Abstract
In addition to their anti-inflammatory properties, nonsteroidal anti-inflammatory drugs (NSAIDs) harbor immunosuppressive activities related to their capacity both to inhibit cyclooxygenases (COXs) and to act as peroxisome proliferator-activated receptor (PPAR) ligands. We have previously shown that the stress-activated kinase p38 is a selective target of NSAIDs in T cells. Here we have investigated the effect of NSAIDs on the signaling pathway triggered by the T-cell antigen receptor (TCR) and leading to stress kinase activation. The results show that nonselective and COX-1–selective NSAIDs also block activation of the stress kinase c-Jun N-terminal kinase (JNK) and that prostaglandin-E2 (PGE2) reverses this block and enhances TCR-dependent JNK activation. Analysis of the activation state of the components upstream of p38 and JNK showed that NSAIDs inhibit the serine-threonine kinase p21-activated protein kinase 1 (Pak1) and the small guanosine 5′-triphosphatase (GTPase) Rac, as well as the Rac-specific guanine nucleotide exchanger, Vav. Furthermore, activation of Fyn, which controls Vav phosphorylation, is inhibited by NSAIDs, whereas activation of lymphocyte-specific protein tyrosine kinase (Lck) and of the Lck-dependent tyrosine kinase cascade is unaffected. Accordingly, constitutively active Fyn reverses the NSAID-dependent stress kinase inhibition. The data identify COX-1 as an important early modulator of TCR signaling and highlight a TCR proximal pathway selectively coupling the TCR to stress kinase activation.
Introduction
Cyclooxygenases (COXs) catalyze the rate-limiting step in the biosynthesis of prostaglandins (PGs) and thromboxanes from arachidonic acid. As the result of sequential reactions implicating first the cyclooxygenase and then the endoperoxidase activities of COX, arachidonic acid is converted to PGH2, which is subsequently acted upon by specific synthases to generate the biologically active prostanoids.1,2 Notwithstanding their largely overlapping features in structure, substrate usage, and catalytic activities, the 2 known COXs, COX-1 and COX-2, display dramatic differences both in their expression profiles and in the panel of cellular responses evoked. COX-1 is constitutively and ubiquitously expressed, whereas COX-2 is inducibly expressed in response to proinflammatory stimuli in monocytes, macrophages, and polymorphonuclear cells. Furthermore, COX-2 is ectopically expressed in some forms of neoplasia, including colon, breast, and prostate cancer.3 Prolonged usage of nonselective nonsteroidal anti-inflammatory drugs (NSAIDs), which inhibit COX activity by reversible or irreversible binding to the active site, results in gastric ulceration and bleeding, supporting a gastroprotective and homeostatic function of COX-1. On the other hand, a key role for COX-2 in inflammation has been established.4 This clear-cut distinction between “good” and “bad” COXs has been more recently challenged both with the generation of mice deficient for either COX-1 or COX-2 and with the development of NSAIDs selective for each COX isozyme. The data suggest a complex interplay of COX-1 and COX-2 in the control of a number of physiologic and pathophysiologic functions, including inflammation and carcinogenesis.5-7 The pleiotropic and in many cases opposite activities of COXs are likely to be dictated by a number of factors, including intracellular localization and coupling to specific prostaglandin synthases, as well as by the specific cellular pattern of expression of prostanoid receptors, which are known to trigger different intracellular signaling pathways.8
Both COX isozymes are expressed in T lymphocytes and appear to play a role in T-cell development, activation, chemotaxis, polarization, and apoptosis.9-11 In the thymus, COX-1 is expressed in developing (CD4-CD8- and CD4+CD8+) thymocytes, whereas COX-2 is expressed in a subset of medullary stromal cells.12 The role of COX isozymes in T-cell development has been elegantly addressed in COX-deficient mice, as well as using selective COX-1 and COX-2 inhibitors in fetal thymic organ cultures. Mice deficient for COX-1 show an early block in thymic development, resulting in a dramatic reduction in double-positive thymocytes, whereas COX-2 deficiency selectively affects maturation of single-positive CD4+ thymocytes.12 Phenocopies of COX deficiency can be obtained by pharmacologic inhibition of specific COX isozymes in the fetal thymus,12 supporting a crucial physiologic role of COX in T-cell development.
Although COX expression has also been documented in mature peripheral T cells, the role of these enzymes has as yet not been fully elucidated, partly because NSAIDs, which have been extensively used as tools to study the function of COXs, display COX-independent activities related to their capacity to act as agonists of the peroxisome proliferator-activated receptor (PPAR) family of transcription factors.13 As opposed to COX-1, which is constitutively expressed in T cells, COX-2 is inducibly expressed as an early response gene following T-cell antigen receptor (TCR) engagement, suggesting a role for COX-2 in T-cell activation.14 This possibility is supported by the inhibitory activity of COX-2–selective NSAIDs on T-cell activation and cytokine production, which correlates with the inhibition of key transcription factors.14 We have recently shown that also COX-1 participates in the process of T-cell activation. Specifically, COX-1 is required for TCR-dependent activation of p38 stress kinase, a member of the mitogen-activated protein (MAP) kinase family activated in response to TCR engagement and required for T-cell activation and differentiation.15 Furthermore, COX-1 inhibition results in impaired COX-2 expression,15 suggesting a sequential role of COX-1 and COX-2 in T-cell activation. Here we address the impact of COX-1 inhibition on the activation of the individual molecular components of the pathway triggered by the TCR and leading to stress kinase activation. The data identify COX-1 as an early component of the tyrosine phosphorylation cascade initiated by the TCR and highlight a TCR-proximal pathway controlled by Fyn selectively coupled to stress kinase activation.
Materials and methods
Cells, antibodies, and reagents
Cells included the Jurkat T-lymphoma line, a stably transfected reporter Jurkat line expressing luciferase under the control of a trimer of the distal nuclear factor of activated T cells (NF-AT) binding site on the human interleukin-2 gene promoter15 and the lymphocyte-specific protein tyrosine kinase (Lck)–defective Jurkat variant JCaM1,16 as well as peripheral blood lymphocytes from healthy donors. Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood by density centrifugation on Ficoll-Paque (Amersham Biosciences, Milan, Italy) and subsequently depleted of macrophages by adherence. Mammalian expression vectors encoding either F505Lck17 or F528Fyn18 and the genes encoding neomycin and hygromycin resistance, respectively, as selectable markers, were introduced into Jurkat cells by electroporation. Stably transfected cells were selected in medium containing 1 mg/mL G418 (Gibco BRL, Life Technologies Italia, Milan, Italy) or 50 μg/mL hygromycin (Sigma Italia, Milan, Italy). Stably transfected cells were checked for CD3 expression using saturating amounts of OKT3 as primary antibody and fluorescein isothiocyanate (FITC)–labeled antimouse antibodies.
Phosphospecific antibodies recognizing the phosphorylated active forms of p38, c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase 1 (Erk1)/Erk2, p21-activated protein kinase 1 (Pak1), and proline-rich tyrosine kinase 2 (Pyk2) were from Cell Signaling Technology (Beverly, MA). Anti-p38, anti-Erk2, and anti-CD3ζ monoclonal antibodies (mAbs) were from Santa Cruz Biotechnology (Santa Cruz, CA); antiphosphotyrosine, anti-Vav, anti–zeta-associated protein of 70KD2 (anti–ZAP-70), anti-Rac, anti-Pyk2, anti–linker of activated T cells (anti-LAT), anti-Fyn, and anti-Lck polyclonal and/or monoclonal antibodies were from Upstate Biotechnology (Boston, MA); and antiactin and antitubulin mAbs were from Amersham Biosciences (Milan, Italy). An mAb suitable for immunoprecipitation of tyrosine phosphorylated CD3ζ was kindly provided by M. Banyiash. Immunoglobulin G (IgG) from OKT3 (anti-CD3; American Type Culture Collection, Rockville, MD) hybridoma supernatants were purified on Mabtrap (Amersham Biosciences) and titrated by flow cytometry. Secondary unlabeled antibodies were purchased from Cappel (Durham, NC), secondary peroxidase-labeled antibodies were from Amersham Biosciences. Agarose-conjugated glutathione-S–transferase (GST)–Pak1 and purified Fyn kinase were purchased from Upstate Biotechnology.
Ibuprofen, NS-398,19 and PGE2 were purchased from Sigma-Aldrich (Milan, Italy). sc-560 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole)20 was synthesized at Searle (St. Louis, MO). Each NSAID was dissolved in dimethyl sulfoxide (DMSO) and used at a concentration resulting in maximal immunosuppression, evaluated using TCR-dependent NF-AT activation as a read-out (Rossi Paccani et al15 ; see Figure S1, available on the Blood website; see the Supplemental Figures link at the top of the online article). The JNK-selective inhibitor SP600125 was purchased from BIOMOL Research Laboratories (Plymouth Meeting, PA). All NSAIDs at the highest concentration used, as well as SP600125, were tested for lack of toxicity by trypan blue exclusion.
Activations, immunoprecipitations, immunoblots, in vitro binding assays, kinase assays, and luciferase assays
Cells were cultured overnight in low serum (0.5%) prior to treatment/activation. One hour before stimulation, cells were transferred to serum-free medium and added with NSAIDs, PGE2, or carrier. Activations by cross-linking of mouse mAbs to TCR/CD3 in solution were carried out as previously described by sequential binding of anti-CD3 mAb on ice and subsequent cross-linking for 30 seconds to 5 minutes with secondary antibodies at 37°C.21 Alternatively, anti-CD3 mAb and secondary antibodies were added simultaneously and cross-linked for 5 minutes at 37°C. Cells (2 × 106 cells/sample for analysis of total cell lysates) were lysed in 1% (vol/vol) Triton X-100 in 20 mM Tris-HCl, pH 8, and 150 mM NaCl (in the presence of 0.2 mg/mL Na orthovanadate; 1 μg/mL pepstatin, leupeptin, and aprotinin; and 10 mM phenyl methyl sulphonyl fluoride) and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). Alternatively, postnuclear supernatants from 2.5 × 107/sample to 5 × 107/sample were immunoprecipitated using the appropriate polyclonal antibodies and protein A Sepharose (Amersham Pharmacia Italia, Milan, Italy) or agarose-conjugated antimouse IgG (Sigma Italia). Rac activity was measured by in vitro binding assays of postnuclear supernatants from 107 cells/sample using a GST-Pak1 p21 binding domain fusion protein, which specifically pulls down GTP-bound, active Rac,22 followed by immunoblot with an anti-Rac mAb. Immunoblots were carried out using peroxidase-labeled secondary antibodies and a chemiluminescence detection kit (Pierce, Rockford, IL). Prestained molecular weight markers were purchased from Life Technologies Italia. Each experiment was repeated 3 to 5 times.
In vitro autophosphorylation assays of Fyn- or Lck-specific immunoprecipitates were carried out in 20 μL of 20 mM Tris-HCl, pH 7.4; 10 mM MgCl2; 10 mM MnCl2; 10 μCi (0.37 MBq) of γ-[32P] adenosine triphosphate (ATP) at room temperature for 16 minutes. Alternatively, Fyn activity was assayed in the same reaction buffer added with 5 μM ATP, using 10 μg acid-denatured enolase (Sigma-Aldrich) per sample as exogenous substrate. The reaction products were subjected to SDS-PAGE, transferred to nitrocellulose, and exposed to a Phosphorimager (Molecular Dynamics, Sunnyvale, CA). The filters were subsequently probed with anti-Fyn or anti-Lck mAb as immunoprecipitation control. The activity of purified Fyn in the presence of sc-560 or PGE2 was evaluated as described for the immune complex kinase assays by in vitro kinase assays using enolase as substrate and unlabeled ATP. Enolase phosphorylation was subsequently analyzed by immunoblot with antiphosphotyrosine antibodies and laser densitometry (Kodak Digital Science Electrophoresis Documentation and Analysis System 120, Rochester, NY).
To assay NF-AT activation, reporter Jurkat cells were activated by CD3 cross-linking on a secondary antibody–coated plate using OKT3 mAb as described.15 NSAID or carrier was added 10 minutes prior to activation. Cells were collected 6 hours after activation and processed for luciferase assays as described previously.15 All samples were in duplicate, and each experiment was repeated 3 to 5 times.
Results
COX-1 is required for p38 and JNK stress kinase activation
The MAP kinase family of proteins includes 2 subfamilies, the “classical” MAP kinases Erk1 and Erk2, which become activated in response to mitogenic stimuli, and the stress-activated kinases stress-activated protein kinase (SAPK)/JNK and p38, which are activated by oxidative or genotoxic stress as well as by mitogens.23,24 Both MAP kinase subfamilies are activated following TCR engagement and participate in the activation of key transcription factors required for initiation of the genetic program of T-cell activation.25 We have previously shown that nonselective and COX-1–selective NSAIDs specifically inhibit TCR-dependent p38 activation in both transformed and normal T cells without affecting the activation of Erk.15
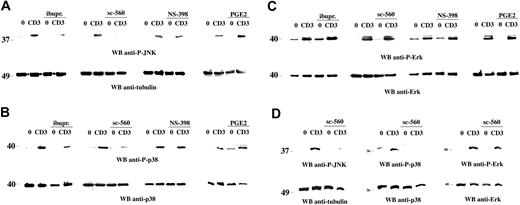
To address the potential role of COX-1 in the activation of JNK, the effect of NSAIDs on TCR-induced JNK activation was investigated. Although the costimulatory receptor CD28 can dramatically enhance JNK activation in T cells,26 engagement of the TCR in the absence of costimulation was sufficient to trigger the activation of the 46-kDa isoform of JNK (Figure 1A), as determined by immunoblot analysis of Jurkat T-cell lysates with phosphospecific antibodies. Treatment of T cells with either the nonselective COX inhibitor ibuprofen or the COX-1–selective inhibitor sc-560 resulted in a strong inhibition of TCR-dependent JNK activation (Figure 1A). As previously reported,15 p38 activation was also inhibited (Figure 1B), whereas Erk activation was unaffected (Figure 1C). Consistent with the lack of COX-2 expression in unstimulated cells,14,15 no effect on either JNK or p38 activation was observed in the presence of the COX-2 inhibitor NS-398 (Figure 1). Similar results were obtained using freshly purified human peripheral blood lymphocytes (PBLs; Figure 1D).
Selective impairment of TCR-dependent JNK and p38 activation by NSAIDs. Immunoblot analysis of postnuclear supernatants from Jurkat cells (A-C) or freshly purified human PBLs (D), nonactivated (0) or activated by CD3 (CD3) ligation for 5 minutes, in the presence or absence of 600 μM ibuprofen (ibupr), 15 μM sc-560, 250 μM NS-398, or 1 μg/mL PGE2. Each filter was sequentially probed by immunoblot with antiphospho–JNK (A,D), antiphospho–p38 (B,D), or antiphospho–Erk (C-D) antibodies and then, after stripping, with control antitubulin, anti-p38, or anti-Erk antibodies as indicated. The migration of molecular mass markers is shown. Anti-P indicates antiphospho; and WB, Western blot.
Selective impairment of TCR-dependent JNK and p38 activation by NSAIDs. Immunoblot analysis of postnuclear supernatants from Jurkat cells (A-C) or freshly purified human PBLs (D), nonactivated (0) or activated by CD3 (CD3) ligation for 5 minutes, in the presence or absence of 600 μM ibuprofen (ibupr), 15 μM sc-560, 250 μM NS-398, or 1 μg/mL PGE2. Each filter was sequentially probed by immunoblot with antiphospho–JNK (A,D), antiphospho–p38 (B,D), or antiphospho–Erk (C-D) antibodies and then, after stripping, with control antitubulin, anti-p38, or anti-Erk antibodies as indicated. The migration of molecular mass markers is shown. Anti-P indicates antiphospho; and WB, Western blot.
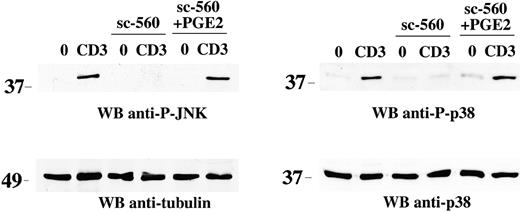
The block by NSAIDs of TCR-dependent JNK and p38 activation suggests that the products of COX-1 activity are required for TCR signaling. In support of this notion, treatment of Jurkat T cells with PGE2, one of the principal prostanoids produced by COXs, resulted in recovery from the block in both JNK and p38 activation by sc-560 (Figure 2). Furthermore, PGE2 enhanced JNK and p38 phosphorylation, but not Erk phosphorylation, following TCR engagement (Figure 1). As previously shown for p38,15 inhibition of JNK affects downstream events triggered by TCR engagement. Indeed, the JNK inhibitor, SP600125, blocked TCR-dependent activation of the transcription factor NF-AT in a dose-dependent manner (Figure S2). Hence COX-1–generated prostanoids are required for activation of both members of the stress kinase subfamily of MAP kinases but not of classical MAP kinases.
Reversal of the NSAID-dependent block in stress kinase activation by PGE2. Immunoblot analysis of postnuclear supernatants from Jurkat cells nonactivated (0) or activated by CD3 (CD3) ligation for 5 minutes, in the presence or absence of 15 μM sc-560, alone or in combination with 1 μg/mL PGE2. Each filter was sequentially probed by immunoblot with antiphospho–JNK or antiphospho–p38 antibodies and then, after stripping, with control antitubulin or anti-p38 antibodies as indicated. The migration of molecular mass markers is shown.
Reversal of the NSAID-dependent block in stress kinase activation by PGE2. Immunoblot analysis of postnuclear supernatants from Jurkat cells nonactivated (0) or activated by CD3 (CD3) ligation for 5 minutes, in the presence or absence of 15 μM sc-560, alone or in combination with 1 μg/mL PGE2. Each filter was sequentially probed by immunoblot with antiphospho–JNK or antiphospho–p38 antibodies and then, after stripping, with control antitubulin or anti-p38 antibodies as indicated. The migration of molecular mass markers is shown.
COX-1 controls an early step in the stress kinase cascade
The impairment in TCR-dependent activation of both JNK and p38 by nonselective and COX-1–selective NSAIDs suggests that COX-1, rather than directly modulating the activity of stress kinases, controls a component upstream of p38 and JNK in the TCR signaling cascade. Furthermore, since tyrosine kinase–dependent pathways leading to Erk and p38/JNK activation diverge at the level of the small GTPases Ras and Rac, respectively,27 the failure of NSAIDs to affect Erk activation suggests a requirement for COX-1 in the serine-threonine cascade initiated by Rac. In T cells, Rac is primarily activated by the guanine nucleotide exchange factor Vav.28 GTP-bound Rac, in addition to promoting reorganization of the actin cytoskeleton, recruits the serinethreonine kinase Pak1, which in turn triggers the serine-threonine kinase cascades leading to JNK and p38 activation.29-31
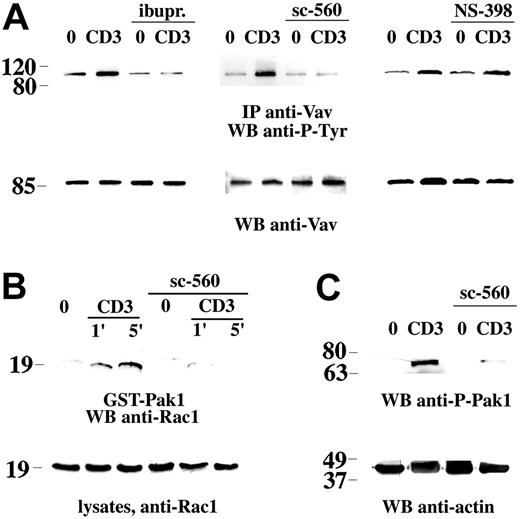
The effect of COX-1 inhibition on Vav activation, as evaluated by its phosphorylation on tyrosine residues, was determined. A significant impairment in TCR-dependent Vav phosphorylation was observed in Jurkat cells (Figure 3A), as well as in human PBLs (data not shown) treated with the COX-1 inhibitor sc-560. A similar inhibition was observed in the presence of ibuprofen, whereas the COX-2 inhibitor NS-398 did not affect Vav phosphorylation (Figure 3A). In agreement with the decrease in Vav phosphorylation, TCR-dependent Rac activation was impaired in cells treated with sc-560 and ibuprofen but not NS-398 (Figure 3B; data not shown). Furthermore, TCR-dependent Pak1 phosphorylation, which occurs following its recruitment to GTP-bound Rac, was also reduced in sc-560– and ibuprofen-treated cells (Figure 3C; data not shown).
Inhibition of TCR-dependent Vav phosphorylation and downstream signaling by NSAIDs. (A) Immunoblot analysis using antiphosphotyrosine mAb of Vav-specific immunoprecipitates from Jurkat cells, nonactivated (0) or activated by CD3 ligation (CD3) for 1 minute, in the presence or absence of 600 μM ibuprofen (ibupr), 15 μM sc-560, or 250 μM NS-398. The filter was stripped and reprobed with anti-Vav mAb. (B) Immunoblot analysis with anti-Rac mAb of in vitro binding assays of postnuclear supernatants from Jurkat cells, using an agarose-conjugated Pak1-GST fusion protein. Equal amounts of postnuclear supernatants from the same samples were separated on the same gel (bottom). Cells were either nonactivated (0) or activated by cross-linking of CD3 (1 minute and 5 minutes) in the presence or absence of 15 μM sc-560. (C) Immunoblot analysis of postnuclear supernatants from Jurkat cells, nonactivated (0) or activated by CD3 ligation as in panel B (1 minute CD3 cross-linking). The filter was probed with antiphospho–Pak1 antibody, followed by antiactin mAb as loading control. The migration of molecular mass markers is shown.
Inhibition of TCR-dependent Vav phosphorylation and downstream signaling by NSAIDs. (A) Immunoblot analysis using antiphosphotyrosine mAb of Vav-specific immunoprecipitates from Jurkat cells, nonactivated (0) or activated by CD3 ligation (CD3) for 1 minute, in the presence or absence of 600 μM ibuprofen (ibupr), 15 μM sc-560, or 250 μM NS-398. The filter was stripped and reprobed with anti-Vav mAb. (B) Immunoblot analysis with anti-Rac mAb of in vitro binding assays of postnuclear supernatants from Jurkat cells, using an agarose-conjugated Pak1-GST fusion protein. Equal amounts of postnuclear supernatants from the same samples were separated on the same gel (bottom). Cells were either nonactivated (0) or activated by cross-linking of CD3 (1 minute and 5 minutes) in the presence or absence of 15 μM sc-560. (C) Immunoblot analysis of postnuclear supernatants from Jurkat cells, nonactivated (0) or activated by CD3 ligation as in panel B (1 minute CD3 cross-linking). The filter was probed with antiphospho–Pak1 antibody, followed by antiactin mAb as loading control. The migration of molecular mass markers is shown.
COX-1 controls Fyn activation and participates in a TCR-proximal pathway selectively coupled to stress kinase activation
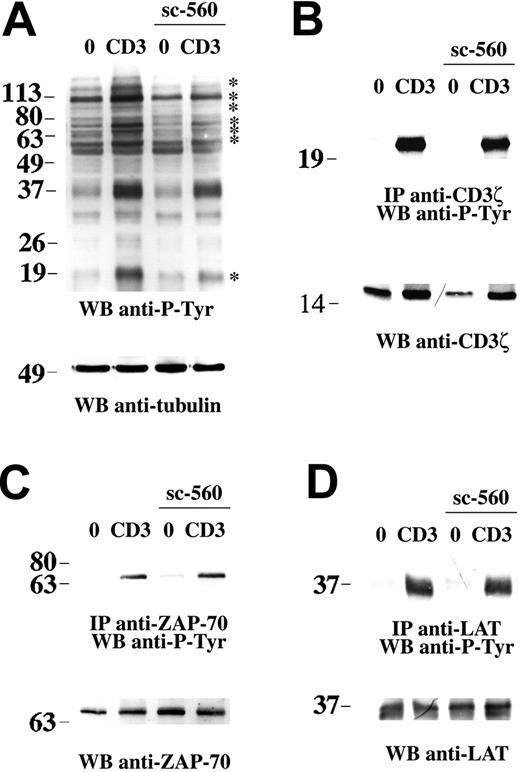
Following TCR engagement, Vav is recruited to the TCR-associated signaling complex both by Src homology 2 (SH2) domain–dependent interactions with proteins such as SLP-76 (SH2 domain–containing leukocyte protein of 76 kDa) and ZAP-70 and by plekstrin homology (PH) domain–dependent interactions with membrane phospholipids.28 In addition, a pool of Vav is constitutively associated to the TCR and has been proposed to participate in an early transient phase of actin reorganization that results in relocalization/stabilization of the TCR in lipid rafts.32 Vav is activated by phosphorylation on tyrosine residues, a process controlled by the tyrosine kinases Fyn and ZAP-70.33 Analysis of total lysates of cells treated with sc-560 showed an impairment in TCR-dependent tyrosine phosphorylation of a subset of proteins (Figure 4A, see asterisks), which suggests that a tyrosine kinase coupled to the TCR might be the target of COX-1.
NSAIDs affect TCR-dependent tyrosine phosphorylation but not CD3ζ, ZAP-70, or LAT activation. (A) Antiphosphotyrosine immunoblot of postnuclear supernatants from Jurkat cells either nonactivated (0) or activated by CD3 ligation for 5 minutes, in the presence or absence of 15 μM sc-560. After stripping, the filter was reprobed with antitubulin mAb as loading control. (B-D) Immunoblot analysis using antiphosphotyrosine mAb of CD3ζ-specific (B), ZAP-70–specific (C), or LAT-specific (D) immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 1 minute, in the presence or absence of 15 μM sc-560. The filter was stripped and reprobed with anti-CD3ζ, anti–ZAP-70, or anti-LAT antibodies as indicated. The migration of molecular mass markers is shown.
NSAIDs affect TCR-dependent tyrosine phosphorylation but not CD3ζ, ZAP-70, or LAT activation. (A) Antiphosphotyrosine immunoblot of postnuclear supernatants from Jurkat cells either nonactivated (0) or activated by CD3 ligation for 5 minutes, in the presence or absence of 15 μM sc-560. After stripping, the filter was reprobed with antitubulin mAb as loading control. (B-D) Immunoblot analysis using antiphosphotyrosine mAb of CD3ζ-specific (B), ZAP-70–specific (C), or LAT-specific (D) immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 1 minute, in the presence or absence of 15 μM sc-560. The filter was stripped and reprobed with anti-CD3ζ, anti–ZAP-70, or anti-LAT antibodies as indicated. The migration of molecular mass markers is shown.
We and others32,34 have previously shown that Lck, while essential for TCR signaling, is partially dispensable for Vav phosphorylation, suggesting that COX-1 might modulate a TCR-proximal pathway triggered by a tyrosine kinase other than Lck and selectively implicated in the activation of stress kinases through Vav. In support of this possibility, despite their capacity to inhibit Vav phosphorylation, no significant effect of sc-560 (Figure 4B; data not shown for PBLs) or ibuprofen (data not shown) was observed on the phosphorylation of CD3ζ, which crucially requires Lck.16,35 Accordingly, neither ZAP-70 phosphorylation (Figure 4C) nor phosphorylation of its target, LAT (Figure 4D), were affected by sc-560, ruling out both Lck and ZAP-70 as targets of COX-1. Furthermore, analysis of MAP kinase activation in JCaM1 cells, a Jurkat subline defective for Lck expression, showed that, notwithstanding a complete block in Erk activation, a weak but reproducible activation of p38 and JNK, as well as of Pak1 and Vav, could be detected (S.R.P., L.P., and C.T.B., unpublished results, 2003-04; Valensin et al,32 Michel et al33 ), indicating that Lck is at least in part dispensable for activation of this pathway.
On the other hand, Fyn plays a key role in Vav activation.33,34 This function is likely to be subserved by a pool of Fyn constitutively associated with CD3ζ,36,37 which might be responsible for initiation of a Vav-dependent pathway regulating stress kinase activation independently of Lck and ZAP-70. The low levels of Fyn expressed in JCaM1 cells35 are in fact likely to underlie the partial TCR-dependent activation of Vav/stress kinases observed in these cells (S.R.P., L.P., and C.T.B., unpublished results, 2003-04; Valensin et al,32 Michel et al33 ). The effect of sc-560 on Fyn activation triggered by TCR engagement was assessed by in vitro kinase assays of Fyn-specific immunoprecipitates. As shown in Figure 5A, a significant inhibition of TCR-dependent Fyn activation was observed in the presence of sc-560 but not NS-398. Similar results were obtained on human PBLs (Figure 5A). Conversely, sc-560 did not affect TCR-dependent activation of Lck (Figure 5B; data not shown for PBLs). The notion that Fyn is a selective target of COX-1 is further supported by the finding that activation of Pyk2, a focal adhesion kinase (FAK) family protein tyrosine kinase associated to Fyn and activated by this kinase,38 was impaired in cells treated with sc-560 and ibuprofen (Figure 5C) but not NS-398 (data not shown). The possibility of an allosteric inhibition of Fyn by sc-560 was ruled out by in vitro kinase assays using purified Fyn (data not shown). In agreement with a role for COX in the control of Fyn, treatment with PGE2 resulted in enhanced Fyn activity (Figure 5D). No effect of PGE2 was observed in in vitro kinase assays using purified Fyn (data not shown), indicating that modulation of Fyn activity by PGE2 results from its interaction with a specific receptor.
NSAIDs inhibit TCR-dependent Fyn activation and Pyk2 phosphorylation. (A) Enolase phosphorylation in in vitro kinase assays of Fyn-specific immunoprecipitates from Jurkat cells or PBLs, nonactivated or activated by CD3 ligation for 1 minute, in the presence of carrier, 15 μM sc-560, or 250 μM NS-398. After the kinase reaction, samples were subjected to SDS-PAGE, transferred to nitrocellulose filters, and analyzed using a Phosphorimager. The filter was subsequently probed with anti-Fyn mAb as immunoprecipitation control. The data (representative of 2 independent experiments) show the fold variation in enolase phosphorylation in stimulated versus unstimulated samples. A representative autoradiograph showing [32P]-labeled enolase by Fyn in Jurkat cells is shown above the graph. The fold variation in Fyn autophosphorylation in CD3-stimulated versus unstimulated Jurkat cells was as follows: carrier, 1.95 ± 0.2-fold; sc-560, 1.05 ± 0.1-fold. (B) Lck autophosphorylation in in vitro kinase assays of Lck-specific immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 1 minute, in the presence of carrier or 15 μM sc-560. The samples were processed as in panel A. The data (representative of 2 independent experiments) show the fold variation in Lck autophosphorylation in stimulated versus unstimulated samples. A representative autoradiograph showing [32P]-labeled Lck is shown above the graph. (C) Immunoblot analysis of postnuclear supernatants from Jurkat cells, nonactivated (0) or activated by CD3 ligation (CD3) for 1 minute in the presence or absence of 15 μM sc-560 or 600 μM ibuprofen (ibupr). The filter was probed with antiphospho–Pyk2 antibodies, followed by anti-Pyk2 mAb as loading control. The migration of molecular mass markers is shown. (D) Enolase phosphorylation in in vitro kinase assays of Fyn-specific immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 30 seconds, in the presence of carrier or 1 μg/mL PGE2. The data from a representative experiment are shown, as well as the respective autoradiograph showing [32P]-labeled enolase. Error bars indicate standard deviation.
NSAIDs inhibit TCR-dependent Fyn activation and Pyk2 phosphorylation. (A) Enolase phosphorylation in in vitro kinase assays of Fyn-specific immunoprecipitates from Jurkat cells or PBLs, nonactivated or activated by CD3 ligation for 1 minute, in the presence of carrier, 15 μM sc-560, or 250 μM NS-398. After the kinase reaction, samples were subjected to SDS-PAGE, transferred to nitrocellulose filters, and analyzed using a Phosphorimager. The filter was subsequently probed with anti-Fyn mAb as immunoprecipitation control. The data (representative of 2 independent experiments) show the fold variation in enolase phosphorylation in stimulated versus unstimulated samples. A representative autoradiograph showing [32P]-labeled enolase by Fyn in Jurkat cells is shown above the graph. The fold variation in Fyn autophosphorylation in CD3-stimulated versus unstimulated Jurkat cells was as follows: carrier, 1.95 ± 0.2-fold; sc-560, 1.05 ± 0.1-fold. (B) Lck autophosphorylation in in vitro kinase assays of Lck-specific immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 1 minute, in the presence of carrier or 15 μM sc-560. The samples were processed as in panel A. The data (representative of 2 independent experiments) show the fold variation in Lck autophosphorylation in stimulated versus unstimulated samples. A representative autoradiograph showing [32P]-labeled Lck is shown above the graph. (C) Immunoblot analysis of postnuclear supernatants from Jurkat cells, nonactivated (0) or activated by CD3 ligation (CD3) for 1 minute in the presence or absence of 15 μM sc-560 or 600 μM ibuprofen (ibupr). The filter was probed with antiphospho–Pyk2 antibodies, followed by anti-Pyk2 mAb as loading control. The migration of molecular mass markers is shown. (D) Enolase phosphorylation in in vitro kinase assays of Fyn-specific immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 30 seconds, in the presence of carrier or 1 μg/mL PGE2. The data from a representative experiment are shown, as well as the respective autoradiograph showing [32P]-labeled enolase. Error bars indicate standard deviation.
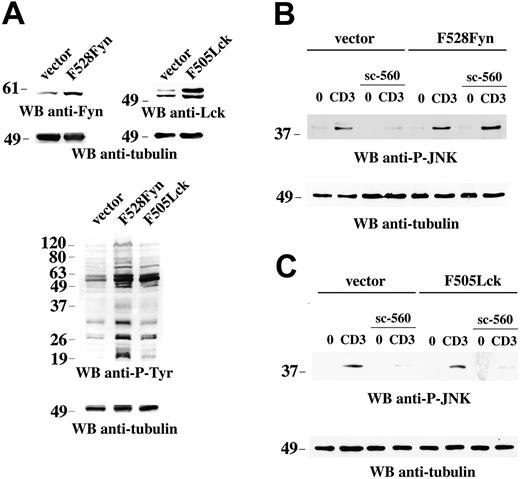
To further address the functional connection between COX-1 and Fyn, we generated Jurkat T-cell transfectants expressing constitutively active Fyn (F528Fyn) or Lck (F505Lck) mutants. Immunoblot analysis of total cell lysates showed that the levels of Fyn and Lck were increased by at least 2-fold in the respective transfectants, which was paralleled by an increase in protein tyrosine phosphorylated proteins (Figure 6A). As shown in Figure 6, sc-560 failed to inhibit JNK phosphorylation in cells expressing F528Fyn (Figure 6B) but not in cells expressing F505Lck (Figure 6C), indicating that active Fyn can selectively bypass COX-1 inhibition. The results identify Fyn as an early target of COX-1 in the TCR signaling cascade.
Constitutively active Fyn bypasses the block of TCR-dependent JNK activation by NSAIDs. (A) Immunoblot analysis of postnuclear supernatants from Jurkat cells stably transfected with empty vector or a construct encoding either F528Fyn or F505Lck. The filters were sequentially probed with anti-Fyn or anti-Lck and antitubulin mAb. Below is an antiphosphotyrosine immunoblot of the 3 lines, subsequently reprobed with antitubulin mAb. (B-C) Immunoblot analysis of postnuclear supernatants from Jurkat cells stably transfected with empty vector or a construct encoding either F528Fyn (B) or F505Lck (C), either nonactivated (0) or activated by CD3 (CD3) ligation for 5 minutes, in the presence or absence of 15 μM sc-560. Each filter was sequentially probed by immunoblot with antiphospho–JNK and then, after stripping, with control antitubulin mAb. The migration of molecular mass markers is shown.
Constitutively active Fyn bypasses the block of TCR-dependent JNK activation by NSAIDs. (A) Immunoblot analysis of postnuclear supernatants from Jurkat cells stably transfected with empty vector or a construct encoding either F528Fyn or F505Lck. The filters were sequentially probed with anti-Fyn or anti-Lck and antitubulin mAb. Below is an antiphosphotyrosine immunoblot of the 3 lines, subsequently reprobed with antitubulin mAb. (B-C) Immunoblot analysis of postnuclear supernatants from Jurkat cells stably transfected with empty vector or a construct encoding either F528Fyn (B) or F505Lck (C), either nonactivated (0) or activated by CD3 (CD3) ligation for 5 minutes, in the presence or absence of 15 μM sc-560. Each filter was sequentially probed by immunoblot with antiphospho–JNK and then, after stripping, with control antitubulin mAb. The migration of molecular mass markers is shown.
Discussion
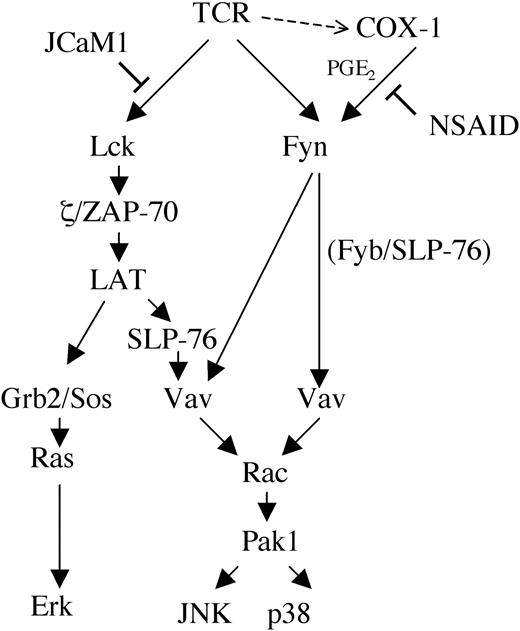
Collectively, the data show that COX-1 participates in TCR signaling by regulating a Fyn-dependent TCR-proximal pathway that controls the activation of stress kinases. This pathway might involve the previously described Fyn/Fyn binding protein (Fyb)/SLP-76/Vav complex that is assembled in response to TCR engagement and required for interleukin 2 (IL-2) gene transcription.39 A complex with a similar composition has been proposed to couple the Fcγ receptor to the actin cytoskeleton in macrophages.40 According to the model presented in Figure 7, stress kinases would be activated both through this complex and through a complex that includes ZAP-70/LAT/SLP-76/Vav and that also controls the activation of Ras/MAP kinases.41 Because of the central role of Fyn in Vav phosphorylation,33,34 inhibition of Fyn by NSAIDs would result in impairment of stress kinase activation through both pathways, whereas no significant effect on Erk activation would be observed. While at this stage the mechanism underlying the modulation of Fyn activity by NSAIDs remains hypothetical, the recent finding that cyclic adenosine monophosphate (cAMP) promotes Src activation through protein kinase A (PKA)–dependent phosphorylation of Ser1742 suggests a potential mechanism of Fyn regulation by prostaglandin receptors coupled to adenylate cyclase. A highly conserved consensus RXXS/T motif for PKA phosphorylation, RDGS, is indeed present in the unique N-terminal domain of Fyn but not of Lck. Furthermore, Fyn has been reported to be phosphorylated both on tyrosine and on serine residues.43
Model of NSAID interference with TCR signal transduction. Grb2 indicates growth factor receptor-bound protein 2; Sos indicates son-of-sevenless.
Model of NSAID interference with TCR signal transduction. Grb2 indicates growth factor receptor-bound protein 2; Sos indicates son-of-sevenless.
Although the immunosuppressive activity of NSAIDs on T-cell activation might be mediated at least in part by PPARs, which block the activity of key transcription factors implicated in this process,13,44 this is not likely to interfere with early events in TCR signaling, which are triggered within minutes following TCR engagement. A COX-independent effect of NSAIDs on TCR signaling is also ruled out by the demonstration that PGE2, one of the principal products of COX activity, effectively reverses JNK and p38 inhibition by NSAIDs and enhances stress kinase activation. Paradoxically, exogenous PGE2 is a potent immunosuppressant, an activity related at least in part to its capacity to increase the levels of intracellular cyclic AMP and interfere with IL-2 gene transcription and cell cycle progression.10 It can be hypothesized that excessive or inappropriate stimulation of the stress kinase pathway through Fyn/Vav/Pak1 might contribute to the immunosuppressive activity of PGE2 by interfering with early TCR signal transduction. Although Fyn is essential for optimal T-cell activation,45 sustained elevation of Fyn activity, as well as selective association of CD3ζ with Fyn, are hallmarks of T-cell anergy.46,47 Increasing the levels of Fyn expression in the Lck-deficient JCaM1 cells, while resulting in partial recovery of intracellular signaling in response to TCR engagement, is however accompanied by reduced IL-2 production and cell growth inhibition.35 These effects correlate with anomalous CD3ζ phosphorylation and defective ZAP-70 activation, a phenotype reminiscent of altered peptide ligand–induced anergy.48,49 Furthermore, TCR engagement by antagonistic peptides results in increased Fyn activation and Vav phosphorylation, with little or no effect on Lck and ZAP-70.34 Another potential target of COX-1 in early Fyn-dependent signaling is C-terminal Src kinase (Csk), which negatively controls Src kinases. Csk has been shown to localize in lipid rafts, where it inhibits Lck activity, as the result of its interaction with phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a transmembrane adaptor implicated in the negative regulation of T-cell activation and a specific substrate of Fyn.50,51 Furthermore, enhancement of Csk activity by PGE2, resulting from PKA-mediated Csk phosphorylation, has been recently demonstrated.52
On the other hand, prostanoids appear to be important positive components in TCR signaling. For example, PGE2 is essential for both the COX-1–dependent transition from the double-negative to double-positive stage of thymocyte development and for the subsequent COX-2–dependent maturation of singe-positive CD4+ cells.12 Despite its delayed cAMP-dependent inhibitory effects on IL-2 gene transcription, mediated at least in part by NF-AT down-modulation through activation of inducible cAMP early repressor (ICER) and glycogen synthase kinase (GSK-3) phosphorylation by PKA,53,54 PGE2 also activates cAMP-response element binding protein (CREB), which is required for CD28 costimulation of IL-2 gene transcription.55 PGB2 has recently been shown to deliver a costimulatory signal in T-cell activation.56 Furthermore, phospholipase A2, which catalyzes the production of arachidonic acid, is essential for T-cell activation and proliferation.57 In this context, while the role of COX-2 in mitogenic signaling and carcinogenesis is well established, data obtained on COX-1-/- mice suggest that COX-1 might also participate in this process.58 It could be hypothesized that, as opposed to the effects evoked by treatment of T cells with exogenous PGE2, physiologic levels of endogenous PGE2, produced in response to TCR engagement by preexisting COX-1 and subsequently sustained by newly expressed COX-2, might contribute, through its interaction with intracellular prostaglandin E receptor 1 (EP) receptors,59 both to the initiation of productive TCR signaling and to subsequent signal extinction, and thereby participate in the orchestration of T-cell activation.
Prepublished online as Blood First Edition Paper, October 28, 2004; DOI 10.1182/blood-2004-04-1299.
Supported by the Italian Association for Cancer Research (AIRC), Telethon (grant E.1161), the European Commission (QLK2-CT-2002-00620), and the University of Siena (Piano di Ateneo per la Ricerca [PAR]).
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
The authors wish to thank John L. Telford for productive discussions and critical reading of the manuscript. The technical assistance of Sonia Grassini and secretarial assistance of Giancarlo Benocci are gratefully acknowledged.

![Figure 5. NSAIDs inhibit TCR-dependent Fyn activation and Pyk2 phosphorylation. (A) Enolase phosphorylation in in vitro kinase assays of Fyn-specific immunoprecipitates from Jurkat cells or PBLs, nonactivated or activated by CD3 ligation for 1 minute, in the presence of carrier, 15 μM sc-560, or 250 μM NS-398. After the kinase reaction, samples were subjected to SDS-PAGE, transferred to nitrocellulose filters, and analyzed using a Phosphorimager. The filter was subsequently probed with anti-Fyn mAb as immunoprecipitation control. The data (representative of 2 independent experiments) show the fold variation in enolase phosphorylation in stimulated versus unstimulated samples. A representative autoradiograph showing [32P]-labeled enolase by Fyn in Jurkat cells is shown above the graph. The fold variation in Fyn autophosphorylation in CD3-stimulated versus unstimulated Jurkat cells was as follows: carrier, 1.95 ± 0.2-fold; sc-560, 1.05 ± 0.1-fold. (B) Lck autophosphorylation in in vitro kinase assays of Lck-specific immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 1 minute, in the presence of carrier or 15 μM sc-560. The samples were processed as in panel A. The data (representative of 2 independent experiments) show the fold variation in Lck autophosphorylation in stimulated versus unstimulated samples. A representative autoradiograph showing [32P]-labeled Lck is shown above the graph. (C) Immunoblot analysis of postnuclear supernatants from Jurkat cells, nonactivated (0) or activated by CD3 ligation (CD3) for 1 minute in the presence or absence of 15 μM sc-560 or 600 μM ibuprofen (ibupr). The filter was probed with antiphospho–Pyk2 antibodies, followed by anti-Pyk2 mAb as loading control. The migration of molecular mass markers is shown. (D) Enolase phosphorylation in in vitro kinase assays of Fyn-specific immunoprecipitates from Jurkat cells, nonactivated or activated by CD3 ligation for 30 seconds, in the presence of carrier or 1 μg/mL PGE2. The data from a representative experiment are shown, as well as the respective autoradiograph showing [32P]-labeled enolase. Error bars indicate standard deviation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/5/10.1182_blood-2004-04-1299/6/m_zh80050574930005.jpeg?Expires=1769128819&Signature=BA5TJdEhv217OoRdcifzZVru3gmNHFfriRsrs5NZ8GTO~i30C7JMGPDTmtpUerAZllAXttSMeh9LI4CsAWwr0MnFapxPwiaHngMxyp6o7HIEcMzoRixJzF1gOamGINU7JjA7aRRlFpccXdwniaz8PYRuhqjtbxzKuVjlxMjWqn4cz-Gi~alybqV7~B1u8iUxoEhqw2~bf9CBQOQkYX7NGworJKn2r46qnSzHWOTiq4yVcgiCtDakDsNz-wDapW7XwfsD1nfAYic9wGIF5tzF3rIzI2XBdwFSclfVdUitjPI5sn9jf5A0zcI6xaHlz4FjCQUDde4FJAm5qYTWWjudfA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal