Abstract
4-1BB, a member of the tumor necrosis factor (TNF) receptor superfamily, is a costimulator for activated T cells. Previous studies have established that treatment with agonistic anti–4-BB monoclonal antibody (3H3) is effective in reversing the progression of spontaneous systemic lupus erythematosus. Its therapeutic effect is mediated by suppression of autoantibody production. In this report, we show that a single injection of 3H3 blocks chronic graft-versus-host disease (cGVHD) in the parent-into-F1 model. In particular, donor CD4+ T cells are rapidly eliminated from host spleens by activation-induced cell death after 4-1BB triggering. Since donor CD4+ T cells are required for the development of cGVHD, and 3H3-mediated inhibition of autoantibody production occurs without donor CD8+ T cells, 3H3 blocks cGVHD by preventing alloreactive donor CD4+ T cells from activating host B cells. Importantly, 3H3 treatment can reverse the progression of advanced cGVHD. Our findings indicate that agonistic anti–4-1BB monoclonal antibody has potential as an immunotherapeutic agent for preventing and treating cGVHD.
Introduction
Chronic graft-versus-host disease (cGVHD) commonly occurs in patients who receive allogeneic stem cell transplants. The clinical complications of cGVHD include virtually all the symptoms of autoimmune disease.1 The pathogenic mechanism may involve the breakdown of tolerance of potential tolerogenic autoreactive T and B cells due to alloreactivity to minor recipient histocompatibility antigens. Accumulating evidence indicates that both alloreactive donor CD4+ T cells and autoreactive host CD4+ T cells are required for cGVHD, and that donor CD8+ T cells are unimportant.2,3 In the clinical setting, however, donor CD8+ T cells play a pivotal role in the induction phase of acute GVHD (aGVHD) by killing host B cells without affecting host CD4+ T cells.4 T helper 1 (Th1) and cytotoxic lymphocyte (CTL) versus Th2 activities of donor T cells may be critical in determining the outcome of GVHD.5,6
Numerous model systems have been developed for examining GVHD. Transfer of parental lymphocytes into unconditioned F1 hybrid mice induces GVHD when the host T cells can't actively resist the donor T cells. For example, (C57BL/6 x DBA/2)F1 (BDF1) mice develop aGVHD or cGVHD by transfer of lymphocytes from C57BL/6 mice or the other parental strain, DBA/2, respectively.7 Since the advent of nonmyeloablative conditioning of transplants to reduce toxicity, the parent-into-unirradiated-F1 model of GVHD may be more appropriate than GVHD models that use extensive conditioning and combinations of strains that induce immune reactivity on the part of both donor and host T cells.8
4-1BB is a member of the tumor necrosis factor (TNF) receptor superfamily that functions mainly as a strong costimulatory molecule for T cells.9-11 In vivo stimulation of 4-1BB with agonistic anti–4-1BB monoclonal antibody (mAb) has a variety of consequences, including promotion of the clonal expansion12 and survival13 of CD8+ T cells, eradication of large established tumors,14,15 abrogation of T-cell–dependent antibody production,16,17 and prevention of inflammatory disease.18 Interestingly, agonistic anti–4-1BB mAb was effective in inhibiting relapse of experimental autoimmune encephalomyelitis (EAE)18 and preventing the progression of spontaneous systemic lupus erythematosus (SLE).19,20 These therapeutic effects of anti–4-1BB mAb appear to occur by deleting autoreactive T and/or B cells.19,20 It has been suggested that agonistic anti–4-1BB mAb may be particularly useful for treating Th2-mediated autoimmune diseases such as SLE, rheumatoid arthritis (RA), and ulcerative colitis.11
In this study we used the murine model of cGVHD that can be induced by transfer of DBA/2 (H-2d) parental spleen/lymph node cells into BDF1 mice (H-2b/d). Since, in the parent-into-F1 model, mice exhibit an SLE-like autoimmune disease involving production of autoantibodies and deposition of an immune complex in the kidney,5,6 we hypothesized that agonistic anti–4-1BB mAb might block cGVHD. We demonstrate that it can prevent cGVHD by inhibiting autoantibody production as a result of the rapid activation-induced cell death (AICD) of donor CD4+ T cells. Most importantly, anti–4-1BB mAb had a beneficial effect on advanced cGVHD.
Materials and methods
Mice
Female DBA/2 (H-2d) and (C57BL/6 x DBA/2)F1 (BDF1) (H-2b/d) mice, 6 to 8 weeks of age, were purchased from Korea Charles River (Seoul, Korea). All mice were maintained in pathogen-free conditions. These studies were approved by the institutional animal care committee.
Antibodies and reagents
Anti–4-1BB mAb (3H3) was described previously21 and purified from ascites. Control rat immunoglobulin G (IgG) was purchased from Sigma (St Louis, MO). The following fluorescein isothiocyanate (FITC)–, phycoerythrin (PE)–, peridinin chlorophyll protein (PerCP)–, or biotin-conjugated mAbs to mouse cell surface molecules were purchased from BD Biosciences Pharmingen (San Diego, CA): CD3, CD4, CD8, CD62L, CD11c, B220, Gr-1, H-2Kb, and annexin V. Horseradish peroxidase (HRP)–conjugated rat anti–mouse IgM, IgG1, IgG2a, IgG2b, IgG3, and IgE antibodies were purchased from Southern Biotechnology (Birmingham, AL).
Induction of cGVHD
Single-cell suspensions in phosphate-buffered saline (PBS) were prepared from the spleens and lymph nodes of normal DBA/2 parental donors, filtered through a sterile mesh (BD Falcon, San Diego, CA), and washed. After the erythrocytes were lysed in hemolysis buffer (144 mM NH4Cl and 17 mM Tris-HCl, pH 7.2), the remaining cells were resuspended at 8 × 107 cells/0.2 mL in PBS. cGVHD was induced by transfer of 8 × 107 of DBA/2 parental cells into the tail vein of normal, unirradiated BDF1 mice. Immediately thereafter, 200 μg of 3H3 or control Ig was administered intraperitoneally. In some experiments, CD4+ or CD8+ T cells were removed by anti-CD4– or anti-CD8–conjugated magnetic beads (Miltenyi Biotech, Auburn, CA) from DBA/2 spleen/lymph node cells. The remaining cells (8 × 107) were transferred into BDF1 mice to induce cGVHD.
Flow cytometry
The spleens of cGVHD mice were harvested on the indicated days after parental cell transfer. After lysis of the erythrocytes, the splenocytes were preincubated in a blocking buffer (PBS containing 2.4G2 mAb/0.2% bovine serum albumin [BSA]/0.1% sodium azide), and then incubated with the relevant mAbs for 30 minutes at 4°C. Finally, they were washed twice with staining buffer (PBS containing 0.2% BSA/0.1% sodium azide) and analyzed by FACscan (BD Biosciences Pharmingen, Mountain View, CA). Cells were stained with annexin V to detect apoptosis, according to the manufacturer's protocol.
Enzyme-linked immunosorbent assay (ELISA)
Mice were bled from the tail vein, and serum titers of anti-DNA IgG1 and other IgG isotypes were assessed by ELISA. Plates (96-well) were incubated overnight at 4°C with 100 μL salmon sperm DNA (Sigma) at a concentration of 10 μg/mL. After blocking with 2% BSA, the plates were incubated with 100 μL serially diluted serum samples for one hour at room temperature. They were washed 3 times with PBS containing 0.1% Tween 20, and HRP-conjugated anti–mouse IgG isotypes were added to each well and the plates were kept at room temperature for one hour. They were washed again with the same solution and color was developed in 100 μL 3,5,3′,5′-tetramethylbenzidine (TMB) substrate for 15 minutes (Pierce, Rockford, IL), and stopped by adding 100 μL of 1 N HCl. The plates were then read at 450 nm with a Wallac Vector 1420 Multilabel Counter (EG&G Wallac, Turku, Finland). Total IgE was measured by coating serially diluted serum sample overnight at 4°C and then following the procedure as described for the titration of anti-DNA IgG. Interferon γ (IFN-γ) was measured from serum and culture supernatant, using an ELISA kit (Endogen, Woburn, MA), according to the manufacturer's protocol.
Enzyme-linked immunospot (ELISPOT)
Serial dilutions of splenocytes were plated in triplicate in a 96-well MultiScreen-IP clean plate (Millipore, Belford, MA) precoated with salmon sperm DNA (10 μg/mL) and incubated overnight at 37°C. Bound anti-DNA IgG1 was detected by incubation with alkaline phosphatase–conjugated rat anti–mouse IgG1 (BD Biosciences Pharmingen) at room temperature for 2 hours. Color was developed with BCIP/NBT color substrate (Promega, Madison, WI).
Histology and immunohistochemistry
Kidneys were collected and immediately immersed in 10% neutral-buffered formalin. The formalin-fixed tissue was embedded in paraffin, and 4-μm sections were stained with hematoxylin and eosin (H&E) and evaluated by light microscopy. For immunohistochemistry, kidneys were embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrence, CA) and snap-frozen in liquid nitrogen. Sections (8 μm) were air-dried, fixed with acetone, and stained with FITC-conjugated anti–mouse IgG (BD Biosciences Pharmingen). Fluorescence was examined by confocal microscopy (Olympus, Tokyo, Japan).
Cell preparation and culture
Cell preparation and culture of CD4+CD25+ regulatory T cells were modified as previously described.22 Briefly, CD25+ T cells were purified from DBA/2 spleens/lymph nodes, using anti-CD25–conjugated magnetic beads (Miltenyi Biotech). Purified CD25+ T cells (2 × 106) were stimulated by 1 × 107 of irradiated BDF1 splenocytes (3000 rad) in the presence of anti-CD3 and anti-CD28 mAbs (1 μg/mL each), and IL-2 (10 U/mL) for 5 days. After removing dead cells, CD4+ T cells were purified using anti-CD4–conjugated magnetic beads (Mitenyi Biotech) (> 90% were CD4+CD25+ T cells). The purified CD4+CD25+ regulatory T cells (1 × 106) were then transferred into BDF1 mice immediately after disease induction.
In vivo depletion and IFN-γ neutralization
For in vivo depletion of CD4+ T cells, CD8+ T cells, granulocytes, or CD4+CD25+ T cells, mice were injected intraperitoneally with anti-CD4 (200 μg per mouse), anti-CD8 (300 μg), anti–Gr-1 (200 μg), or anti-CD25 (250 μg) mAbs, respectively, on days -2, 0, and +2 (for the former 3 mAbs) or on days -3, 0, +2, and +5 (for anti-CD25 mAb). Disease induction was on day 0. More than 99% of each subset was depleted in spleens and lymph nodes by the corresponding mAb. For neutralization of IFN-γ, 500 μg anti–IFN-γ mAb was administered every 4 days starting from 2 days after disease induction or from the day of 3H3 treatment in the curing model of cGVHD.
In vivo expansion and activation of donor CD4+ T cells
Spleen/lymph node cells were isolated from DBA/2 mice and a single-cell suspension made in PBS. Cell debris was removed by passage through a sterile mesh. The erythrocytes were lysed with hemolysis buffer, and the cells (2 × 107/mL in PBS) were incubated for 10 minutes at room temperature with 5 μM 5,6-carboxyfluorescein diacetate (CFSE). The reaction was quenched with an equal volume of ice-cold fetal bovine serum (FBS; Hyclone, Logan, UT). The cells were then washed 2 times in PBS/10% FBS and once in PBS, and 8 × 107 of the CFSE-labeled cells were injected through the lateral tail vein into BDF1 mice. Control Ig or 3H3 (200 μg) was injected intraperitoneally immediately after the cell transfer. Forty hours later, the mice were killed and their spleens collected. Single-cell suspensions were prepared for flow cytometry in 5% FBS/0.09% NaN3/PBS. Where indicated, the cells were stained with anti-CD62L–PE plus anti-CD4–biotin followed by streptavidin–cychrome (Cy). Cell division was analyzed with a FACScan (BD Biosciences Pharmingen).
Statistical analysis
Student t test was used to determine the statistical significance of differences between groups. In addition, a Kaplan-Meier survival graph was constructed for the control Ig- and 3H3-treated groups, and log-rank comparisons of the 2 groups were used to calculate P values.
Results
3H3 treatment abolishes autoantibody production in cGVHD
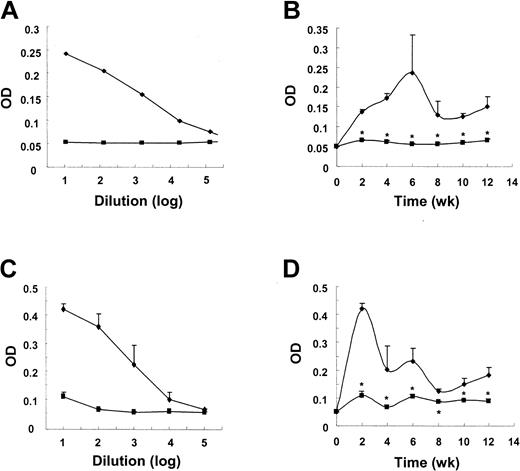
3H3 treatment blocks the humoral immune response against both particulate and soluble antigens.16 In DO11.10 T-cell receptor (TCR) transgenic mice, the production of all isotypes of epitope-specific IgG was also abolished by 3H3 treatment (J.K. and B.K., manuscript in preparation, December 2004) in agreement with previous observations.16,17 Since anti-4-1BB–mediated inhibition of antibody production is achieved by inducing CD4+ T-cell tolerance16 or deletion,17 we expected that 3H3 treatment would prevent alloreactive donor CD4+ T cells from breaking host B-cell tolerance to self-antigens in the cGVHD mouse model. cGVHD was induced by transfer of parental spleen/lymph node cells and levels of IgG1 anti-DNA autoantobody were measured. As expected, a one-time administration of 3H3 sufficed to completely block the production of anti-DNA IgG1 (Figure 1B). The production of other isotypes of anti-DNA autoantibody was also inhibited in 3H3-treated cGVHD mice compared with control Ig–treated cGVHD mice, even though their levels were much lower than those of IgG1 (not shown). Similarly, total IgE production was strongly inhibited by 3H3 treatment (Figure 1D). Our data demonstrate that 3H3 treatment can inhibit production of anti-DNA IgG and total IgE, markers of cGVHD.7,23
3H3 treatment inhibits the production of IgG1 anti-DNA autoantibody and total IgE in cGVHD. cGVHD was induced by transferring 8 × 107 DBA/2 spleen/lymph node cells into BDF1 mice. Immediately thereafter, 200 μg of 3H3 (▪) or control Ig ( ) was injected. Serum samples were collected every 2 weeks and assayed in duplicate by ELISA for IgG1 anti-DNA autoantibody (B) and total IgE (D). The optical density (OD) of duplicate samples for each mouse was measured at 450 nm, using serially diluted serum samples. Titration curves for anti-DNA IgG1 are shown in panel A and those for total IgE at week 2 in panel C. OD values are means ± SD (n = 10 per group) of 10-fold dilutions of samples and are representative of more than 3 independent experiments. *P < .05, between the 2 groups at the indicated time points.
) was injected. Serum samples were collected every 2 weeks and assayed in duplicate by ELISA for IgG1 anti-DNA autoantibody (B) and total IgE (D). The optical density (OD) of duplicate samples for each mouse was measured at 450 nm, using serially diluted serum samples. Titration curves for anti-DNA IgG1 are shown in panel A and those for total IgE at week 2 in panel C. OD values are means ± SD (n = 10 per group) of 10-fold dilutions of samples and are representative of more than 3 independent experiments. *P < .05, between the 2 groups at the indicated time points.
3H3 treatment inhibits the production of IgG1 anti-DNA autoantibody and total IgE in cGVHD. cGVHD was induced by transferring 8 × 107 DBA/2 spleen/lymph node cells into BDF1 mice. Immediately thereafter, 200 μg of 3H3 (▪) or control Ig ( ) was injected. Serum samples were collected every 2 weeks and assayed in duplicate by ELISA for IgG1 anti-DNA autoantibody (B) and total IgE (D). The optical density (OD) of duplicate samples for each mouse was measured at 450 nm, using serially diluted serum samples. Titration curves for anti-DNA IgG1 are shown in panel A and those for total IgE at week 2 in panel C. OD values are means ± SD (n = 10 per group) of 10-fold dilutions of samples and are representative of more than 3 independent experiments. *P < .05, between the 2 groups at the indicated time points.
) was injected. Serum samples were collected every 2 weeks and assayed in duplicate by ELISA for IgG1 anti-DNA autoantibody (B) and total IgE (D). The optical density (OD) of duplicate samples for each mouse was measured at 450 nm, using serially diluted serum samples. Titration curves for anti-DNA IgG1 are shown in panel A and those for total IgE at week 2 in panel C. OD values are means ± SD (n = 10 per group) of 10-fold dilutions of samples and are representative of more than 3 independent experiments. *P < .05, between the 2 groups at the indicated time points.
3H3 treatment prevents glomerulonephritis in cGVHD
Immune complex glomerulonephritis is a typical manifestation of cGVHD. As 3H3 treatment completely inhibited autoantibody production, we investigated whether it also prevented glomerulonephritis. Gross observation revealed intact kidney morphology in the 3H3-treated cGVHD mice 12 weeks after disease induction, whereas the kidneys of control Ig–treated cGVHD mice were edematous. Consistent with the gross observations, histologic examination revealed severe glomerulosclerosis in virtually all the glomeruli of control kidneys, and pronounced perivascular inflammatory cell infiltrates (Figure 2A) indicative of the chronic phase of glomerulonephritis. In contrast, the morphology of the kidneys of the 3H3-treated cGVHD mice was healthy and hardly differed from naive kidneys (Figure 2A). Since immune complexes are the direct cause of tissue injury in glomerulonephritis, we asked whether the 3H3-treated mice had lower deposits of immune complexes. As expected, we could barely detect IgG antibody in the kidneys of the 3H3-treated cGVHD mice, whereas the glomeruli of control cGVHD mouse kidneys had heavy immune complex deposits (Figure 2B). Finally, we confirmed that the 3H3-treated cGVHD mice had increased survival (Figure 2C); 60% of the control Ig–treated cGVHD mice died by week 15, whereas all the 3H3-treated mice remained healthy to the end of the experiment. Thus, 3H3 treatment has an effect on cGVHD mortality as well as morbidity. Taken together, our findings suggest that 3H3 treatment prevents cGVHD by inhibiting the production of autoantibodies, and so avoiding the induction and development of glomerulonephritis.
3H3 inhibits immune complex formation and glomerulonephritis. (A) Kidneys were collected from cGVHD mice at week 12 after disease induction and were fixed in formalin. Sections from control Ig– or 3H3-treated mice were stained with H&E. (B) Kidneys from week-12 cGVHD mice were collected and snap-frozen, and sections were stained with FITC-labeled rat anti–mouse IgG. (C) 3H3 treatment completely prevented death due to cGVHD (n = 10 per group). *P < .05, between the 2 groups.
3H3 inhibits immune complex formation and glomerulonephritis. (A) Kidneys were collected from cGVHD mice at week 12 after disease induction and were fixed in formalin. Sections from control Ig– or 3H3-treated mice were stained with H&E. (B) Kidneys from week-12 cGVHD mice were collected and snap-frozen, and sections were stained with FITC-labeled rat anti–mouse IgG. (C) 3H3 treatment completely prevented death due to cGVHD (n = 10 per group). *P < .05, between the 2 groups.
3H3 treatment inhibits cGVHD-associated B-cell expansion and donor CD4+ T-cell engraftment
To address the mechanism underlying the inhibition of cGVHD by 3H3 treatment, we first examined the lymphocyte populations in cGVHD mouse spleens. We found that 4-1BB stimulation affected splenocyte numbers (Table 1). Total splenocyte numbers increased in the control Ig–treated cGVHD mice until day 3 after parental cell transfer and slowly declined thereafter. 3H3-treated cGVHD mice followed a similar pattern except that a reduction in the total splenocyte populations was seen at day 14 (33% reduction relative to the control Ig–treated cGVHD). At this time, the number of host B cells had fallen by 35% or more in the 3H3-treated cGVHD mice compared with the control Ig–treated cGVHD mice. The marked reduction in total splenocyte numbers in the 3H3-treated cGVHD mice was due in part to a decline in host CD4+ T-cell as well as host B-cell numbers (Figure S1, which can be found at the Blood website; see the Supplemental Figure link at the top of the online article). Annexin V staining revealed that the reduction in absolute numbers of those cells could result from active apoptosis (Figure S2). Interestingly, 3H3 treatment caused a reduction in donor CD4+ T-cell engraftment that was especially obvious by day 5 (Table 1).
4-1BB stimulation promotes elimination of donor CD4+ T cells
. | . | Donor . | . | Host . | |
|---|---|---|---|---|---|
| Group . | Total splenocytes . | CD4+ . | CD8+ . | B220+ . | |
| Day 1 | |||||
| Control Ig | 68.5 ± 13.3 | 1.3 ± 0.3 | 0.7 ± 0.2 | 43.5 ± 8.2 | |
| 3H3 | 64.8 ± 3.1 | 1.0 ± 0.3 | 0.6 ± 0.1 | 41.8 ± 4.0 | |
| Day 3 | |||||
| Control Ig | 110.0 ± 16.9 | 4.2 ± 1.9 | 2.1 ± 0.7 | 68.0 ± 10.3 | |
| 3H3 | 159.0 ± 35.8 | 2.6 ± 0.6 | 3.1 ± 1.2 | 80.3 ± 11.0 | |
| Day 5 | |||||
| Control Ig | 105.0 ± 17.3 | 2.5 ± 0.5 | 5.1 ± 0.7 | 45.3 ± 7.6 | |
| 3H3 | 117.5 ± 35.0 | 0.54 ± 0.5* | 2.9 ± 0.2 | 46.0 ± 9.5 | |
| Day 14 | |||||
| Control Ig | 90.8 ± 15.9 | 1.8 ± 1.2 | 5.6 ± 2.9 | 43.6 ± 14.9 | |
| 3H3 | 30.3 ± 13.1* | 0.1 ± 0.1* | 3.5 ± 1.3 | 15.0 ± 10.6* | |
| Day 21 | |||||
| Control Ig | 77.0 ± 12.6 | 2.2 ± 1.9 | 3.7 ± 2.8 | 35.6 ± 3.9 | |
| 3H3 | 64.0 ± 2.8 | 0.6 ± 0.0* | 4.8 ± 0.8 | 40.3 ± 1.0 | |
| Day 14 | |||||
| CD8-depleted† | |||||
| Control Ig | 290.8 ± 72.4 | 7.6 ± 0.6 | NT | 152.0 ± 36.3 | |
| 3H3 | 113.0 ± 12.8 | 0.6 ± 0.1* | NT | 52.2 ± 4.5* | |
. | . | Donor . | . | Host . | |
|---|---|---|---|---|---|
| Group . | Total splenocytes . | CD4+ . | CD8+ . | B220+ . | |
| Day 1 | |||||
| Control Ig | 68.5 ± 13.3 | 1.3 ± 0.3 | 0.7 ± 0.2 | 43.5 ± 8.2 | |
| 3H3 | 64.8 ± 3.1 | 1.0 ± 0.3 | 0.6 ± 0.1 | 41.8 ± 4.0 | |
| Day 3 | |||||
| Control Ig | 110.0 ± 16.9 | 4.2 ± 1.9 | 2.1 ± 0.7 | 68.0 ± 10.3 | |
| 3H3 | 159.0 ± 35.8 | 2.6 ± 0.6 | 3.1 ± 1.2 | 80.3 ± 11.0 | |
| Day 5 | |||||
| Control Ig | 105.0 ± 17.3 | 2.5 ± 0.5 | 5.1 ± 0.7 | 45.3 ± 7.6 | |
| 3H3 | 117.5 ± 35.0 | 0.54 ± 0.5* | 2.9 ± 0.2 | 46.0 ± 9.5 | |
| Day 14 | |||||
| Control Ig | 90.8 ± 15.9 | 1.8 ± 1.2 | 5.6 ± 2.9 | 43.6 ± 14.9 | |
| 3H3 | 30.3 ± 13.1* | 0.1 ± 0.1* | 3.5 ± 1.3 | 15.0 ± 10.6* | |
| Day 21 | |||||
| Control Ig | 77.0 ± 12.6 | 2.2 ± 1.9 | 3.7 ± 2.8 | 35.6 ± 3.9 | |
| 3H3 | 64.0 ± 2.8 | 0.6 ± 0.0* | 4.8 ± 0.8 | 40.3 ± 1.0 | |
| Day 14 | |||||
| CD8-depleted† | |||||
| Control Ig | 290.8 ± 72.4 | 7.6 ± 0.6 | NT | 152.0 ± 36.3 | |
| 3H3 | 113.0 ± 12.8 | 0.6 ± 0.1* | NT | 52.2 ± 4.5* | |
cGVHD was induced as described in “Materials and methods” and mice received control Ig or 3H3 on day 0. Splenocytes were analyzed by flow cytometry at the indicated days after parental cell transfer.
Values for lymphocyte subsets are shown as (mean ± SD) × 10-6. n = 4 to 6 mice per group.
ND indicates not detected.
Values of P < .05 versus the control Ig-treated cGVHD group
Donor CD8+ T cells were depleted before transfer
3H3 treatment eliminates donor CD4+ T cells by AICD
It was previously reported that cGVHD results from the selective activation of alloreactive donor CD4+ T cells that provide cognate help to host B cells, leading to their activation and to autoantibody production.2,5,6 Consistent with this, deletion of donor CD4+ T cells during the phase of disease induction totally abolished the production of anti-DNA IgG1 in both control Ig– and 3H3-treated cGVHD mice (see the next section). This, together with the data in Table 1, demonstrates that 3H3 treatment blocks the development of cGVHD by eliminating donor CD4+ T cells.
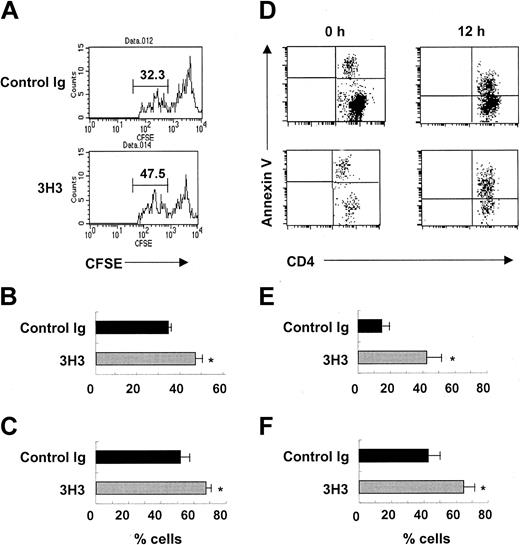
We next explored how 3H3 treatment eliminated donor CD4+ T cells during the induction phase of cGVHD. We hypothesized that 4-1BB costimulation could induce the AICD of donor CD4+ T cells if they received strong allo-stimulation. As expected, most of the donor DBA/2 CD4+ T cells initially divided more actively in the control Ig–treated and 3H3-treated cGVHD mice 40 hours after parental cell transfer (Figure 3A) than in syngeneic BDF1 CD4+ T cells (not shown). In the 3H3-treated mice, 46% of donor CD4+ T cells divided for more than 4 generations compared with 33% in the control mice (n = 3, P < .05; Figure 3B). A larger proportion of the fast-proliferating cells showed an activation phenotype (a situation in which CD62L is down-regulated in effector T cells) in the 3H3-treated than in the control Ig–treated mice (67% vs 52%; n = 3, P < .05; Figure 3C). These results suggest that 3H3 treatment promotes the proliferation and activation of donor CD4+ T cells at the initial phase of cGVHD. By contrast, on day 5, a greater number of donor CD4+ T cells underwent apoptosis in the 3H3-treated than in the control Ig–treated mouse spleens (42% vs 15%; n = 4, P < .01; Figure 3D-E). We observed a similar pattern of donor CD4+ T-cell apoptosis in splenocytes cultured for 12 hours (65% vs 43%; n = 4, P < .05; Figure 3D,F). Taken together, these results indicate that 4-1BB costimulation results in the elimination of donor CD4+ T cells by AICD and the ensuing inhibition of cGVHD development.
Donor CD4+ T cells are deleted by AICD after 4-1BB stimulation in cGVHD. (A-C) CFSE-labeled DBA/2 spleen/lymph node cells (8 × 107) were adoptively transferred into BDF1 mice. Immediately thereafter, 200 μg 3H3 or control Ig was injected. Forty hours later, splenocytes were isolated and stained with anti-CD4 with or without anti-CD62L mAb. (A) Representative FACS plots of CFSE staining of donor CD4+ T cells. (B) Percent of donor CD4+ T cells that divided for more than 4 generations (indicated by lines in the FACS plots in panel A). (C) Percent CD62Llow donor CD4+ T cells in these populations (n = 3). *P < .05 between the 2 groups. (D-F) Splenocytes prepared on day 5 after disease induction were analyzed for annexin V staining of donor CD4+ T cells immediately after isolation (0 h) or after in vitro culture for 12 hours (12 h). H-2Kb–negative CD4+ T cells were gated and analyzed for annexin V staining. (D) Representative FACS plots of annexin V+ donor CD4+ T cells. (E,F) Percent of annexin V+ donor CD4+ T cells at 0 hours (E) and 12 hours (F); n = 4. *P < .05 between the 2 groups at the indicated times.
Donor CD4+ T cells are deleted by AICD after 4-1BB stimulation in cGVHD. (A-C) CFSE-labeled DBA/2 spleen/lymph node cells (8 × 107) were adoptively transferred into BDF1 mice. Immediately thereafter, 200 μg 3H3 or control Ig was injected. Forty hours later, splenocytes were isolated and stained with anti-CD4 with or without anti-CD62L mAb. (A) Representative FACS plots of CFSE staining of donor CD4+ T cells. (B) Percent of donor CD4+ T cells that divided for more than 4 generations (indicated by lines in the FACS plots in panel A). (C) Percent CD62Llow donor CD4+ T cells in these populations (n = 3). *P < .05 between the 2 groups. (D-F) Splenocytes prepared on day 5 after disease induction were analyzed for annexin V staining of donor CD4+ T cells immediately after isolation (0 h) or after in vitro culture for 12 hours (12 h). H-2Kb–negative CD4+ T cells were gated and analyzed for annexin V staining. (D) Representative FACS plots of annexin V+ donor CD4+ T cells. (E,F) Percent of annexin V+ donor CD4+ T cells at 0 hours (E) and 12 hours (F); n = 4. *P < .05 between the 2 groups at the indicated times.
Neither donor CD8+ T cells nor CD11b+Gr-1+ granulocytes are required for 3H3-mediated inhibition of autoantibody production
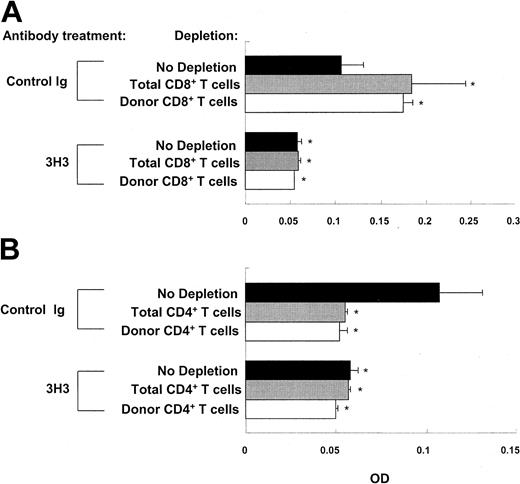
aGVHD can be induced by transferring C57BL/6 parental cells into BDF1 mice. Donor CD8+ T cells play a pivotal role in the unirradiated C56BL/6-into-F1 model of aGVHD by eliminating activated autoreactive host B cells.5 Selective blockade of donor CD8+ T-cell activity can promote the development of cGVHD in this model.4 Conversely, inducing donor CD8+ T cells to develop into mature anti-host CTLs may result in aGVHD instead of cGVHD in the DBA/2-into-F1 model. In addition, 4-1BB is known to be a strong costimulator for CD8+ T cells.13,21 To test the hypothesis that 4-1BB stimulation induces aGVHD in the DBA/2-into-F1 model, BDF1 mice were injected with DBA/2 spleen/lymph node cells depleted of CD8+ T cells or were deleted in vivo of host plus donor (total) CD8+ T cells after the transfer of DBA/2 parental cells. Depletion of either donor or total CD8+ T cells elevated autoantibody levels in the control Ig–treated cGVHD mice (Figure 4A). Furthermore, we found a greater engraftment of donor CD4+ T cells and a greater expansion of B cells in the donor CD8+ T cell–depleted cGVHD mice than in the nondepleted mice on week 2 after disease induction (Table 1). Long-term observations suggest that donor CD8+ T cells are important for regulating cGVHD (Figure S3). Nonetheless, depletion of donor CD8+ T cells did not affect 3H3-mediated inhibition of autoantibody production (Figure 4A) and deletion of donor CD4+ T cells (Table 1), as seen in nondepleted cGVHD mice. Two observations further demonstrated that the aforementioned hypothesis is not valid; first, in contrast to aGVHD in which the elimination of host B cells is sustained, 4 numbers of host B cells were restored by week 3 in the 3H3-treated nondepleted cGVHD mice. Second, we saw no sign of aGVHD in the 3H3-treated nondepleted cGVHD mice during long-term observations. Collectively, our data indicate that 3H3-mediated inhibition of cGVHD is not due to the conversion of cGVHD to aGVHD. Depletion of donor CD4+ T cells or donor plus host (total) CD4+ T cells totally blocked autoantibody production in 3H3-treated as well as in control Ig–treated cGVHD mice (Figure 4B), as seen in the SLE mouse model.24
CD8+ T cells are not required for 3H3-mediated inhibition of autoantibody production. CD4+ and CD8+ T cells were depleted in vivo with 200 μg anti-CD4 mAb and 300 μg anti-CD8 mAb, respectively, on days -2, 0, and 2. Donor CD4+ T cells or CD8+ T cells were depleted before transfer as described in “Materials and methods.” Two weeks after disease induction, anti-DNA IgG1 levels were measured. Neither donor nor total (host plus donor) CD8+ T cells influenced the effect of 3H3 (A), whereas depletion of either donor or total (host plus donor) CD4+ T cells prevented the development of cGVHD in both groups (B). OD values are shown as means ± SD of n = 5 per group at 10-fold dilution of samples, and are representative of 2 experiments. *P < .05 between the control Ig–treated group with no depletion and the indicated group.
CD8+ T cells are not required for 3H3-mediated inhibition of autoantibody production. CD4+ and CD8+ T cells were depleted in vivo with 200 μg anti-CD4 mAb and 300 μg anti-CD8 mAb, respectively, on days -2, 0, and 2. Donor CD4+ T cells or CD8+ T cells were depleted before transfer as described in “Materials and methods.” Two weeks after disease induction, anti-DNA IgG1 levels were measured. Neither donor nor total (host plus donor) CD8+ T cells influenced the effect of 3H3 (A), whereas depletion of either donor or total (host plus donor) CD4+ T cells prevented the development of cGVHD in both groups (B). OD values are shown as means ± SD of n = 5 per group at 10-fold dilution of samples, and are representative of 2 experiments. *P < .05 between the control Ig–treated group with no depletion and the indicated group.
Sun et al19 have shown that 4-1BB stimulation markedly increases the percentage of CD11b+Gr-1+ granulocytes associated with B-cell killing in the spleens of MRL/lpr mice. Even though, in our cGVHD, 3H3 treatment seemed, from our measurements of FasL expression (Figure S4B), to activate the granulocytes, it failed to substantially increase their percentages (Figure S4A) and absolute numbers (Figure S1C). Moreover, in vivo depletion of granulocytes had no effect on 3H3-mediated inhibition of autoantibody production (Figure S4C).
CD4+CD25+regulatory T cells are not involved in 3H3-mediated inhibition of cGVHD
Even though the data in Figure 3 indicated that 3H3 treatment inhibited cGVHD by deleting donor CD4+ T cells, it seemed possible that it also could generate regulatory T cells. To test this hypothesis, we first reinduced cGVHD by transfer of DBA/2 parental cells into 3H3-treated cGVHD mice 6 weeks after the initial induction, when B cell numbers had recovered. We found that reintroduction of cGVHD induced the production of high levels of IgG1 anti-DNA autoantibody after a 3-week delay (Figure 5A). This indicates that 3H3 treatment may not evoke the active regulatory environment that suppresses the breakdown of B-cell tolerance to self-antigens that occurs as a result of transfer of parental cells. We next examined involvement of CD4+CD25+ regulatory T cells in 3H3-mediated inhibition of autoantibody production in cGVHD. Even though allo-stimulated CD4+CD25+ regulatory T cells were able to suppress autoantibody production in BDF1 mice that received parental cells (Figure 5B), in vivo depletion of CD4+CD25+ regulatory T cells had no influence on the inhibition of autoantibody production, deletion of donor CD4+ T cells and B cells, and reduction of autoreactive B-cell numbers that were caused by 3H3 (Figure 5C). Our results suggest that 3H3 is capable of inhibiting cGVHD by a route that is independent of CD4+CD25+ regulatory T cells.
CD4+CD25+ regulatory lymphocytes are not involved in 3H3-mediated inhibition of cGVHD. (A) cGVHD was induced by transferring 8 × 107 DBA/2 parental cells into BDF1 mice. 3H3 was administered immediately afterward. The mice were divided 6 weeks later into 2 groups (n = 5 per group); one group was infused with 8 × 107 DBA/2 parental cells (▪) and the other remained without infusion ( ). Autoantibody production was measured from 2 weeks thereafter. OD values are shown as means ± SD of n = 5 per group at 10-fold dilution of samples. *P < .05 between the 2 groups. (B) Purified DBA/2 CD4+CD25+ T cells were stimulated in vitro by irradiated BDF1 splenocytes in the presence of 1 μg/mL anti-CD3 plus anti-CD28 mAbs for 5 days. A quantity of 1 × 106 of the allo-stimulated CD4+CD25+ T cells (> 90% purity) was infused into BDF1 mice immediately after parental cell transfer. Autoantibody production was measured at day 14. OD values are shown as means plus or minus SD of n = 5 per group at 10-fold dilution of samples. *P < .05, between the 2 groups. (C-F) BDF1 mice were depleted of CD4+CD25+ T cells as described in “Materials and methods.” On day 14, serum and splenocytes were harvested from cGVHD mice. (C) OD values are shown as means ± SD of n = 4 per group at 10-fold dilution of samples. *P < .05, between the control Ig–treated group without depletion and the indicated group. (D-E) Donor CD4+ T-cell and B-cell numbers were counted by staining splenocytes with anti–H-2Kb plus anti-CD4 (D) or anti-B220 (E), respectively. (F) Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. Data are presented as means ± SD of n = 4 per group. *P < .05, between the control Ig–treated group without depletion and the indicated group.
). Autoantibody production was measured from 2 weeks thereafter. OD values are shown as means ± SD of n = 5 per group at 10-fold dilution of samples. *P < .05 between the 2 groups. (B) Purified DBA/2 CD4+CD25+ T cells were stimulated in vitro by irradiated BDF1 splenocytes in the presence of 1 μg/mL anti-CD3 plus anti-CD28 mAbs for 5 days. A quantity of 1 × 106 of the allo-stimulated CD4+CD25+ T cells (> 90% purity) was infused into BDF1 mice immediately after parental cell transfer. Autoantibody production was measured at day 14. OD values are shown as means plus or minus SD of n = 5 per group at 10-fold dilution of samples. *P < .05, between the 2 groups. (C-F) BDF1 mice were depleted of CD4+CD25+ T cells as described in “Materials and methods.” On day 14, serum and splenocytes were harvested from cGVHD mice. (C) OD values are shown as means ± SD of n = 4 per group at 10-fold dilution of samples. *P < .05, between the control Ig–treated group without depletion and the indicated group. (D-E) Donor CD4+ T-cell and B-cell numbers were counted by staining splenocytes with anti–H-2Kb plus anti-CD4 (D) or anti-B220 (E), respectively. (F) Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. Data are presented as means ± SD of n = 4 per group. *P < .05, between the control Ig–treated group without depletion and the indicated group.
CD4+CD25+ regulatory lymphocytes are not involved in 3H3-mediated inhibition of cGVHD. (A) cGVHD was induced by transferring 8 × 107 DBA/2 parental cells into BDF1 mice. 3H3 was administered immediately afterward. The mice were divided 6 weeks later into 2 groups (n = 5 per group); one group was infused with 8 × 107 DBA/2 parental cells (▪) and the other remained without infusion ( ). Autoantibody production was measured from 2 weeks thereafter. OD values are shown as means ± SD of n = 5 per group at 10-fold dilution of samples. *P < .05 between the 2 groups. (B) Purified DBA/2 CD4+CD25+ T cells were stimulated in vitro by irradiated BDF1 splenocytes in the presence of 1 μg/mL anti-CD3 plus anti-CD28 mAbs for 5 days. A quantity of 1 × 106 of the allo-stimulated CD4+CD25+ T cells (> 90% purity) was infused into BDF1 mice immediately after parental cell transfer. Autoantibody production was measured at day 14. OD values are shown as means plus or minus SD of n = 5 per group at 10-fold dilution of samples. *P < .05, between the 2 groups. (C-F) BDF1 mice were depleted of CD4+CD25+ T cells as described in “Materials and methods.” On day 14, serum and splenocytes were harvested from cGVHD mice. (C) OD values are shown as means ± SD of n = 4 per group at 10-fold dilution of samples. *P < .05, between the control Ig–treated group without depletion and the indicated group. (D-E) Donor CD4+ T-cell and B-cell numbers were counted by staining splenocytes with anti–H-2Kb plus anti-CD4 (D) or anti-B220 (E), respectively. (F) Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. Data are presented as means ± SD of n = 4 per group. *P < .05, between the control Ig–treated group without depletion and the indicated group.
). Autoantibody production was measured from 2 weeks thereafter. OD values are shown as means ± SD of n = 5 per group at 10-fold dilution of samples. *P < .05 between the 2 groups. (B) Purified DBA/2 CD4+CD25+ T cells were stimulated in vitro by irradiated BDF1 splenocytes in the presence of 1 μg/mL anti-CD3 plus anti-CD28 mAbs for 5 days. A quantity of 1 × 106 of the allo-stimulated CD4+CD25+ T cells (> 90% purity) was infused into BDF1 mice immediately after parental cell transfer. Autoantibody production was measured at day 14. OD values are shown as means plus or minus SD of n = 5 per group at 10-fold dilution of samples. *P < .05, between the 2 groups. (C-F) BDF1 mice were depleted of CD4+CD25+ T cells as described in “Materials and methods.” On day 14, serum and splenocytes were harvested from cGVHD mice. (C) OD values are shown as means ± SD of n = 4 per group at 10-fold dilution of samples. *P < .05, between the control Ig–treated group without depletion and the indicated group. (D-E) Donor CD4+ T-cell and B-cell numbers were counted by staining splenocytes with anti–H-2Kb plus anti-CD4 (D) or anti-B220 (E), respectively. (F) Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. Data are presented as means ± SD of n = 4 per group. *P < .05, between the control Ig–treated group without depletion and the indicated group.
3H3-mediated inhibition of cGVHD is IFN-γ independent
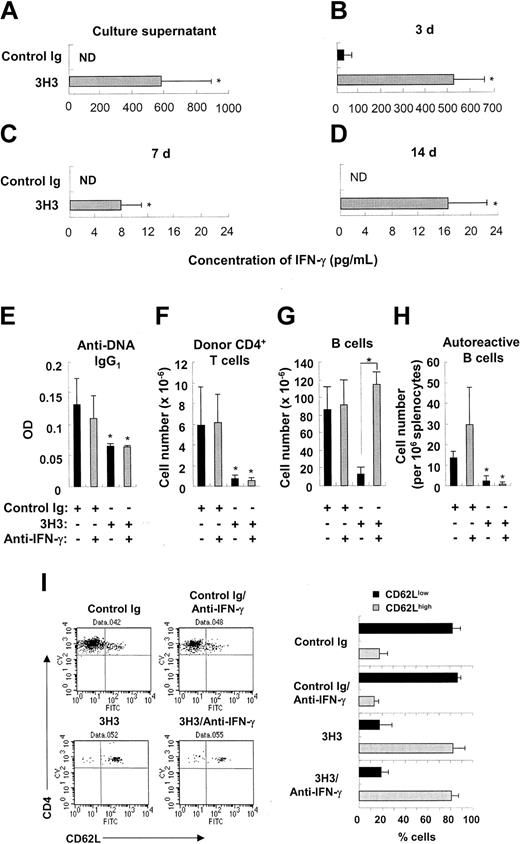
Stimulation of 4-1BB17,25 or CD27,26 another costimulatory molecule belonging to the TNF receptor superfamily, has been shown to deplete B cells, and there is evidence that this is driven by the excessive production of IFN-γ. In cGVHD, 3H3 treatment increased production of IFN-γ, derived mainly from activated CD8+ T cells (Figure 6A-D and Figure S5). Neutralization of IFN-γ reduced B-cell deletion in 3H3-treated cGVHD mice (Figure 6G). Despite the significant recovery of B-cell numbers, however, few autoreactive B cells producing anti-DNA IgG1 were detectable in the 3H3-treated cGVHD mice after IFN-γ neutralization (Figure 6H). This is consistent with the results showing that neutralization of IFN-γ does not reverse 3H3-mediated inhibition of autoantibody production (Figure 6E), or the deletion of activated donor CD4+ T cells (Figure 6F,I). Overall, our findings indicate that 3H3-mediated inhibition of autoantibody production is due to deletion of donor CD4+ T cells and that autoreactive B cells can't be generated without their help.
Effects of IFN-γ neutralization on autoantibody production and deletion of donor CD4+ T cells and B cells. (A-D) cGVHD was induced by transferring 8 × 107 DBA/2 parental cells into BDF1 mice, and 3H3 or control Ig was administered immediately afterward. (A) Three days later, splenocytes were harvested and stimulated with PMA/ionomycin. Twenty-four hours thereafter, the concentration of IFN-γ in culture supernatants was measured. (B-D) Serum was harvested from cGVHD mice at days 3 (B), 7 (C), and 14 (D) after disease induction. Serum IFN-γ levels were measured by ELISA. ND indicates not detectable. Data are presented as means ± SD of n = 3-4 per group. *P < .05 between the 2 groups. (E-I) Starting from day 2, 500 μg anti–IFN-γ mAb was administered to cGVHD mice every 4 days. On day 14, serum and splenocytes were harvested from the cGVHD mice. (E) OD values for anti-DNA IgG1 levels are shown as means ± SD at 10-fold dilution of samples. (F-G) Donor CD4+ T-cell and B-cell numbers were counted by staining splenocytes with anti–H-2Kb plus anti-CD4 (F) or anti-B220 (G), respectively. (H) Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. (I) Splenocytes were triple-stained with anti–H-2Kb, anti-CD4, and anti-CD62L, and H-2Kb–negative CD4+ T cells were gated and analyzed for CD62L expression. Representative FACS plots are shown in the left column, and percentages of CD62Llow versus CD62Lhigh donor CD4+ T cells are summarized in the right column. Data are presented as means ± SD of n = 3-5 per group. *P < .05 between the control Ig–treated group without neutralization and the indicated group (E, F, H), or between the indicated groups (G).
Effects of IFN-γ neutralization on autoantibody production and deletion of donor CD4+ T cells and B cells. (A-D) cGVHD was induced by transferring 8 × 107 DBA/2 parental cells into BDF1 mice, and 3H3 or control Ig was administered immediately afterward. (A) Three days later, splenocytes were harvested and stimulated with PMA/ionomycin. Twenty-four hours thereafter, the concentration of IFN-γ in culture supernatants was measured. (B-D) Serum was harvested from cGVHD mice at days 3 (B), 7 (C), and 14 (D) after disease induction. Serum IFN-γ levels were measured by ELISA. ND indicates not detectable. Data are presented as means ± SD of n = 3-4 per group. *P < .05 between the 2 groups. (E-I) Starting from day 2, 500 μg anti–IFN-γ mAb was administered to cGVHD mice every 4 days. On day 14, serum and splenocytes were harvested from the cGVHD mice. (E) OD values for anti-DNA IgG1 levels are shown as means ± SD at 10-fold dilution of samples. (F-G) Donor CD4+ T-cell and B-cell numbers were counted by staining splenocytes with anti–H-2Kb plus anti-CD4 (F) or anti-B220 (G), respectively. (H) Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. (I) Splenocytes were triple-stained with anti–H-2Kb, anti-CD4, and anti-CD62L, and H-2Kb–negative CD4+ T cells were gated and analyzed for CD62L expression. Representative FACS plots are shown in the left column, and percentages of CD62Llow versus CD62Lhigh donor CD4+ T cells are summarized in the right column. Data are presented as means ± SD of n = 3-5 per group. *P < .05 between the control Ig–treated group without neutralization and the indicated group (E, F, H), or between the indicated groups (G).
3H3 treatment ameliorates advanced cGVHD
3H3 is effective in blocking antibody responses only when given simultaneously with immunization.16 Since alloreactive CD4+ T cells and autoreactive CD4+ T cells appear to be persistently exposed to alloantigens and autoantigens in full-blown cGVHD, respectively,6,26 3H3 treatment alone may suffice to abolish autoantibody production by eliminating alloreavtive and/or autoreactive CD4+ T cells by AICD. In fact, 2 groups have shown that agonistic anti–4-1BB mAbs can ameliorate spontaneous SLE19,20 and relapse of EAE.18 We wished to determine whether, by analogy, 3H3 treatment could reverse advanced cGVHD. We divided cGVHD mice into 2 groups. One was administered with 3H3 6 weeks after the initial induction of cGVHD, and the other received DBA/2 parental cells at the same time. There was a marked reduction in anti-DNA IgG1 levels in both groups of mice (Figure 7A), and similar results were obtained in other similar experiments (Figure 7B). In this curing model of cGVHD, 3H3 administration resulted in rapid deletion of donor CD4+ T cells and B cells, including autoreactive B cells (Figure 7C). These findings indicate that 3H3 treatment ameliorates advanced cGVHD by deleting both alloreactive CD4+ T cells and autoreactive B cells.
3H3 treatment reverses advanced cGVHD. (A-B) At 6 weeks after disease induction, cGVHD mice were administered 200 μg 3H3 alone ( ) or infusion of 8 × 107 DBA/2 spleen/lymph node cells (▪) (A). In panel B, one group of cGVHD mice was administered control Ig (
) or infusion of 8 × 107 DBA/2 spleen/lymph node cells (▪) (A). In panel B, one group of cGVHD mice was administered control Ig ( ), and the other, 3H3 (▴). Autoantibody production was measured from 1 to 2 weeks thereafter. OD values are shown as means ± SD of 10-fold dilution of samples of n = 4-5 per group. *P < .05 between the 2 groups at the indicated times. (C) Control Ig or 3H3 was injected into cGVHD mice 2 weeks after disease induction. Splenocytes were harvested 7 days after antibody treatment, and donor CD4+ T cells and B cells were counted by staining with anti–H-2Kb plus anti-CD4 or anti-B220, respectively. Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. *P < .05 between the 2 groups.
), and the other, 3H3 (▴). Autoantibody production was measured from 1 to 2 weeks thereafter. OD values are shown as means ± SD of 10-fold dilution of samples of n = 4-5 per group. *P < .05 between the 2 groups at the indicated times. (C) Control Ig or 3H3 was injected into cGVHD mice 2 weeks after disease induction. Splenocytes were harvested 7 days after antibody treatment, and donor CD4+ T cells and B cells were counted by staining with anti–H-2Kb plus anti-CD4 or anti-B220, respectively. Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. *P < .05 between the 2 groups.
3H3 treatment reverses advanced cGVHD. (A-B) At 6 weeks after disease induction, cGVHD mice were administered 200 μg 3H3 alone ( ) or infusion of 8 × 107 DBA/2 spleen/lymph node cells (▪) (A). In panel B, one group of cGVHD mice was administered control Ig (
) or infusion of 8 × 107 DBA/2 spleen/lymph node cells (▪) (A). In panel B, one group of cGVHD mice was administered control Ig ( ), and the other, 3H3 (▴). Autoantibody production was measured from 1 to 2 weeks thereafter. OD values are shown as means ± SD of 10-fold dilution of samples of n = 4-5 per group. *P < .05 between the 2 groups at the indicated times. (C) Control Ig or 3H3 was injected into cGVHD mice 2 weeks after disease induction. Splenocytes were harvested 7 days after antibody treatment, and donor CD4+ T cells and B cells were counted by staining with anti–H-2Kb plus anti-CD4 or anti-B220, respectively. Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. *P < .05 between the 2 groups.
), and the other, 3H3 (▴). Autoantibody production was measured from 1 to 2 weeks thereafter. OD values are shown as means ± SD of 10-fold dilution of samples of n = 4-5 per group. *P < .05 between the 2 groups at the indicated times. (C) Control Ig or 3H3 was injected into cGVHD mice 2 weeks after disease induction. Splenocytes were harvested 7 days after antibody treatment, and donor CD4+ T cells and B cells were counted by staining with anti–H-2Kb plus anti-CD4 or anti-B220, respectively. Autoreactive B cells producing anti-DNA IgG1 were counted using ELISPOT. *P < .05 between the 2 groups.
Discussion
We found that 4-1BB was expressed on both parental CD4+ and CD8+ T cells 12 hours after their transfer into BDF1 mice and before they had begun to divide.27 Forty hours later, the donor CD4+ and CD8+ T cells divided 4 to 7 times and 4-1BB was preferentially expressed on these dividing donor T cells. This rapid 4-1BB expression may reflect the fact that strong allo-stimulation is associated with earlier 4-1BB expression than occurs in other clinical settings such as EAE.18 In combination with strong allo-stimulation, 4-1BB costimulation seems to promote early activation28 and subsequent AICD of donor CD4+ T cells (Figure 3). Because cGVHD cannot develop without donor CD4+ T-cell help for a sustained effector B-cell response capable of mediating systemic autoimmunity,29 we conclude that 3H3-mediated inhibition of cGVHD is due to deletion of donor CD4+ T cells via AICD. This conclusion is supported by the findings of Sun and colleagues.18 It is not entirely clear how agonistic anti–4-1BB mAb inhibits humoral immune responses. Other studies16,18-20 have provided several possible explanations. One is that agonistic anti–4-1BB mAb induces CD4+ T-cell tolerance by anergy16 or deletion18 of that T-cell subset. Unlike CD8+ T cells, CD4+ T cells may be more susceptible to AICD after 4-1BB stimulation,9,30 and this may lead to peripheral deletion of autoreactive CD4+ T cells. A second possibility is that anti–4-1BB mAb abolishes antibody responses by deleting autoreactive B cells. Anti–4-1BB treatment caused an absolute reduction in B-cell numbers in the MRL/lpr SLE model.19 It seems that, in this disease model, IFN-γ–activated macrophages can kill B cells, which suggests that IFN-γ is primarily responsible for the suppression of autoantibody production by anti–4-1BB mAb. We also found (Figure S5) that, in RA, 4-1BB stimulation expanded the number of CD11c+CD8+ T cells capable of producing a larage amount of IFN-γ that in turn down-regulated autoimmunity by inducing indoleamine 2,3-dioxygenase expression in the antigen-presenting cells.31
Our results appear to support the hypothesis that 4-1BB stimulation inhibits autoantibody production by deleting autoreactive CD4+ T cells. It seems that 4-1BB stimulation does not lead to CD4+ T-cell anergy or tolerance. The 3H3-mediated abrogation of antibody responses that was observed by Mittler et al16 might have been due to deletion of antigen-specific CD4+ T cells rather than to the induction of CD4+ T-cell anergy; they adoptively transferred CD4+ T cells from antigen-immunized and anti-4-1BB–treated mice and B cells from naive mice into severe combined immunodeficient (SCID) mice, and did not observe a normal humoral response against the antigen. They also showed that the B cells of anti-4-1BB–treated mice had the ability to produce antibody to the same antigen that was used for the initial immunization. This idea is further supported by the observation that adoptive transfer of primed CD4+ T cells bypasses 3H3-induced suppression of autoantibody production in NZB/W F1 mice.20 In our cGVHD, 3H3 treatment suppressed autoantibody production by deleting donor CD4+ T cells before they had a chance to activate potential autoreactive B cells (Figures 3 and 6). Therefore, the massive deletion of B cells induced by 3H3 treatment is unrelated to inhibition of autoantibody production (Figure 6). In the curing model of cGVHD, however, 3H3 treatment deletes both donor CD4+ T cells and B cells, including autoreactive B cells (Figure 7C). In this case, the large amount of IFN-γ secreted by CD8+ T cells that are hyperactivated by anti–4-1BB treatment may have a beneficial effect on cGVHD, as seen in other autoimmune diseases (presumably via nonspecific killing of normal and autoreactive B cells by activated macrophages19 and by inducing indoleamine 2,3-dioxygenase expression31 ).
It is possible that anti–4-1BB mAb can promote T-cell clearance by complement- or Fc receptor (FcR)–mediated depletion of the activated T cells that express 4-1BB, or by blocking 4-1BB/4-1BB ligand interactions. 3H3 treatment enhances potent CD8+ T-cell expansion and tumor immunity (Sung-A Ju and B.-S.K., manuscript submitted May 2004). Interestingly, blockade of 4-1BB/4-1BB ligand interactions aggravates cGVHD by augmenting autoantibody production.32 In our hands, treatment with anti–4-1BB ligand mAb failed to abrogate the production of autoantibody in our cGVHD model (Figure S6). These findings, and the observation that 3H3 treatment specifically deletes donor CD4+ T cells, exclude both of the above explanations (ie, FcR-mediated depletion or blockade of 4-1BB/4-1BB ligand) for the effect of 3H3 on cGVHD and we conclude that 3H3 delivers an agonistic signal through the 4-1BB receptor.
It has been shown recently that effector CD8+ T cells acquire the ability to suppress CD4+ T-cell proliferation after 4-1BB triggering.33 Our data provide evidence that donor CD8+ T cells are important regulators of cGVHD (Figure 4A and Figure S4). However, 3H3-mediated inhibition of autoantibody production and the apoptosis of donor CD4+ T cells was independent of donor CD8+ T cells (Figure 4A). Currently, we are investigating the possibility that donor CD8+ T cells can function as suppressor T cells in cGVHD, as reported by others.34
4-1BB stimulation may have different effects depending on the type of immune response initiated. First, it seems that it augments the CTL-dominant immune response as seen in aGVHD,35 and in viral12 and tumor14,15 immunity. Second, it reduces or abolishes the Th2-dominant immune response, including antibody responses to nominal antigens16,17 and to self-antigens.19,20 Thus far, agonistic 4-1BB mAb has been shown to be highly effective in treating autoimmune diseases such as SLE,19,20 cGVHD (in this study), and RA,31 and shows promise of preventing asthma (Yoo Suk Cho and B.K., manuscript submitted September 2004). Third, there is controversy regarding the effect of agonistic anti–4-1BB mAb on the Th1-dominant immune response; agonistic 4-BB mAb is reported to aggravate aGVHD,35 but to ameliorate EAE.18 It is thought currently that the effect of agonistic anti–4-1BB mAb depends on the strength of the immune response and on the affinity of anti–4-1BB mAb for 4-1BB molecules. Further studies are needed to clarify how 4-1BB stimulation affects Th1-mediated immune responses.
Agonistic anti–4-1BB mAb has many potential advantages for treating autoimmune diseases. First, it has both preventive and therapeutic effects, as shown in our present study. In fact, the preventive and therapeutic effects of anti–4-1BB mAb on autoimmune diseases may be indistinguishable from each other mechanistically, as it is believed that CD4+ T and B cells continue to be exposed to autoantigens, and that exposure of mice with advanced autoimmune diseases to anti–4-1BB mAb may have the same effect as during disease induction. Second, while treatment with anti–4-1BB mAb can reverse the progression of autoimmune diseases, physiologic immune responses are restored without major side effects. It has been shown that mice that are cured of SLE by treatment with anti–4-1BB mAb normally respond to nominal antigens.19,20 Third, it seems that anti–4-1BB mAb is effective in treating autoimmune diseases in which autoantibodies are major pathogenic factors, regardless of their etiology. Thus far, anti–4-1BB mAb has been shown to suppress the development of EAE,18 RA,31 and SLE-like diseases in 3 experimental models: cGVHD (this study), in MRL/lpr,19 and in NZB/W
In summary, we have shown that anti–4-1BB mAb inhibits the induction and progression of cGVHD, and that deletion of alloreactive CD4+ T cells is mainly responsible for this effect. Our findings appear to provide a sound basis for immunotherapy of autoimmune disease with costimulatory targeting.
Prepublished online as Blood First Edition Paper, November 2, 2004; DOI 10.1182/blood-2004-06-2080.
Supported by grants from the Science Research Center (SRC) Fund to the Immunomodulation Research Center from the Korean Science and Engineering Foundation (KOSEF) and the Korean Ministry of Science and Technology, the Korea Research Foundation (KRF-2003-041-E00 130), and the Asan Institute for Life Sciences (2004-119), Seoul, Korea.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Dr Robert Mittler for providing the 3H3 hybridoma clone and Dr Young Chul Sung for technical advice.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal