Abstract
Evidence suggests that infusional therapy is a more effective means for administering cytotoxic therapy than intravenous bolus therapy for lymphoma and offers greater potential for therapeutic synergy with rituximab, which has a long half-life. We pooled the results of 3 prospective phase 2 trials evaluating rituximab in combination with 96-hour infusion of cyclophosphamide (187.5-200 mg/m2 per day), doxorubicin (12.5 mg/m2 per day), and etoposide (60 mg/m2 per day) (R-CDE) plus granulocyte–colony-stimulating factor (G-CSF) in 74 patients with HIV-associated, B-cell non-Hodgkin lymphoma, of whom 56 (76%) patients received concurrent highly active antiretroviral therapy (HAART). The complete remission (CR) rate was 70% (95% confidence interval [CI], 59%-81%), and the estimated 2-year failure-free survival and overall survival rates were 59% (95% CI, 47%-71%) and 64% (95% CI, 52%-76%), respectively. Ten (14%) patients had opportunistic infections during or within 3 months of the end of R-CDE, and 17 (23%) patients developed nonopportunistic infections after that time. Six (8%) patients died because of infection; 2 (3%) of those infections were bacterial sepsis during R-CDE, and 4 (5%) were opportunistic infections that occurred between 2 and 8 months after the completion of R-CDE. R-CDE produced a 70% CR rate and a 59% 2-year failure-free survival rate in patients with HIV-associated lymphoma. Consistent with other reports, adding rituximab to cytotoxic therapy in this population may increase the risk for life-threatening infection. Further studies evaluating rituximab in combination with infusional chemotherapy are warranted, but caution is advised.
Introduction
Since 1996, the use of highly active antiretroviral therapy (HAART) in patients with HIV infection has resulted in substantial declines in morbidity and mortality rates.1 Although HAART also has been associated with a decline in the incidence of HIV-associated cancers, systemic non-Hodgkin lymphoma (NHL) has become a more common initial manifestation of the AIDS syndrome.2
Previous reports published in the pre-HAART era indicate that HIV-associated non-Hodgkin lymphoma typically manifested with intermediate- or high-grade histology, advanced-stage disease with extranodal involvement, and poor response and poor tolerance to chemotherapy. Patients experienced a high incidence of treatment-associated complications and generally poor survival.3 For example, in 1 large multicenter trial performed in the pre-HAART era that evaluated a conventional chemotherapy regimen (methotrexate, bleomycin, doxorubicin, vincristine, and dexamethasone [m-BACOD]) given at a standard dose or at a reduced dose, the median survival time was 8 months, and only approximately 10% of patients survived at least 2 years.4 Results were similarly poor in other multicenter trials evaluating other commonly used lymphoma regimens, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (ie, CHOP) or more intensive CHOP-like regimens (eg, ACVBP).5,6 Recent studies have indicated, however, that prognosis has improved in the HAART era at a rate similar to the improvement noted for patients with HIV infection without lymphoma.7-9 Several reasons may account for this, including decreased occurrence of extranodal disease and higher CD4 count at presentation, improved bone marrow reserve, fewer opportunistic infections, and fewer deaths from lymphoma or the complications of HIV infection. Previous studies have demonstrated that low CD4 count (less than 100/μL) at presentation is the single most important prognostic factor, predicting more myelosuppression, serious infections, and treatment-associated deaths, reduced likelihood of achieving CR, and worse chance for survival.10,11
Given the positive impact of HAART on the survival of patients with HIV infection, it is logical to evaluate whether more aggressive treatment approaches may further improve survival in those with HIV-associated lymphoma. When this study was initiated in 1998, evidence showed that the infusional administration of cytotoxic therapy was effective in patients with relapsed lymphoma12 and as initial therapy for HIV-associated lymphoma.13 In addition, it had been demonstrated that combining it with the anti-CD20 monoclonal antibody rituximab was an effective therapy for low-grade B-cell lymphoma, produced activity in relapsed intermediate-grade B-cell lymphoma,14 and was highly effective and safe when combined with standard cytotoxic chemotherapy for intermediate-grade B-cell lymphoma.15 In addition, preclinical evidence shows that rituximab sensitized lymphoma cell lines to the effects of cytotoxic agents in vitro.16 Given the long half-life of rituximab,17 we hypothesized that the prolonged infusion of cytotoxic therapy would provide greater opportunity for therapeutic synergy with rituximab compared with administration by a conventional intravenous bolus schedule. Based on these considerations, our 3 groups initiated a phase 2 trial that evaluated the safety and efficacy of rituximab in combination with infusional cyclophosphamide-doxorubicin-etoposide (CDE) in patients with HIV-associated B-cell NHL; the pooled results of these 3 trials, which used nearly identical eligibility criteria and treatment plans, form the basis for this report.
Patients, materials, and methods
Inclusion criteria
Patients were enrolled in the study if they met the following inclusion criteria: biopsy-proven CD20+ NHL according to World Health Organization (WHO) classification18 ; age 18 or older; confirmed HIV infection; WHO performance status (PS) 3 or lower; no previous chemotherapy for lymphoma; no concomitant or previous malignancy except for non–melanoma skin cancer or in situ cervical carcinoma; total bilirubin level 1.5 mg/dL or lower (unless from antiretrovirals or lymphoma producing biliary obstruction or infiltrating the liver); creatinine level 2.0 mg/dL or lower; granulocyte count 1500 cells/dL or higher; platelet count 100 000 cells/dL or higher (unless the last 2 findings were caused by bone marrow involvement); and had provided written informed consent.
This study was a collaborative effort that included the Italian Cooperative Group on AIDS and Tumors (GICAT), the Division of Hematology at the University of Vienna, and the Albert Einstein Cancer Center in New York. Each group or center was performing a study with nearly identical eligibility criteria and treatment plan. The institutional review board of the participating center approved each study, and all patients signed written informed consent. Data from the 3 studies were pooled for this analysis. Findings from a preliminary analysis have been reported19 ; the present analysis represents an updated report after the final accrual goal was achieved and after all patients were monitored for at least 1 year after closure of the study. Thirty-nine patients were enrolled at the Aviano Cancer Center, 17 were enrolled from 9 centers within the GICAT, 12 were enrolled at the University of Vienna, and 6 were enrolled at the Albert Einstein Cancer Center.
Staging
All patients were evaluated through history taking and physical examination for parameters that included height, weight, and WHO performance status; measurements of all involved palpable lesions; complete blood cell count; blood chemistry profile; chest radiography; computed tomography of the thorax, abdomen, and pelvis; bone marrow aspiration and biopsy; electrocardiography and left ventricular ejection fraction evaluation; lumbar puncture; gastroscopy, if indicated; CD4 cell count; and HIV viral load. The Ann Arbor staging system was applied.20
Treatment
The treatment plan is outlined in Table 1. Rituximab-CDE (R-CDE) therapy consisted of rituximab 375 mg/m2 intravenously on day 1 followed by a 4-day (96-hour) continuous intravenous infusion of cyclophosphamide 187.5 to 200 mg/m2 per day, doxorubicin 12.5 mg/m2 per day, and etoposide 60 mg/m2 per day. Central nervous system (CNS) prophylaxis consisted of 12 mg intrathecal methotrexate on day 1 of every cycle (Italy, Vienna) or intrathecal cytarabine 50 mg on the first and fourth days of cycles 1 and 2 (New York). A central venous catheter (CVC) was used in all patients. Supportive care included oral trimethoprim/sulfamethoxazole (160 mg/800 mg) and fluconazole 100 mg daily. Granulocyte–colony-stimulating factor (G-CSF) was given prophylactically (5 μg/kg per day) from day 6 until neutrophil recovery. HAART was strongly recommended for all patients participating in the trial. R-CDE was given every 4 weeks for a maximum of 6 cycles and was repeated if the granulocyte count was at least 1000/μL, the platelet count was at least 50 000/μL, and the patient had adequately recovered from treatment-associated toxicity. The doses of each agent in the CDE regimen were reduced by 25% if the patient had febrile neutropenia or grade 4 nonhematologic toxicity in the previous cycle.
Treatment plan
Agents and doses/routes . | Schedule . |
|---|---|
| R-CDE | |
| Rituximab, 375 mg/m2 | Every 28 d just before the initiation of each cycle of CDE |
| Cyclophosphamide, 187.5-200 mg/m2/d for 4 d* | Repeat every 28 d for a maximum of 6 cycles |
| Doxorubicin, 12.5 mg/m2/d for 4 d* | |
| Etoposide, 60 mg/m2/d for 4 d, continuous intravenous infusion over 96 h | |
| Colony-stimulating factor filgrastim (G-CSF), 5 μg/kg/d, subcutaneous injection | D 6 until neutrophil recovery |
| Infection prophylaxis required | |
| Trimethoprim-sulfamethoxazole, 160 mg/800 mg by mouth | 3 times/wk |
| Fluconazole, 100 mg by mouth | Daily |
| Central nervous system prophylaxis | |
| Intrathecal methotrexate, 12 mg | D 1 of each cycle (n = 68) |
| OR | |
| Intrathecal cytarabine, 50 mg | D 1 and 4 of cycles 1 and 2 for patients with Burkitt lymphoma or bone marrow involvement only (n = 6) |
Agents and doses/routes . | Schedule . |
|---|---|
| R-CDE | |
| Rituximab, 375 mg/m2 | Every 28 d just before the initiation of each cycle of CDE |
| Cyclophosphamide, 187.5-200 mg/m2/d for 4 d* | Repeat every 28 d for a maximum of 6 cycles |
| Doxorubicin, 12.5 mg/m2/d for 4 d* | |
| Etoposide, 60 mg/m2/d for 4 d, continuous intravenous infusion over 96 h | |
| Colony-stimulating factor filgrastim (G-CSF), 5 μg/kg/d, subcutaneous injection | D 6 until neutrophil recovery |
| Infection prophylaxis required | |
| Trimethoprim-sulfamethoxazole, 160 mg/800 mg by mouth | 3 times/wk |
| Fluconazole, 100 mg by mouth | Daily |
| Central nervous system prophylaxis | |
| Intrathecal methotrexate, 12 mg | D 1 of each cycle (n = 68) |
| OR | |
| Intrathecal cytarabine, 50 mg | D 1 and 4 of cycles 1 and 2 for patients with Burkitt lymphoma or bone marrow involvement only (n = 6) |
The daily dose of cyclophosphamide and doxorubicin was admixed in the same bag of intravenous fluid (1 L) and was infused through a central venous catheter, and etoposide was diluted in a separate liter of intravenous fluid and infused through a separate central venous catheter or peripheral line.
Evaluation of antitumor response and toxicity
Complete response (CR) was defined as the complete absence of clinically detectable tumors and normalization of previously abnormal radiographic findings persisting for at least 4 weeks. Partial response (PR) was at least a 50% reduction in the product of the perpendicular diameters of all tumors assessed by physical examination or radiographic check-up persisting for more than 1 month with no new lesions occurring. Stable disease (SD) was described as change less than 25% in the product of the perpendicular diameters of all tumors. Progressive disease (PD) was defined as increase greater than 25% increase in the measured lesions or in the occurrence of new lesions. Toxicity was rated according to WHO criteria.21
Statistical methods
With the sample size used, the trial had 90% power (10% 1-sided alpha-level test) to detect a 20% improvement in CR rate (from 50% to 70%). Overall survival (OS) was computed from the initiation of therapy until the last visit at which the patient was known to be alive or until death from any cause. Failure-free survival (FFS) was defined as the time from the initiation of therapy to progression, relapse, or death from any cause. Disease-free survival (DFS) was calculated for patients who achieved CR from the first CR recorded until relapse or the last known date on which the patient was disease free. Event-free survival (EFS) was computed from the initiation of treatment until the onset of a clinically significant event, defined as death from any cause, relapse, progression, or development of an opportunistic infection.
OS, FFS, DFS, and EFS were evaluated according to the Kaplan-Meier method,22 and differences among subgroups were assessed by means of the log-rank test.23 Multivariate analysis of survival was performed using the Cox proportional hazards model24 (hazard ratio [HR]) and the 95% confidence interval (CI), including all variables that were significantly associated with prognosis in the univariate analysis. Differences between qualitative parameters were performed by χ2 and Fisher exact tests. In all cases, statistical significance was claimed for P ≤ .05 (2-sided). A statistical evaluation for heterogeneity was performed to determine whether there was an interaction between treatment center and outcome.
Results
Patient characteristics
From June 1998 to June 2003, 74 patients with HIV-associated lymphoma were enrolled, and all were evaluable for response, toxicity, and survival. Characteristics of the study population are outlined in Table 2. When classified by the age-adjusted International Prognostic Index,25 57% were at high or high-intermediate risk. The median CD4 cell count was 161 cells/dL (range, 3-691 cells/dL), and 56 (84%) of 67 evaluable patients had a detectable HIV viral load (median, 67 000 copies/mL; range 0-750 000 copies/mL). Forty-two (57%) patients had been receiving HAART for at least 6 months before the diagnosis of lymphoma. Fifty-six (76%) patients received HAART concomitantly with R-CDE. Eighteen (24%) patients did not receive concurrent HAART because of either physician (n = 12) or patient (n = 6) choice. Of the 56 who received concurrent HAART, 39 (70%) patients received 2 nucleoside reverse transcriptase inhibitors (NRTIs) plus a protease inhibitor, whereas the remaining 17 (30%) patients received 2 NRTIs plus a non-nucleoside reverse transcriptase inhibitor (NNRTI). There were no differences in the characteristics of the patient population in the 3 trials
Patient characteristics
. | Value . |
|---|---|
| No. patients | 74 |
| Median age, y (range) | 38 (29-65) |
| Median CD4 cell count/dL (range) | 161 (3-691) |
| Median HIV viral load, copies/dL (range)* | 67000 (0-750 000) |
| Sex, no.(%) | |
| Male | 60 (81) |
| Female | 14 (19) |
| Risk group for HIV infection, no.(%) | |
| Intravenous drug use | 26 (35) |
| Homosexual male | 22 (30) |
| Heterosexual contact | 21 (28) |
| Unknown | 5 (7) |
| Previous AIDS defining event, no. (%) | 8 (11) |
| Previous HAART, no. (%) | 42 (57) |
| Histology, no. (%) | |
| Large noncleaved cell | 48 (65) |
| Small noncleaved, Burkitt | 21 (28) |
| Immunoblastic | 3 (4) |
| Anaplastic large cell | 2 (3) |
| Stage, no. (%) | |
| 1-2 | 22 (30) |
| 3-4 | 52 (70) |
| Age-adjusted IPI, no. (%) | |
| Low risk (0 factors) | 8 (11) |
| Low-intermediate risk (1 factor) | 24 (32) |
| High-intermediate risk (2 factors) | 25 (34) |
| High risk (3 factors) | 17 (23) |
| No. patients with extranodal involvement (%) | 55 (74) |
| Site of extranodal involvement, no. (%) | |
| Gastrointestinal tract | 29 (39) |
| Lung | 9 (12) |
| Bone marrow | 9 (12) |
| Liver | 9 (12) |
| Spleen | 9 (12) |
. | Value . |
|---|---|
| No. patients | 74 |
| Median age, y (range) | 38 (29-65) |
| Median CD4 cell count/dL (range) | 161 (3-691) |
| Median HIV viral load, copies/dL (range)* | 67000 (0-750 000) |
| Sex, no.(%) | |
| Male | 60 (81) |
| Female | 14 (19) |
| Risk group for HIV infection, no.(%) | |
| Intravenous drug use | 26 (35) |
| Homosexual male | 22 (30) |
| Heterosexual contact | 21 (28) |
| Unknown | 5 (7) |
| Previous AIDS defining event, no. (%) | 8 (11) |
| Previous HAART, no. (%) | 42 (57) |
| Histology, no. (%) | |
| Large noncleaved cell | 48 (65) |
| Small noncleaved, Burkitt | 21 (28) |
| Immunoblastic | 3 (4) |
| Anaplastic large cell | 2 (3) |
| Stage, no. (%) | |
| 1-2 | 22 (30) |
| 3-4 | 52 (70) |
| Age-adjusted IPI, no. (%) | |
| Low risk (0 factors) | 8 (11) |
| Low-intermediate risk (1 factor) | 24 (32) |
| High-intermediate risk (2 factors) | 25 (34) |
| High risk (3 factors) | 17 (23) |
| No. patients with extranodal involvement (%) | 55 (74) |
| Site of extranodal involvement, no. (%) | |
| Gastrointestinal tract | 29 (39) |
| Lung | 9 (12) |
| Bone marrow | 9 (12) |
| Liver | 9 (12) |
| Spleen | 9 (12) |
Other disease sites included bone (6 patients), soft tissue (4 patients), Waldeyer ring (4 patients), gingiva/tongue (3 patients), meninges (3 patients), kidney (3 patients), skin (3 patients), pleura (3 patients), and adrenal gland (2 patients).
n = 67.
Response and survival data
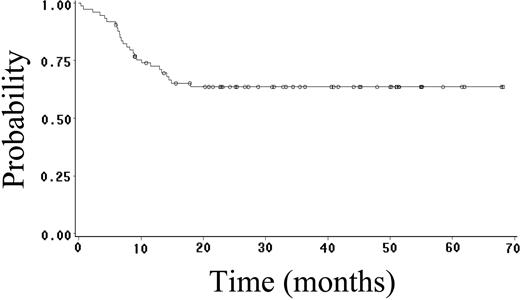
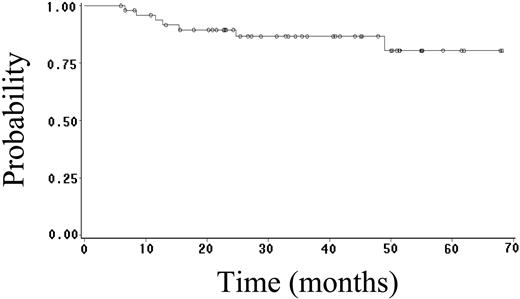
Fifty-two (70%) patients achieved CR (95% CI, 59%-81%), and 4 (5%) patients achieved PR, yielding an overall response rate of 75%. Twenty-seven (36%) patients have had progressive or relapsed disease, including 7 (14%) of 52 patients who achieved CR. After a median follow-up of 23 months (range, 1-68 months), 48 (65%) patients are alive, and 41 (55%) patients are alive and disease free. The estimated 2-year OS, FFS, DFS, and EFS rates are 64% (95% CI, 52%-76%; Figure 1), 59% (95% CI, 47%-71%; Figure 2), 89% (95% CI, 81%-97%; Figure 3), and 52% (95% CI, 42%-62%; Figure 4), respectively. Of the 26 patients who died, the cause of death was lymphoma in 20 patients (27% of total population), opportunistic infection in 4 patients (5% of total population), and treatment-associated sepsis in 2 patients (3% of total population). Statistical evaluation for heterogeneity demonstrated no interaction between treatment center and outcome.
Prognostic factor analysis
We evaluated a variety of prognostic factors for their effect on response, FFS, and OS, including demographic factors (age, sex), lymphoma-specific factors (stage, histology, IPI score, bone marrow involvement, serum lactate dehydrogenase, performance status), and HIV-specific factors (eg, risk factor for HIV, CD4 count, prior opportunistic infection, prior HAART, detectable HIV viral load at diagnosis and at the end of treatment) (Table 3). In the univariate analysis, factors associated with a statistically significant increased risk for treatment failure and death included Burkitt histologic subtype, male homosexuality, and detectable HIV viral load after the completion of R-CDE. Factors associated with a borderline significant increase in the risk for treatment failure or death in the univariate analysis included advanced-stage disease, elevated serum lactate dehydrogenase level, and elevated IPI risk. In the multivariate analysis, the only factors associated with a significant increased risk for death and treatment failure included Burkitt histology (HR for death, 2.24; 95% CI, 1.01-4.97; P = .05) and male homosexual risk factor (HR for death, 2.34; 95% CI, 1.07-5.08; P = .03). Median OS was significantly worse among patients with Burkitt lymphoma (14 months vs not reached; P = .01) and among male homosexual patients (15 months vs not reached; P = .02). Patients with diffuse large B-cell lymphoma (DLBCL) or its variants were more likely to achieve CR than patients with Burkitt lymphoma (77% vs 52%; P = .05), and the median survival for patients with DLBCL or its variants has not been reached after a median follow-up of 23 months.
Influence of demographic, lymphoma-specific, and HIV-specific factors on outcome
. | Overall survival . | . | . | ||
|---|---|---|---|---|---|
| Prognostic factors . | No. . | Univariate HR*(95% CI) . | Multivariate HR†(95% CI) . | ||
| Risk factor for HIV infection | |||||
| Intravenous drug use | 47‡ | 1§ | 1§ | ||
| Male homosexuality | 22 | 2.41 (1.11-5.23)∥ | 2.34 (1.07-5.08)∥ | ||
| Histology | |||||
| DLBCL | 52‡ | 1§ | 1§ | ||
| Burkitt lymphoma | 21 | 2.60 (1.20-5.64)¶ | 2.24 (1.01-4.97)# | ||
| HIV viral load at the end of R-CDE | |||||
| Undetectable | 36‡ | 1§ | 1§ | ||
| Detectable | 26 | 2.39 (1.10-5.18)∥ | 1.96 (0.88-4.36)** | ||
. | Overall survival . | . | . | ||
|---|---|---|---|---|---|
| Prognostic factors . | No. . | Univariate HR*(95% CI) . | Multivariate HR†(95% CI) . | ||
| Risk factor for HIV infection | |||||
| Intravenous drug use | 47‡ | 1§ | 1§ | ||
| Male homosexuality | 22 | 2.41 (1.11-5.23)∥ | 2.34 (1.07-5.08)∥ | ||
| Histology | |||||
| DLBCL | 52‡ | 1§ | 1§ | ||
| Burkitt lymphoma | 21 | 2.60 (1.20-5.64)¶ | 2.24 (1.01-4.97)# | ||
| HIV viral load at the end of R-CDE | |||||
| Undetectable | 36‡ | 1§ | 1§ | ||
| Detectable | 26 | 2.39 (1.10-5.18)∥ | 1.96 (0.88-4.36)** | ||
Cox proportional hazards model (HR and 95% CI).
Cox proportional hazards model, including only significant (P ≤ .05) prognostic factors in univariate analysis.
The sum does not add up to the total because of some missing values.
Reference category.
P = .03.
P = .02.
P = .05.
P = .10
Toxicity
In total, 338 treatment cycles were given to all 74 patients. The median number of treatment cycles given was 5 (range, 1-6). Six (8%) patients discontinued R-CDE before the completion of 6 planned cycles (or disease progression) because of persistent thrombocytopenia (n = 2), pulmonary tuberculosis (n = 1), cytomegalovirus (CMV) retinitis (n = 1), cerebral hemorrhage (n = 1), and patient refusal (n = 1). The dose of CDE was reduced in 1 or more treatment cycles because of toxicity in 31 (42%) patients. The initiation of a second or subsequent treatment cycles was delayed 1 week or less in 25 (34%) patients and more than 1 week in 14 (19%) patients, primarily because of bone marrow toxicity. Grades 3 to 4 toxicity included neutropenia in 58 (78%) patients, anemia in 24 (32%) patients, thrombocytopenia in 18 (24%) patients, and infection in 23 (31%) patients. In particular, 10 (14%) patients had opportunistic infections during chemotherapy or within 3 months from the end of R-CDE, including CMV retinitis (n = 3), cryptosporidiosis (n = 3), pulmonary tuberculosis (n = 2), Pneumocystis carinii pneumonia (n = 1), and salmonellosis (n = 1). However, in 3 patients (2 with cryptosporidiosis and 1 with CMV retinitis), the onset of opportunistic infection occurred more than 1 month after the end of chemotherapy. In addition, 17 (23%) patients developed complications of nonopportunistic infections, including bacterial pneumonia (n = 7), sepsis caused by bacterial pathogens (n = 3), or other infections (n = 3). In addition, 4 (5%) patients developed febrile neutropenia without a documented source of infection. Grades 3 to 4 nonhematologic toxicity including mucositis was recorded in 8 (11%) patients and otherwise occurred in less than 10% of patients. Six deaths were related to infection, including bacterial sepsis attributed to R-CDE (n = 2) and 4 opportunistic infections that occurred after the completion of CDE. The opportunistic infections included cryptosporidiosis (n = 2), pulmonary aspergillosis (n = 1), and pulmonary tuberculosis (n = 1), and they developed 5, 8, 2, and 8 months after the completion of R-CDE, respectively. There was no difference in the incidence of neutropenia or of infection when comparing patients with detectable or undetectable HIV viral load at baseline.
Immune function and viral load
The median CD4 count 1 month after the last cycle of R-CDE was 108/dL, compared with 161/dL at baseline. None of 5 patients with undetectable HIV viral load before chemotherapy had a detectable viral load after treatment, whereas 25 of 62 (40%) evaluable patients with a detectable viral load at the beginning of R-CDE had an undetectable viral load 1 month after the last cycle of R-CDE. In univariate analysis (Table 3), patients with detectable HIV viral load at the completion of R-CDE had a significantly increased risk for treatment failure (HR, 1.97; 95% CI, 0.99-3.91; P = .05]) and death (HR, 2.39; 95% CI, 1.10-.5.18; P = .03]); this was of borderline significance in the multivariate analysis. The median CD4 cell count was similar in patients with detectable HIV viral load (median, 235 copies/dL; range, 91-691 copies/dL) and in patients with undetectable HIV viral load (median, 250 copies/dL, range, 3-608 copies/dL) at baseline.
Discussion
We report the results of a multi-institutional phase 2 trial of infusional CDE plus the anti-CD20 monoclonal antibody rituximab in 74 patients with HIV-associated, B-cell NHL. The cytotoxic agents were given at conventional doses but were administered in an unconventional manner by protracted intravenous infusion over 96 hours. All patients received filgrastim and infection prophylaxis, and most (76%) patients received HAART concurrently with R-CDE. All patients had intermediate- or high-grade lymphoma, 57% were considered at intermediate-high or high risk by the age-adjusted IPI index, and the median CD4 count was 161/μL. The CR rate was 70%, and the estimated 2-year FFS and OS rates were 59% and 64%, respectively. For patients with DLBCL or its variants, 77% achieved CR, and median survival has not been reached after a median follow-up of 23 months. This is one of the most effective regimens reported thus far for the treatment of HIV-associated B-cell lymphoma. Given the multicenter nature of the study, it is likely that these findings are generalizable to patients with HIV-associated lymphoma diagnosed in the community.
The prognosis for patients with advanced HIV infection has improved considerably in the past 15 years, initially because of infection prophylaxis and more recently because of the widespread use of HAART. HAART has also been associated with improved response and survival in patients with HIV-associated lymphoma. The improved outcome observed in this study is not related purely to the routine use of HAART; the results reported here are also superior to those of more recently reported HAART-era studies that included standard CHOP chemotherapy.26
Potential explanations for the improved results observed in this trial include the addition of rituximab, the infusional administration of chemotherapy, a combination of both, or other factors. Although adding rituximab to CHOP has resulted in improved response and survival in immunocompetent patients with intermediate-grade lymphoma,27,28 a similar phase 3 trial in patients with HIV-associated lymphoma comparing R-CHOP with CHOP demonstrated no improvement in CR rate (57% vs 49%).29 In a multicenter trial conducted by the Eastern Cooperative Oncology Group (ECOG), infusional CDE resulted in a 45% CR rate, a 38% 2-year FFS rate, and a 45% 2-year OS rate in 55 patients treated during the HAART era. These findings have been confirmed by another group, who reported a 51% CR rate in 46 patients treated with CDE concurrently with HAART.30 In the trial that is the subject of this report, we combined rituximab with infusional CDE in a manner identical to that used in the ECOG trial. The efficacy and toxicity of these 2 studies, both of which were conducted in the HAART era, are contrasted in Table 4. R-CDE was associated with improved CR, 2-year FFS, and OS rates. Although there are limitations to the conclusions that may be drawn from comparing the results of 2 contemporaneously conducted phase 2 trials, the data suggest that adding rituximab to infusional CDE may augment the effectiveness of infusional cytotoxic therapy, an observation that merits further evaluation.
R-CDE compared with CDE
. | CDE . | R-CDE . |
|---|---|---|
| Reference | Sparano et al 11 | Present report |
| No. patients | 55 | 74 |
| Median age, y | 40 | 38 |
| Median CD4 count/μL | 227 | 161 |
| Histology, % | ||
| Burkitt or Burkitt-like | 22 | 28 |
| DLBCL or variants | 78 | 72 |
| Age-adjusted IPI, % | ||
| Low or low intermediate | 42 | 43 |
| High or high intermediate | 58 | 57 |
| CR rate, % (95% CI) | 45 (30-58) | 70 (59-81) |
| 2-year FFS, % (95% CI) | 38 (25-51) | 59 (47-71) |
| 2-year OS, % (95% CI) | 45 (20-58) | 64 (52-76) |
. | CDE . | R-CDE . |
|---|---|---|
| Reference | Sparano et al 11 | Present report |
| No. patients | 55 | 74 |
| Median age, y | 40 | 38 |
| Median CD4 count/μL | 227 | 161 |
| Histology, % | ||
| Burkitt or Burkitt-like | 22 | 28 |
| DLBCL or variants | 78 | 72 |
| Age-adjusted IPI, % | ||
| Low or low intermediate | 42 | 43 |
| High or high intermediate | 58 | 57 |
| CR rate, % (95% CI) | 45 (30-58) | 70 (59-81) |
| 2-year FFS, % (95% CI) | 38 (25-51) | 59 (47-71) |
| 2-year OS, % (95% CI) | 45 (20-58) | 64 (52-76) |
When this trial was initiated, there was concern that rituximab might increase the risk for infection in this population because of its known effects in depleting B lymphocytes. Indeed, Kaplan and Scadden29 reported a significantly higher risk for infectious death when R-CHOP was compared with CHOP (15% vs 2%; P = .027) in patients with HIV-associated lymphoma; this study included rituximab given at a standard dose (375 mg/m2) before each cycle of CHOP, followed by monthly rituximab for 3 months after the completion of R-CHOP. Maintenance therapy with rituximab, a strategy not used in our trial, might have contributed to some of the R-CHOP–associated deaths because 6 of the 14 deaths in the R-CHOP arm occurred after the completion of R-CHOP during or after maintenance rituximab therapy. In addition, another single-arm phase 2 trial evaluating R-CHOP without maintenance rituximab in 52 patients with HIV-associated lymphoma demonstrated no apparent increase in the risk for infectious death.31 In comparing infection rates in the current trial of R-CDE with the ECOG trial of CDE alone, R-CDE was associated with a higher incidence of grades 3 to 4 infection (31% vs 20%) and lethal infection (2% vs 0%), though there were no apparent differences in the proportions of patients who developed grades 3 to 4 neutropenia (78% vs 90%) or who required CDE dose reduction for toxicity (42% vs 48%). Taken together with the results reported by Kaplan and Scadden,29 these findings are consistent with the notion that rituximab may increase the risk for severe and life-threatening infection when used in combination with chemotherapy for HIV-associated lymphoma, and they suggest that further evaluation of this strategy should proceed cautiously.
Another group has reported encouraging results with a 96-hour infusional regimen of doxorubicin, etoposide, and vincristine used in conjunction with intravenous bolus cyclophosphamide plus oral prednisone (EPOCH).32 In this trial, conducted at the United States National Cancer Institute (NCI), EPOCH therapy without rituximab resulted in a 74% CR rate and a 72% survival rate (after a median follow-up of 53 months) in 39 patients with HIV-associated lymphoma. Similarly, the same group reported that EPOCH without rituximab resulted in a 92% CR rate and a 70% OS rate (after a median follow-up of 62 months) in immunocompetent patients with intermediate-grade lymphoma (of whom 52% had at least 2 poor prognostic factors by the age-adjusted IPI index).33 Finally, a multicenter ECOG trial that evaluated infusional CDE in immunocompetent patients with age-adjusted poor IPI risk intermediate-grade lymphoma (at least 2 adverse prognostic factors) demonstrated a trend toward improved FFS and OS compared with patients treated with matched historical controls.34 Given that rituximab is known to sensitize lymphoma cells to cytotoxic agents in vitro and that it has a long-half life of approximately 7 days, infusional administration of cytotoxic therapy may offer greater potential for therapeutic synergy than the same agents given by a conventional intravenous bolus route.
Several differences between the infusional EPOCH and CDE regimens may explain the difference in efficacy and toxicity. First, the doses of doxorubicin and etoposide are lower in EPOCH (20% and 17%, respectively) than in CDE. Second, the starting dose of cyclophosphamide is 50% to 75% lower in EPOCH than in CDE, with the initial dose in the EPOCH regimen adjusted based on baseline CD4 counts and subsequent doses based on the neutrophil nadir. Third, EPOCH includes additional antilymphoma-active drugs that are not myelosuppressive, such as vincristine and prednisone. Fourth, in the NCI trial, all patients discontinued HAART therapy when EPOCH was administered, which might have contributed to the lower risk for neutropenia (as suggested by the report by Bower et al).30 Fifth, EPOCH therapy was given every 3 weeks compared with every 4 weeks for CDE. The 4-week schedule for the latter regimen was used because previous studies conducted in the pre-HAART era demonstrated that bone marrow recovery was usually insufficient after 3 weeks to permit additional treatment.35 For patients treated in the HAART era, however, marrow recovery is usually sufficient to permit repeated dosing. In addition, the 4-week schedule may an inferior schedule for high-grade lymphoma and may provide an explanation for the inferior outcome for R-CDE observed in the current study for patients with Burkitt lymphoma.
In conclusion, our study demonstrated that R-CDE is an effective treatment for patients with HIV-associated B-cell lymphoma, resulting in a 70% CR rate and a 64% 2-year OS rate. Based on our clinical results and the premise that infusional drug administration offers greater potential for therapeutic synergy with rituximab, rituximab in combination with infusional therapy merits further investigation. Given the potential for rituximab to increase the risk for life-threatening infections in this population, however, such evaluations must proceed cautiously. For example, the AIDS Malignancy Consortium is performing a randomized phase 2 trial comparing concurrent administration of rituximab immediately before each cycle of EPOCH (R-EPOCH) for 4 to 6 cycles compared with the sequential administration of EPOCH for 4 to 6 cycles until maximal response, followed by weekly rituximab for 6 weeks (EPOCH-R).36 All patients receive G-CSF and quinolone prophylaxis, and blood counts are monitored twice a week, or more often, until the nadir has resolved. The trial includes an early stopping rule of an excessive risk for infectious death in either arm. Additional studies such as these will be required to verify the efficacy and safety of rituximab combined with infusional therapy.
Prepublished online as Blood First Edition Paper, November 18, 2004; DOI 10.1182/blood-2004-08-3300.
Supported by grants from the Istituto Superiore di Sanità (ISS) and the Associazione Italiana per la Ricerca sul Cancro (AIRC).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Ms Paola Favetta for her expert assistance in the manuscript preparation. We also thank Bernardino Allione (Division of Hematology, Santi Antonio e Biagio e Cesare Arrigo Hospital, Alessandria, Italy), Antonio Benci (Department of Haematology, San Donato Hospital, Arezzo, Italy), Guido Chichino (Institute of Infectious Diseases, Policlinico San Matteo, Pavia, Italy), Anna Marina Liberati (Institute of Internal Medicine and Oncological Sciences, Policlinico Monteluce, Perugia, Italy), Filippo Lipani (Division of Infectious Diseases A, Amedeo di Savoia Hospital, Turin, Italy), Giorgio Perboni (Division of Infectious Diseases, Carlo Poma Hospital, Mantova), and Lilj Uziel (S. Paolo Hospital, Internal Medicine I, Milan, Italy) for their contributions to the study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal