GVHD. While several reports have investigated and shown a role for host APCs,12,13,31,35-37 there have been few reports of a role for donor APCs.
Here, we have directly addressed this question by using a CD4-dependent, MHC-matched model of GVHD. We found important roles for donor APCs in promoting the skin manifestations of cGVHD, such as fibrosis and dropout of adnexal structures. Intact host APCs were also sufficient to induce cGVHD but dispensable as long as donor APCs were competent. However, when host APCs alone were impaired, the penetrance of cutaneous cGVHD was reproducibly reduced, indicating a partially exclusive role for host APCs. The induction of cGVHD in hosts lacking CD80/86 also indicates that expression of these molecules on any host tissue is not required for GVHD and thus allows us to restrict our discussion to the effects of CD80/86 on APC function.
These results raise the question of why APC requirements differ in the CD4-dependent model of cGVHD we used and the CD8-mediated aGVHD model we previously reported.12 One simple explanation is that the MHC II antigen presentation pathway incorporates exogenous antigens by design, thus facilitating presentation of host-derived miHAs by donor-derived APCs. While presentation of exogenously acquired antigen can also occur on MHC I (cross-presentation),38 it is less efficient and operationally is insufficient to initiate GVHD when CD8 cells alone are given in a miHA-mismatched model.12 MHC II–mediated presentation of host-derived miHAs by donor-derived APCs can even enable GVHD to occur when the host hematopoietic system has been replaced by the donor-type bone marrow (Figure 2). In this case, only donor-type APCs exist, and they must present host antigens from nonhematopoietic tissues; similar evidence for the importance of miHA expressed on nonhematopoietic tissue has been obtained by Korngold and colleagues (Jones et al).39 In contrast, analogous chimeras in the CD8-mediated system we studied did not get GVHD.12 Aside from differences in presentation pathways, CD4 T cells may differ from CD8 T cells in their trafficking, activation requirements, and survival requirements. However, at present there is no information on which if any of these might affect differential APC requirements.
In addition to demonstrating the role of donor APCs, we also showed that the function of both donor and host APCs requires CD80/86. Thus, at some stage, for GVHD to ensue, CD4 T cells must receive CD80/86-mediated signals, presumably transduced through CD28 expressed on the CD4 T cells themselves. Costimulation by CD80/CD86 is particularly important in the activation of naive CD4 T cells.40-42 The dependence on CD80/CD86 we demonstrate is consistent with our recent finding that GVHD in this model is mediated only by naive T cells,29 a result which has been extended to several different murine systems (our unpublished data, B.A., January 2004, and Chen et al43 ). Furthermore, when resident host APCs were CD80/86 deficient, GVHD incidence was reduced even though donor APCs were wild type, again arguing that CD80/86 is probably required for initial priming. However, we should emphasize that our results do not mean that CD80/86 is a critical T-cell activator throughout the GVHD course. For example, it is plausible that initial priming could occur in the host in a CD80/86-dependent fashion, but subsequent T-cell activation required for frank GVHD could occur on donor APCs without CD80/86 function. Nonetheless, the critical role of CD80/86 at some point in the process is clearly established by the complete absence of GVHD when both donor and host are deficient.
The important role of costimulation in various models of GVHD has been studied by a number of others, mainly in MHC-disparate models. APC and costimulatory requirements, which can depend on antigen dose,40,44 may differ from the miHC-mismatched situation we studied. Nonetheless, in these studies, GVHD has been reduced by using CTLA4 immunoglobulin, anti-CD80/86 antibodies, or CD28-deficient T cells.45-49 Because these prior studies used inhibitor or CD28-deficient T cells, they could not distinguish the differential roles of donor and host APCs, as we do in the present work. Blazar et al50 were the first to show a role for CD80/86 in a miHA-incompatible model. They used spleen cells to elicit GVHD in a setting in which CD8 cells cause GVHD that can be augmented by CD4 cells, although the latter do not cause GVHD by themselves. CTLA4-immunoglobulin delayed GVHD induced by unfractionated splenocytes, although all mice eventually succumbed to GVHD. CTLA4-immunoglobulin had no effect when GVHD was induced by CD8 cells alone. Thus, one can infer a role for CD80/86 costimulation, albeit a modest one, in priming CD4 cells that “help” CD8 responses. Again, since inhibitors were used, the roles of donor and host APCs were not distinguished. The use of knock-out mice in the present studies does allow such distinction; moreover, the current results demonstrate a primary role for CD28:CD80/86 stimulation when CD4 cells alone are directly pathogenic, rather than functioning solely as helpers of CD8-mediated GVHD.
During normal immune responses to pathogens, both CD80 and CD86 are up-regulated upon APC maturation, and this plays an important role in their function to activate naive CD4 T cells.51 Whether up-regulation (as opposed to expression) is required in GVHD is not known. Nonetheless, one might expect that maturation of DCs, with its attendant up-regulation of CD80/86, would be important for GVHD induction. Signals through CD40 on the DCs, delivered by CD154 on CD4 T cells, can play an important role in DC maturation52 as well as enable DCs to more optimally stimulate CD8 T cells.53 This might be particularly important in CD4-mediated GVHD. We, therefore, studied whether the requirement for CD80/86 was downstream of CD40 signals. However, skin-targeted GVHD progressed normally even in the absence of CD40 on either donor or host APCs (Figure 3). Thus, for skin GVHD, CD40 signaling is not obligatorily upstream of increased CD80/86 expression or other aspects of DC maturation. Presumably other means of causing DC maturation are operative, including inflammatory and Toll-like receptor signals that could be present because of tissue damage or breach of the gut barrier.54,55
In studying the roles of CD80/86 and CD40 in APC function, we were surprised to find that CD40 and CD80/86 both had non-redundant functions on donor APCs when it came to inducing gut GVHD. This finding illustrates a surprising principle thatAPC requirements can differ depending on the site or type of disease. In this case, donor APC function (as indicated by ability to express CD80/86) was required to mediate disease in the gut, in contrast to skin disease, even in the presence of wild-type host APCs. Without it, disease was markedly reduced, as measured by weight gain and pathologic assessment. Moreover, in contrast to the case with skin disease for which CD40 expression on either donor or host APCs was dispensable, CD40 played an important role in mediatingAPC activation necessary for gut GVHD. Thus, CD40 signaling is required in this setting for optimal activation of donor T cells to cause disease in the gut, albeit that a small amount of residual disease was seen in the absence of either CD40 or CD80/86 on donor cells. Only in the case in which host APCs were inactivated did a partial role emerge for CD40 on donor APCs in mediating skin disease.
We do not yet know why APC requirements would differ depending on the target tissue even within the same mouse. There could be differences in the rate of APC engraftment in different tissues, leading to differential donor APC residence; for example, Langerhans cells in the skin are reported to remain largely recipient type after syngeneic transplantation while LN DCs are mainly donor type.31 Activation of gut-homing T cells in secondary lymphoid tissues could be CD80/86 dependent whereas this may not be the case for T cells that traffic to other tissues. Finally, disease in the gut may be more dynamic than in the skin, requiring persistent T-cell activation for pathogenesis. Perhaps the fibrotic reaction that ensues in the skin becomes independent of further T cell activation; this would explain why skin GVHD is relatively independent of donor APC engraftment compared with the gut. Future experiments will test these possibilities with a chance to better define different local pathogenesis mechanisms.
In addition to the mechanistic implications of our findings, there are some clinically relevant conclusions. First, our data suggest that depletion of host APCs will be effective in moderating CD4-mediated GVHD, a significant extension of the prior work that showed an essential role for host APCs in CD8-mediated GVHD. Importantly, results for CD4 and CD8 T cells were obtained in minor antigen-mismatched models, suggesting their applicability to the most common type of human stem cell transplantation. Since the model studied here also has features of cGVHD, it is possible that depletion or inhibition of host APCs at the time of transplantation will also have an effect on late complications like cGVHD, although this remains to be better tested. Second, the results add a new rationale for targeting donor APCs in vivo after transplantation, either as a means of preventing GVHD or as a method for treating established GVHD, particularly that of the gut. This could be through costimulatory molecule blockade, as demonstrated using inhibitors of CD80/86 in a variety of GVHD models.45-49 Alternatively, this could be accomplished via reagents that physically deplete APCs. Finally, if donor APCs do play a role, particularly in gut disease, then it may be effective to deplete them at later stages as a therapy for ongoing GVHD. This concept is further supported by our recent finding that donor APCs are required for maximal CD8-mediated GVHD across only miHAs.56 Direct tests of these therapeutic approaches will have to await models in which APC depletion can be carried out via reagents rather than genetically.
Prepublished online as Blood First Edition Paper, November 2, 2004; DOI 10.1182/blood-2004-08-3032.
Supported by grants from the National Institutes of Health (R01 HL66279 [M.J.S.] and R01 AI34495 and R37 HL56067 [B.R.B.]).
M.J.S. and W.D.S. contributed equally to this work.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Catherine Matte and Yanna Athanasiadis for expert technical assistance. We thank Arlene Sharpe for generously providing the CD80/86 double knock-out mice. We thank Michelle Horniak and the staff of the Yale Animal Resources Center for outstanding animal husbandry that made these studies possible. We thank Dan Kaplan for critical reading of the manuscript.

![Figure 1. CD80/86 expression is required for skin cGVHD. Combined data from 3 experiments are shown. On day 0 recipient mice were lethally irradiated and reconstituted with 8 × 106 BM cells WT BM or CD80/86-/- [CD80/86] BM) alone or with 2 × 106 WT CD4 cells (BM + CD4). All BM controls (ctrls): WT or CD80/86-/- recipients of WT or CD80/86-/- BM (n = 20); WT recipients of WT BM + CD4 (n = 17); WT recipients of CD80/86-/- BM + CD4 (n = 8); CD80/86-/- recipients of WT BM + CD4 (n = 28); CD80/86-/- recipients of CD80/86-/- BM + CD4 (n = 16). (A) Incidence of cGVHD. †P < .01 for CD80/86-/- recipients of WT BM + CD4 as compared with all other experimental groups. (B) Average clinical disease score for mice affected with cGVHD (unaffected mice are excluded). †P < .01 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with all other CD4 recipients. BM control mice and CD80/86-/- recipients of CD80/86-/- BM + CD4 did not get cGVHD and are represented on the graph as scoring “0.” (C) Pathology scores for representative mice. Mean score is indicated by a horizontal bar. †P < .01 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with all other CD4 recipients. P = .1715 for CD80/86-/- recipients of CD80/86-/- BM + CD4 as compared with BM control recipients.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-08-3032/6/m_zh80050575210001.jpeg?Expires=1766067557&Signature=0uvopv5Z4LJPR0UpXROyFZUX7GZXmqlMcorYLOk7BvwHTtTnmgAx~5PpUdmdHvwT-1sRZB1bEwS1z4gRIMhOczdoAi90aYZYGw1C7DT5d~Xezkx2bkpIUX7ANVPjYUBd0WK2qpJC20SgUQK9jD0ULOSG6Ju8ImydP7Aj-VAtTebgSAuBJIGT4RCTtu3-2GU7XsrT1fJ5Im~BiT3fR-0zkb5nfExmxQdt1rh-ogk2~JfjuY7BRxFJHgAknMZ4BRzqtRmCcll4Jre1tjXmmCo-IbAdYFdb8F6TXs-yJ--ZuLQmLhYYx6YBu0EMQA7DCNXeJPcmCAF9D9xeKrGyANN38w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal