Abstract
Most studies of mammalian reticulocyte maturation have used blood reticulocytes.Nascent reticulocytes, as found in bone marrow, have not been available in developmentally synchronized populations. Nascent murine reticulocytes formed in vitro by enucleation of Friend virus–infected erythroblasts were purified and recultured for 110 hours. At 0 hours, all recultured cells were lobulated and contained dense, centralized reticulin. By 110 hours, about 20% to 25% of the cells became biconcave erythrocytes. Most ribosomes and cellular RNAs were degraded within 20 hours, and during that period, heme synthesis declined from a rate equal to that of late erythroblasts to less than 10% of that rate. Many mitochondria appeared normal until they showed outer membrane swelling, degradation, and apparent fusion with intracellular vacuoles at 40 hours of culture. During the period of mitochondrial loss, Bcl-XL, an antiapoptotic protein that accumulates during erythroblast differentiation and maintains mitochondrial membrane integrity, demonstrated progressive decreases and changes consistent with deamidation. Nevertheless, the reticulocytes did not undergo apoptosis, because their apoptotic machinery was degraded. This experimental system that provides a developmentally synchronized population of nascent murine reticulocytes that mature into biconcave erythrocytes in vitro should be useful in further investigations of the cellular events involved in reticulocyte maturation.
Introduction
During definitive mammalian erythropoiesis, late-stage erythroblasts enucleate and thereby become reticulocytes. Over a period of a few days, these newly formed reticulocytes mature by (1) completing the syntheses of hemoglobin and other proteins that characterize mature erythrocytes; (2) changing from larger, motile, irregular-shaped cells to smaller, uniform, biconcave discoid cells; and (3) degrading internal organelles and shedding specific plasma membrane proteins and residual portions of the organelles. In addition to these intrinsic cellular changes, reticulocytes migrate into the circulation from the bone marrow or other hematopoietic organ in which they are formed. Because the reticulocyte stage of erythroid differentiation only lasts a few days, the number of reticulocytes in the blood is a useful clinical indicator of the rate of erythropoiesis. However, reticulocyte populations themselves are considerably heterogeneous due to differences in the stage of maturation of individual reticulocytes. Immature reticulocytes have more reticulin, motility, and irregular shapes compared to mature reticulocytes.1-6 With increasing erythropoietic stimulation, such as following bleeding or hemolysis, the number and proportion of immature or stress reticulocytes increase in the marrow and blood, but they remain a small minority of the circulating reticulocytes in the blood.1-6
Relatively few studies have examined reticulocytes in the bone marrow prior to their migration into the circulating blood. In the marrow, the reticulocytes are a very minor population among the various types of hematopoietic cells. At the level of individual cells, bone marrow reticulocytes have been examined in situ by electron microscopy7,8 and autoradiography9 or in vitro by physiological function.4,10,11 One study estimated that reticulocyte residency in the bone marrow prior to entering the circulation varies from 17 hours in normal rats to 6.5 hours in anemic rats.9 Although marrow reticulocytes have been partially purified by separations based upon cell density and/or size,4,12,13 these populations are contaminated by reticulocytes and erythrocytes from the blood. Flow cytometry can distinguish marrow reticulocytes from contaminating erythrocytes,14 but the permeabilization and fixation required with this method preclude subsequent studies of physiological function or maturation.
Peripheral blood of erythropoietically stimulated patients or laboratory rodents has been the source of reticulocytes for most studies of their maturation, because of the increased numbers of reticulocytes and greater percentages of immature reticulocytes in these blood samples. Reticulocyte populations prepared from blood samples have been isolated from erythrocytes using a variety of separation media and techniques.15-21 Because these preparations of enriched reticulocytes from the blood have residual heterogeneity of reticulocyte maturity and persistent contamination with mature erythrocytes, some investigators have obtained developmentally synchronized populations of reticulocytes for in vivo studies by exchange hypertransfusion of blood from timed, erythropoietically stimulated rats into erythropoietically suppressed recipients.22,23 Also, for in vitro or in vivo studies, developmentally synchronous populations of reticulocytes were obtained by density purification of reticulocytes from erythropoietically stimulated rats that were recovering from thiamphenicol-induced hematopoietic suppression.24,25 In all of these studies, however, the stages of reticulocyte development prior to entering the circulation were not examined. Depending upon methods used, species studied, and the levels of erythropoietic stress, the maturation time of circulating reticulocytes has been measured or calculated to be in the range of 2 to 5 days.6,22-24,26-30
No system has permitted the continuous evaluation of mammalian definitive reticulocyte maturation from the time of enucleation through the development of the biconcave erythrocyte. We report here a murine system of erythroid differentiation that permits studies of large numbers of nascent reticulocytes that mature synchronously in vitro with about 20% to 25% reaching the biconcave erythrocyte stage on the fifth day of culture.
Materials and methods
Mice and procurement of nascent reticulocytes
Two weeks after infection with 104 spleen focus-forming units of the anemia-inducing strain of Friend leukemia virus (FVA), female CD2F1 mice (Harlen-Sprague Dawley, Indianapolis, IN) were killed, and their splenic proerythroblasts were purified by velocity sedimentation at unit gravity.31 The proerythroblasts were cultured at 37°C in a humidified atmosphere of 5% CO2 in air at 1 × 106 cells/mL. The culture medium was Iscoves modified Dulbecco medium (IMDM; Gibco, Grand Island, NY) with 30% heat-inactivated, fetal bovine serum (Hyclone, Logan, UT), 1% deionized bovine albumin (Intergen, Purchase, NY), 100 units/mL penicillin G (Sigma, St Louis, MO), 100 μg/mL streptomycin (Sigma), 0.1 mM α-thioglycerol (Sigma), and 2 units/mL human recombinant erythropoietin (EPO; OrthoBiotech, Bridgewater, NJ). After 44 hours of culture, the proerythroblasts had differentiated into orthochromatic erythroblasts, reticulocytes, and extruded nuclei. The cells and nuclei of the 44-hour cultures were harvested, centrifuged, washed once in phosphate-buffered saline (PBS), and separated by unit gravity sedimentation for 6 hours in a 1% to 2% gradient of deionized bovine albumin in PBS at 4°C. The gradient volume of 1 liter was collected in 50-mL fractions for the first 9 fractions and then in 25-mL fractions for the remaining 22 fractions as shown in Figure 1. Aliquots of each fraction were used to make cytospin preparations, which were stained with 3,3′-dimethoxybenzidine and hematoxylin.
Separation and culture of reticulocytes derived in vitro from FVA erythroblasts. (A) Velocity sedimentation profiles of erythroblasts (▪), reticulocytes (○), and extruded nuclei (⬡) derived from the 44-hour cultures of murine proerythroblasts from FVA-infected mice. Fractions collected from the bottom of the gradient were 50 mL for fractions 1 to 9 and 25 mL for the remaining fractions. Fraction 6 represents pooled fractions 1 to 6, and fraction 29 represents pooled fractions 29 to 31. Corresponding sedimentation rates in mm/hour are shown along the top of the figure. Cytospin preparations were stained with 3,3′-dimethoxybenzidine and hematoxylin, and 300 cells were counted for each fraction. Data are ± SEM of 5 separate experiments. (B) Viable cells per mL in cultures initiated with 1 × 106 nascent reticulocytes per mL at 0 hour. Data are ± SEM of 4 separate experiments. (C) Percentage of cells scored as reticulocytes in cultures of nascent reticulocytes. Data are ± SEM of 5 separate experiments. (D) Total RNA per 1 × 108 cells in cultures of nascent reticulocytes. Data are ± SEM of 5 separate experiments. (E) Percentage of biconcave cells in cultures of nascent reticulocytes. Data are ± SEM of 4 separate experiments. (F) 59Fe-incorporation into heme (cpm) in cultures of nascent reticulocytes. Data are ± SEM of 3 separate experiments.
Separation and culture of reticulocytes derived in vitro from FVA erythroblasts. (A) Velocity sedimentation profiles of erythroblasts (▪), reticulocytes (○), and extruded nuclei (⬡) derived from the 44-hour cultures of murine proerythroblasts from FVA-infected mice. Fractions collected from the bottom of the gradient were 50 mL for fractions 1 to 9 and 25 mL for the remaining fractions. Fraction 6 represents pooled fractions 1 to 6, and fraction 29 represents pooled fractions 29 to 31. Corresponding sedimentation rates in mm/hour are shown along the top of the figure. Cytospin preparations were stained with 3,3′-dimethoxybenzidine and hematoxylin, and 300 cells were counted for each fraction. Data are ± SEM of 5 separate experiments. (B) Viable cells per mL in cultures initiated with 1 × 106 nascent reticulocytes per mL at 0 hour. Data are ± SEM of 4 separate experiments. (C) Percentage of cells scored as reticulocytes in cultures of nascent reticulocytes. Data are ± SEM of 5 separate experiments. (D) Total RNA per 1 × 108 cells in cultures of nascent reticulocytes. Data are ± SEM of 5 separate experiments. (E) Percentage of biconcave cells in cultures of nascent reticulocytes. Data are ± SEM of 4 separate experiments. (F) 59Fe-incorporation into heme (cpm) in cultures of nascent reticulocytes. Data are ± SEM of 3 separate experiments.
Reticulocyte cultures and cell processing
Gradient fractions containing the greatest proportions of reticulocytes were pooled such that nascent reticulocytes accounted for more than 95% of the cells. Almost all of the contaminants were enucleated nuclei. The purified, nascent reticulocytes were then recultured under the same conditions as the proerythroblast cultures, except that the cell concentration was 2 × 106 cells/mL. EPO was not added to the medium, because preliminary studies had demonstrated no effect of EPO on survival or maturation of the cultured reticulocytes. At various times, aliquots of the cultured reticulocytes were harvested, and viable cells were counted using trypan blue dye exclusion. Cytospin preparations stained with 3,3′-dimethoxybenzidine and hematoxylin were examined by light microscopy using an Olympus BH-2 microscope with an SPlan 100, oil-immersion, 100 × objective lens having a 1.25 numeric aperture (Olympus America, Melville, NY). For reticulocyte counts, aliquots of harvested cells were washed once with PBS and stained for 10 minutes with 0.5% new methylene blue prior to making cytospin preparations. Five hundred cells were counted in each cytospin preparation, and those with 2 or more granules were scored as reticulocytes.3 Cell shapes and surfaces were evaluated by examining concentrated cell samples in culture medium using Nomarski differential interference contrast (DIC) microscopy with the same Olympus microscope and 100 × objective used for light microscopy. Photomicroscopy was performed with an Olympus C-35AD-4 camera (Olympus America) using Ektachrome 64T file (Eastman Kodak, Rochester, NY) for the stained preparations and Pan F 50 Plus film (Ilford Imaging, Mobberley, United Kingdom) for the DIC-examined preparations. The developed film was scanned with a Nikon Coolscan V ED (Nikon, Melville, NY), and the scanned images were processed in Adobe Photoshop 7.0 (Adobe Systems, San Jose, CA). For electron microscopy, the harvested cells were resuspended in glutaraldehyde fixative and processed as described in the section on electron microscopy.
In other experiments the harvested cells were washed once with PBS, counted using a hemocytometer, and pelleted by centrifugation. The washed pellets were resuspended in guandinium-thiocyanate lysis buffer for RNA extraction with phenol-chloroform32 or in double-strength Laemmli sample buffer for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE).33 Some nascent (0-hour) reticulocyte pellets were lysed in 0.2% Triton X-100 in 0.02 M sodium phosphate buffers with pH values varying between 7 and 10.34 These reticulocyte lysates were incubated for 18 hours at 37°C and then mixed with an equal volume of double-strength Laemmli sample buffer for subsequent gel electrophoresis. To measure heme synthesis, 0.5-mL cultures of reticulocytes were incubated for 2 hours prior to harvest with 50 μL of a 2.4-mg/mL solution of human apotransferrin (Sigma) in IMDM containing 10 μCi (0.37 MBq)/mL of 59FeCl3 (1.70 Ci [62.9 GBq]/mmol Fe; Amersham Bioscience, Piscataway, NJ). The harvested cells were pelleted by centrifugation, lysed in Drabkin solution, extracted into cyclohexanone, and counted with a gamma counter.
Electron microscopy
The harvested cells were fixed for 30 minutes at 37°C in 2.0% glutaraldehyde/0.1% tannic acid in 0.1 M sodium cacodylate (pH 7.4), washed with 0.05 M sodium cacodylate, pelleted, and gently resuspended in melted 2.0% agar in glass-distilled water at 42°C. The agar was allowed to solidify, cut into 1 mm3 blocks, rinsed in 0.05 M sodium cacodylate, and postfixed in 0.5% osmium tetroxide/0.1 M sodium cacodylate (pH 6.0) on ice for 1.5 hours. The blocks were rinsed once in 0.05 M sodium cacodylate (pH 7.4), once in distilled water, and stained en bloc with 2.0% aqueous uranyl acetate. Blocks were then dehydrated in acetone and embedded in low viscosity resin. Thin sections were stained with 1.5% uranyl acetate in 50% methanol and lead citrate and viewed on a JEOL JEM 100S electron microscope at 80 kV (JEOL USA, Peabody, MA). Electron photomicrographs were made by direct exposure of Kodak 4489 Electron Microscopy Film (Eastman Kodak), scanning of the developed negatives with a ScanMaker 6800 scanner equipped with a transparency adapter (Microteck USA, Carson, CA), and processing of the scanned images in Adobe Photoshop 7.0 (Adobe Systems).
Western blotting of reticulocyte proteins
The harvested reticulocytes lysed in double-strength Laemmli sample buffer were sonicated, heated to 95°C for 10 minutes, and aliquots equivalent to 2.5 × 106 cells were diluted 1:1 with water and separated by SDS-PAGE. The gels containing the separated proteins were electroblotted onto polyvinylidene difluoride membranes (Schleicher and Scheull, Keene, NH) and processed for Western blotting as described previously.35 Briefly, the membranes were incubated with primary antibodies: anti–Bcl-X (BD Biosciences, Lexington, KY, cat #610212); anti–cytochrome c (BD Biosciences, San Diego, CA, cat #65981A); anti-Bax (Upstate Biotechnology, Lake Placid, NY, cat #06-499); anti–caspase 3 (Calbiochem, San Diego, CA, cat #235412); anti–Apaf-1 (Upstate Biotechnology cat#06-957); anti–Ter-119 (BD Biosciences, San Jose, CA, cat#55371). After washing, the membranes were reacted with species-specific secondary antibodies conjugated to horseradish peroxidase (Amersham Pharmacia Biotech, Piscataway, NJ) and detected by enhanced chemiluminescence (ECL) using a kit (Amersham Pharmacia Biotech).
Results
Previous studies with FVA-infected proerythroblasts showed that between 36 and 44 hours of culture, most cells enucleate, giving rise to reticulocytes.36 However, these newly formed reticulocytes are mixed with the extruded nuclei and erythroblasts that have not yet enucleated. Nascent reticulocytes were purified from the extruded nuclei and the erythroblasts by sedimentation at unit gravity. Figure 1A shows the distributions of the 3 cellular populations harvested from the 44-hour erythroblast cultures by sedimentation rate at the top of the figure and by fraction number on the abscissa. By pooling fractions 22 through 27, corresponding to sedimentation velocities between 1.2 and 2.0 mm/hour, we routinely obtained 2 to 3 × 108 reticulocytes that were at least 95% pure, with extruded nuclei as essentially the only contaminants. When these in vitro–derived, nascent reticulocytes were cultured, the numbers of viable cells decreased such that about 60% of the input numbers were present at 110 hours (Figure 1B).
The purified, nascent reticulocytes showed an intense, centralized reticulin that progressively dispersed and decreased during the first 3 days of culture (Figure 2). The decrease in reticulin per cell was accompanied by a parallel decrease in total RNA content such that by 40 hours almost all cells were still scored as reticulocytes, but they contained less than 10% of the total RNA that they had had as nascent (0 hours) reticulocytes (Figure 1C-D). By 60 hours, only about half of the cells were scored as reticulocytes, and many had decreased size and more intense benzidine staining (Figure 2). Less than 20% of cells at 75 hours and 10% of cells at 90 hours were scored as reticulocytes (Figure 1C). At 110 hours, essentially all cells lacked stainable reticulin, but many retained vacuoles of variable size, some of which appeared to communicate with the cell surface (Figure 2).
Morphological changes in light microscopy of cytospin preparations of cultured nascent reticulocytes. Aliquots of cultured nascent reticulocytes were harvested for cytospin preparations at the times shown. Upper row shows 3, 3′-dimethoxybenzidine staining, and lower row shows new methylene blue supravital staining for reticulin. For reference, each field of the 0-hour reticulocytes shows an extruded nucleus (arrows). Magnification, × 450.
Morphological changes in light microscopy of cytospin preparations of cultured nascent reticulocytes. Aliquots of cultured nascent reticulocytes were harvested for cytospin preparations at the times shown. Upper row shows 3, 3′-dimethoxybenzidine staining, and lower row shows new methylene blue supravital staining for reticulin. For reference, each field of the 0-hour reticulocytes shows an extruded nucleus (arrows). Magnification, × 450.
When examined by DIC microscopy, all of the nascent (0-hour) reticulocytes had irregular, lobulated shapes, but in culture they progressively acquired discoid shapes such that by 60 hours only a minority of the cells had lobulations (Figure 3). By 86 hours and 110 hours of culture, almost all cells had discoid shapes, and some demonstrated biconcavity similar to that of peripheral blood erythrocytes (Figure 3). The percentages of biconcave cells reached 20% to 25% at 110 hours (Figure 1E). However, these biconcave cells found at the late times of culture had small vacuoles and slight irregularities in contour when compared to the blood erythrocytes (Figure 3).
Morphological changes in differential interference contrast microscopy of cultured nascent reticulocytes. Aliquots of cultured nascent reticulocytes were concentrated by centrifugation, and wet mount preparations in tissue culture medium were prepared at the times shown. Biconcave cells are shown as arrows in the 86-hour cells and as arrowheads in the 110-hour cells. RBC is a wet mount preparation of blood erythrocytes from a normal mouse. Magnification, × 390.
Morphological changes in differential interference contrast microscopy of cultured nascent reticulocytes. Aliquots of cultured nascent reticulocytes were concentrated by centrifugation, and wet mount preparations in tissue culture medium were prepared at the times shown. Biconcave cells are shown as arrows in the 86-hour cells and as arrowheads in the 110-hour cells. RBC is a wet mount preparation of blood erythrocytes from a normal mouse. Magnification, × 390.
To evaluate further the sequence of morphological changes during in vitro reticulocyte maturation, transmission electron microscopy was performed at various times of culture. All of the nascent (0-hour) reticulocytes had irregular cell shapes, abundant ribosomes, intact mitochondria, and active endocytosis as shown by clathrin-coated pits and vesicles (Figure 4). A relatively high rate of 59Fe incorporation into heme in these 0-hour cells (Figure 1F), as compared to later times of culture, confirmed the endocytic activity involving Fe-transferrin and mitochondrial activity involving heme synthesis. The rate of 59Fe incorporation into heme by the 0-hour reticulocytes was similar to that of cultured erythroblasts from fraction 10 in Figure 1A (data not shown). However, during the culture of the cells, the 59Fe incorporation into heme rapidly declined (Figure 1F) in parallel with the marked decline in total RNA (Figure 1D). The ribosomes, endocytic vesicles, and clathrin-coated pits also rapidly decreased such that they were essentially absent from the cells at 40 hours (Figure 5).
Electron micrographs of nascent (0-hour) reticulocytes. Nascent reticulocytes were placed in culture medium and incubated for 15 minutes at 37°C prior to fixation and processing for transmission electron microscopy. Images of 2 reticulocytes demonstrating endocytic vesicles (arrowheads), mitochondria (m), and intracellular vacuoles (v). Inserted images in lower left corner of each panel show lower-power magnification of the same respective cells. Image in the lower center of the top panel shows a higher-power magnification of the coated pit in the upper right of the same reticulocyte. The clathrin coating the pit is shown by white arrows. Magnification, × 23 000.
Electron micrographs of nascent (0-hour) reticulocytes. Nascent reticulocytes were placed in culture medium and incubated for 15 minutes at 37°C prior to fixation and processing for transmission electron microscopy. Images of 2 reticulocytes demonstrating endocytic vesicles (arrowheads), mitochondria (m), and intracellular vacuoles (v). Inserted images in lower left corner of each panel show lower-power magnification of the same respective cells. Image in the lower center of the top panel shows a higher-power magnification of the coated pit in the upper right of the same reticulocyte. The clathrin coating the pit is shown by white arrows. Magnification, × 23 000.
Electron micrographs of reticulocytes cultured for 40 hours. Images of reticulocytes demonstrating mitochondria (m) in various states of degradation: in close association with a vacuole (white arrowheads in left panel), with ballooning of the outer membrane (black arrowheads in center panel), and intact (right panel). Intracellular vacuoles (v) contain cellular debris, and the reticulocytes demonstrate exocytic activity (asterisks). Magnification: left, × 20 000; center, × 24 000; right, × 35 000.
Electron micrographs of reticulocytes cultured for 40 hours. Images of reticulocytes demonstrating mitochondria (m) in various states of degradation: in close association with a vacuole (white arrowheads in left panel), with ballooning of the outer membrane (black arrowheads in center panel), and intact (right panel). Intracellular vacuoles (v) contain cellular debris, and the reticulocytes demonstrate exocytic activity (asterisks). Magnification: left, × 20 000; center, × 24 000; right, × 35 000.
Mitochondria remained intact and appeared normal during the first 20 hours, but some mitochondria showed major structural changes including blebbing of their membranes and close association and apparent fusion with vacuoles by 40 hours (Figure 5). By 90 hours and later, all mitochondria were greatly decreased in number. Those rare mitochondrial remnants present at these late times had very few internal membranes and were often filled with hemoglobin (Figure 6 right panel). During these later times when mitochondrial degradation was apparent, the cells showed prominent compaction of hemoglobin in the cytoplasm and more intracellular vacuoles (Figures 5, 6). These vacuoles, which have been described as autophagic, are present in erythroid cells as early as the enucleating erythroblasts and the bone marrow reticulocytes in vivo,8 and they have been shown to persist throughout reticulocyte maturation in vitro.37-40 In nascent (0-hour) reticulocytes, these vacuoles were present (Figure 4), but at 40 hours and later they were more prominent and were associated with exocytosis of cellular material (Figure 5 left and right panels). At 110 hours, most cells had prominent vacuoles, some of which contained variable quantities of undigested material (Figure 6 left and central panels).
Electron micrographs of reticulocytes cultured for 110 hours. Images of reticulocytes showing large intracellular vacuoles (v) containing cellular debris (left panel) or without cellular debris (center panel). A biconcave cell (right panel) without autophagic vacuoles contains faint, residual intracellular membranes (arrowhead) that are filled with hemoglobin. Magnification: left, × 7200; center, × 7500; right, × 6800.
Electron micrographs of reticulocytes cultured for 110 hours. Images of reticulocytes showing large intracellular vacuoles (v) containing cellular debris (left panel) or without cellular debris (center panel). A biconcave cell (right panel) without autophagic vacuoles contains faint, residual intracellular membranes (arrowhead) that are filled with hemoglobin. Magnification: left, × 7200; center, × 7500; right, × 6800.
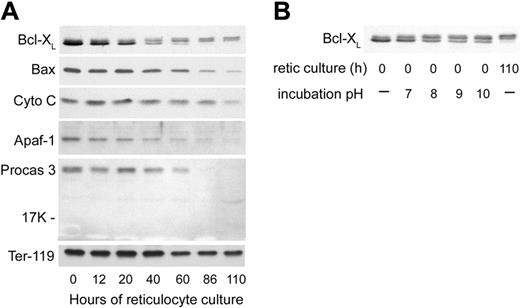
The swelling and degradation of the mitochondria that became evident at 40 hours of reticulocyte culture prompted us to examine several proteins involved in the mitochondria-associated pathway of apoptosis41 to determine whether or not apoptosis-related events may play a role in reticulocyte maturation. We first examined the antiapoptotic protein Bcl-XL, which is important for functional integrity of the outer mitochondrial membrane,42,43 as well as Bax, the proapoptotic binding partner of Bcl-XL. Bcl-XL is a protein that exists in substantial amounts as a homodimer in the cytoplasm but translocates to the mitochondrial outer membrane, where it prevents apoptosis by binding to Bax.44 Bax, also a cytoplasmic protein, translocates to the mitochondrial outer membrane upon induction of some apoptosis mechanisms. When not bound by Bcl-XL, Bax can participate in the formation of a pore in the mitochondrial membrane that releases cytochrome c, initiates caspase activation, and results in apoptosis.41 Previous results with FVA erythroblasts had shown dramatic increases in Bcl-XL prior to enucleation, while Bax levels remained stable.45 For the first 20 hours of reticulocyte culture, the total amount of Bcl-XL decreased slightly while Bax levels were relatively stable (Figure 7A). At 40 hours of culture, the fastest migrating band of Bcl-XL that had accounted for the significant majority of the protein was decreased (Figure 7A). Lesser amounts of 2 more slowly migrating Bcl-XL bands were maintained after 40 hours. The slower migrating of these 2 increased at later times of reticulocyte maturation (Figure 7A). These 2 slowly migrating bands of Bcl-XL on Western blots were very similar to changes in Bcl-XL that have been attributed to its deamidation in cell lines that were induced to undergo apoptosis by agents that damage DNA.34 When we incubated aliquots of a lysate of nascent (0-hour) reticulocytes in a series of alkaline buffers and then analyzed them by Western blotting (Figure 7B), increases in the 2 slowly migrating forms of Bcl-XL with increasing pH replicated the apparent changes in molecular weight observed during maturation of reticulocytes. These changes in Bcl-XL migration following incubations at increasing pH were previously demonstrated to be consistent with deamidation of asparagines 52 and 66 of Bcl-XL.34 Therefore, during reticulocyte maturation, Bcl-XL showed progressive changes consistent with loss of the majority of the total protein and deamidation of the remaining forms. By 40 hours of culture, approximately one half of the Bcl-XL had one asparagine deamidated (Figure 7A). By 90 hours, all of the Bcl-XL had changes consistent with deamidation of one or both asparagines. At 40 hours and later, Bax also showed a progressive decline. Thus, at the time that mitochondria were undergoing degradation by electron microscopy, both Bcl-XL and Bax showed decreases, and the Bcl-XL that persisted had changes consistent with deamidation.
Western blot analyses of apoptosis-related proteins in cultured reticulocytes. In vitro–derived reticulocytes were purified by sedimentation at unit gravity and cultured for 110 hours. At various times, aliquots of cells were harvested, washed in PBS, and the total cells were processed for SDS-PAGE and Western blotting. Each lane was loaded with lysate of 2.5 × 106 cells. (A) Proteins associated with mitochondrial damage and apoptosis. Abbreviations: Cyto C, cytochrome C; Apaf-1, apoptosis protease activating factor-1; Procas 3, procaspase 3; Ter-119, an erythroid-specific protein found on the surface of erythroblasts and mature erythrocytes (included as a positive control that is present in all stages of reticulocyte maturation). (B) Effects of incubation pH on the appearance of Bcl-XL in Western blots of 0-hour reticulocyte lysates. Pelleted, nascent (0-hour) reticulocytes were lysed and incubated at 37°C in hypotonic phosphate buffer with the pH shown prior to separation by SDS-PAGE for Western blotting. For comparison, nonincubated lysates of reticulocytes at 0 hours and 110 hours of culture are shown in the first and last lanes, respectively.
Western blot analyses of apoptosis-related proteins in cultured reticulocytes. In vitro–derived reticulocytes were purified by sedimentation at unit gravity and cultured for 110 hours. At various times, aliquots of cells were harvested, washed in PBS, and the total cells were processed for SDS-PAGE and Western blotting. Each lane was loaded with lysate of 2.5 × 106 cells. (A) Proteins associated with mitochondrial damage and apoptosis. Abbreviations: Cyto C, cytochrome C; Apaf-1, apoptosis protease activating factor-1; Procas 3, procaspase 3; Ter-119, an erythroid-specific protein found on the surface of erythroblasts and mature erythrocytes (included as a positive control that is present in all stages of reticulocyte maturation). (B) Effects of incubation pH on the appearance of Bcl-XL in Western blots of 0-hour reticulocyte lysates. Pelleted, nascent (0-hour) reticulocytes were lysed and incubated at 37°C in hypotonic phosphate buffer with the pH shown prior to separation by SDS-PAGE for Western blotting. For comparison, nonincubated lysates of reticulocytes at 0 hours and 110 hours of culture are shown in the first and last lanes, respectively.
Because of ultrastructural changes that involved the outer mitochondrial membrane, we examined the cultured reticulocytes for the levels of cytochrome c and some of the other proteins involved in apoptosis triggered by mitochondrial release of cytochrome c. The reticulocytes showed stable levels of cytochrome c during their maturation in vivo, even at late times when all mitochondria had been degraded (Figure 7A). Because cytochrome c, when released from mitochondria, can facilitate apoptosis through its association with Apaf-1 and the subsequent activation of procaspases 9 and 3, we investigated these proteins in the maturing reticulocytes. In parallel to the decreases in Bcl-XL and Bax, the levels of Apaf-1 and procaspase 3 also declined significantly during reticulocyte maturation (Figure 7A). The decrease in procaspase 3 was not accompanied by the appearance of the 17-kDa and 12-kDa cleavage fragments that constitute the active form of caspase 3 (Figure 7A), indicating that procaspase 3 declined by degradation rather than by activation. Neither procaspase 9 nor caspase 9 were detected at any time during reticulocyte maturation (data not shown).
Discussion
Reticulocytes with abundant reticulin concentrated in the central part of the cell, like the nascent reticulocytes obtained here by enucleation in vitro, are essentially absent from the blood of healthy individuals. Even in erythropoietically stressed individuals, these cells at the earliest stages of reticulocyte development represent only a minority of all reticulocytes in the blood.2,4-6,24 Because most previous studies of reticulocyte maturation have used reticulocytes isolated from peripheral blood, the time of reticulocyte residency in the hematopoietic organs prior to entry into the blood was not determined. As a result, the total duration of the reticulocyte stage of erythroid differentiation has not been accurately assessed. The time required in this present study for nascent murine reticulocytes to mature to erythrocytes, as defined by loss of reticulin staining, is about 3 days (Figures 1C, 2). However, the achievement of biconcave shape, at least in vitro, required an additional day or 2 after the reticulin is completely lost (Figures 1E,3). The reticulocytes derived in vitro by enucleation of FVA erythroblasts do not interact with stromal and cellular elements of the hematopoietic organs as blood-derived reticulocytes do prior to and during their passage into the circulation. Differentiation into biconcave erythrocytes by these cultured reticulocytes indicates that passage from the hematopoietic tissue into the blood and subsequent circulation in the blood are not essential for achieving this late stage of erythrocyte development. Thus, our results demonstrating in vitro maturation of reticulocytes into biconcave erythrocytes are more consistent with results reported for rat blood reticulocytes by Gronowicz et al37 than with results reported by Noble et al,24 who did not find in vitro maturation of rat blood reticulocytes to biconcave erythrocytes.
Although 1 or 2 days were required in vitro for maturation into biconcave erythrocytes following the complete loss of reticulin, this period of completion of maturation may well be shorter in vivo. Furthermore, the retention of vacuoles and organelle remnants in cells that have achieved biconcavity at 110 hours (Figures 3, 6) suggests that significant maturational changes in addition to the assumption of the biconcave shape occur after erythrocytes lose their reticulin. Previous studies have indicated by biochemical analyses23,46 and by membrane physical properties10,11,47,48 that significant membrane remodeling occurs in late-stage reticulocytes and in erythrocytes that have recently lost their reticulin. In vitro studies have demonstrated that maturing reticulocytes lose cellular components by 2 processes: (1) vesicular fusion with the plasma membrane and externalization of the vesicular contents,38,49-51 and (2) exocytosis of the contents of autophagic vacuoles1,8,37,52 as shown in Figure 5. The numbers of erythrocytes containing autophagic vacuoles are increased in patients with elevated reticulocytes and absent spleens,40 indicating that the spleen facilitates the removal of the vacuoles in reticulocytes and in erythrocytes that have recently lost their reticulin. These results suggest that the autophagic vacuoles might be removed at a slower rate from the reticulocytes maturing in vitro than they would be in vivo, where the spleen or another organ with reticuloendothelial function might facilitate the process. However, this potential splenic role in reticulocyte maturation cannot be assessed in our in vitro system. Another possible difference between the in vitro and in vivo environments that might contribute to the rate or completeness of reticulocyte maturation in vivo is the presence of a soluble factor that stabilizes the maturing reticulocyte or enhances its maturation.
The purified reticulocytes in the present study had progressive losses of ribosomes and total RNA similar to those reported earlier with blood reticulocytes.37,52-54 The rapid ribosomal and RNA losses as well as the rapid decline in 59Fe incorporation into heme in cultured nascent reticulocytes were largely completed by 20 hours. The mitochondria, on the other hand, remained intact with normal appearance at 20 hours and first showed swelling, degradation, and apparent fusion with vacuoles at 40 hours of reticulocyte culture. Previous ultrastructural studies, however, have provided evidence for loss of mitochondria from reticulocytes not only by autolysis in the cytoplasm,37,52 but also by exocytosis of apparently intact mitochondria37 or degraded mitochondria8,37,52 that are enclosed in vacuoles. The temporal sequence of these events in the maturation process is not known. Although autophagic vacuoles containing mitochondria have been seen in a small minority of bone marrow reticulocytes, suggesting that the process of autophagy may begin as early as the nascent reticulocyte stage,8 another study found that some blood reticulocytes had no abnormal mitochondria and that progressive increases in degraded mitochondria accompanied the maturation stage of the reticulocytes.52 Although we did not see abnormal mitochondria in the reticulocytes at 0 hours or 20 hours of culture, reticulocytes at these times did have vacuoles that could have contained degraded mitochondria. Thus, at these early times as well as at the later times of reticulocyte maturation when the autophagic vacuoles became very prominent, some of the mitochondrial proteins such as Bcl-XL and cytochrome c may have been present in vacuoles containing degraded mitochondria. Alternatively, the morphologically normal mitochondria at early times of maturation may have initiated mitochondrial degradation that could not be detected by electron microscopy. Thus, a more accurate temporal relationship between the degradation of mitochondria and other events in the maturation of nascent reticulocytes will require further study with specific probes for degraded mitochondrial proteins.
A study with rabbit reticulocytes demonstrated that when compared to ribosomal degradation, mitochondrial degradation is delayed and mechanistically unrelated.55 Mitochondria of the youngest reticulocytes were not susceptible to proteolysis, while those of older reticulocytes were.55 This delayed mitochondrial susceptibility to proteolysis appears to be due to the onset of 15-lipoxygenase expression during maturation of reticulocytes, because 15-lipoxygenase inhibition prevented mitochondrial degradation but not ribosomal degradation in rabbit reticulocytes.56 The lipoxygenase activity, in turn, precedes an ubiquitin-mediated proteolysis55 that has been well characterized in reticulocyte maturation.57 An induction of this ubiquitin/proteosomal system has been demonstrated in the late stages of in vitro maturation in FVA erythroid cells.58,59
Some recent reports have suggested that caspases that mediate apoptosis also may play a role in the differentiation of erythroblasts.60-62 These caspases have not been investigated in reticulocyte development. Immediately prior to erythroblast enucleation, Bcl-XL, an outer membrane protein of the mitochondrion that regulates mitochondrial-associated apoptosis, accumulates to very high levels.45 Murine erythroid cells that are bcl-x null demonstrate that Bcl-XL is required for survival in the terminal stages of definitive erythropoiesis.63,64 However, mice with a conditional null deletion of bcl-x allowing survival into adulthood have been reported to have hemolytic anemia indicating that Bcl-XL may have a role in the survival of reticulocytes.65 At 40 hours of reticulocyte culture, the amount of Bcl-XL was decreased, and slightly slower migrating forms consistent with deamidated Bcl-XL were found on Western blots (Figure 7A). These changes in Bcl-XL occurred at the same time as electron microscopic changes showing mitochondrial membrane blebbing and apparent fusion with vacuoles (Figure 5). In apoptosis induced by DNA damage, changes in Bcl-XL consistent with deamidation were associated with loss of Bcl-XL function.34 Loss of Bcl-XL function usually leads to apoptosis, but some protection from apoptosis in the maturing reticulocyte may be related to reduced amounts of Bax (Figure 7A), the proapoptotic binding partner of Bcl-XL. The persistent levels of cytochrome c in the reticulocytes during later stages, 86 hours and 110 hours of culture (Figure 7A), when the mitochondria are mostly degraded, would trigger apoptosis in many types of cells.41 However, the cultured reticulocytes did not have detectable caspase 9, and they had progressive decreases in 2 other effectors, Apaf-1 and procaspase 3, that are associated with apoptosis induced by cytochrome c released from mitochondria. Caspase 3, a key protease involved in apoptosis, was lost from the maturing reticulocytes without becoming activated. Thus, the apoptosis that would normally accompany mitochondrial outer membrane disruption and cytochrome c release appears to be prevented in the maturing reticulocyte because the machinery of apoptosis is also undergoing degradation. Our results, therefore, do not support a role for apoptosis-related caspases in the proteolytic remodeling of reticulocyte maturation.
Purification and culture of nascent reticulocytes produced in vitro with the FVA proerythroblast model provides a developmentally synchronized population of mammalian erythroid cells for studies of differentiation in the postenucleation stages. Combining the FVA system of proerythroblast differentiation in vitro with this extension to include the reticulocyte and erythrocyte stages should permit the study of pathological events that lead to abnormal shape, size, or life span of erythrocytes in many murine models of human anemias. Most strains of mice are susceptible to Friend virus infection and can be used in the FVA system. A significant exception is the C57BL/6 strain, which has resistance to Friend virus disease conferred by their homozygosity for the Fv2r/Ron gene.66 Although some models of anemia have been established in the C57BL/6 strain, these mice can be backcrossed to a Friend disease–sensitive strain such as BALB/c or DBA/2 and then used in the FVA system.
Supported by merit review awards from the Department of Veterans Affairs (M.J.K., M.C.B.) and National Institutes of Health grant R15-HL67059 (S.T.K.).
Prepublished online as Blood First Edition Paper, November 4, 2004; DOI 10.1182/blood-2004-02-0616.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.







This feature is available to Subscribers Only
Sign In or Create an Account Close Modal