Abstract
Umbilical cord blood transplantation is applied as treatment for mainly pediatric patients with hematologic malignancies. The clinical results show a relatively low incidence of graft-versus-host disease and leukemia relapse. Since maternal cells traffic into the fetus during pregnancy, we questioned whether cord blood has the potential to generate cytotoxic T cells specific for the hematopoietic minor histocompatibility (H) antigen HA-1 that would support the graft-versus-leukemia effect. Here, we demonstrate the feasibility of ex vivo generation of minor H antigen HA-1-specific T cells from cord blood cells. Moreover, we observed pre-existing HA-1-specific T cells in cord blood samples. Both the circulating and the ex vivo-generated HA-1-specific T cells show specific and hematopoietic restricted lysis of human leukocyte antigen-A2pos/HA-1pos (HLA-A2pos/HA-1pos) target cells, including leukemic cells. The cord blood-derived HA-1-specific cytotoxic T cells are from child origin. Thus, the so-called naive cord blood can comprise cytotoxic T cells directed at the maternal minor H antigen HA-1. The apparent immunization status of cord blood may well contribute to the in vivo graft-versus-leukemia activity after transplantation. Moreover, since the fetus cannot be primed against Y chromosome-encoded minor H antigens, cord blood is an attractive stem cell source for male patients. (Blood. 2005;105:1823-1827)
Introduction
In the last decade, umbilical cord blood transplantation (CBT) has been available as an alternative to human leukocyte antigen (HLA)-matched sibling or unrelated donor stem cell transplantation (SCT) for the treatment of hematologic malignancies.1-7 The clinical outcome of transplanted CB grafts with 1 or 2 HLA antigen mismatches demonstrates a similar risk of developing graft-versushost disease (GvHD) as compared to HLA-matched unrelated SCT.5,6 A significant lower incidence of acute and chronic GvHD was reported after HLA-identical CBT when compared to sibling SCT.4 Collectively, these clinical results point to a decreased incidence of GvHD after CBT.
GvHD is often associated with a curative graft-versus-leukemia (GvL) response. Minor H antigen disparities between donor and recipient play important roles in both the GvH and GvL reactivity after HLA-matched SCT as reviewed.8 One of the well-described minor H antigens is HA-1. The immunodominant minor H antigen HA-1 is encoded by a diallelic gene with a single amino acid polymorphism.9 The HA-1 “positive” allele (HA-1pos) contains a histidine at position 3 (HA-1H), whereas the HA-1 “negative” allele (HA-1neg) contains an arginine (HA-1R). The HA-1H peptide is recognized by HLA-A2-restricted CD8pos cytotoxic T cells from HA-1neg donors.10-12 The expression of HA-1 is restricted to the hematopoietic lineage and to epithelial carcinomas.13,14 This restricted expression makes HA-1 an attractive target antigen for GvL and graft-versus-tumor responses.15
Despite the lower incidence of GvHD after CBT, there is no indication of increased leukemia relapse rates when compared with sibling or unrelated donor SCT.3-5 Comparable survival rates point to an as-yet-unexplored GvL potential of cord blood. Relatively little is known about the development of antigen-specific T-cell responses around birth.16 Mature monocyte-derived neonatal dendritic cells (DCs) are able to efficiently prime antigen-specific cytotoxic T cells in vitro.17 In bulk cultures, cord blood T cells proliferate in response to alloantigen.18 Yet the development of functional alloreactive cytotoxic T cells is impaired.18-20 Limiting dilution studies have, however, reported normal precursor frequencies of cytotoxic T cells specific for allo-HLA class I and class II in cord blood.20-22 Thus, the capacity to develop allogeneic cytotoxic T cells is intact at birth, despite overall diminished magnitude of responses.23
It is known that feto-maternal hemorrhage occurs during pregnancy. Fetal cells expressing paternal minor H antigens can prime maternal T cells.12,24 Since cells of the mother also traffic into the fetus during pregnancy, we tested the hypothesis that maternal minor H antigens can prime fetal T cells. Fifteen HLA-A2pos/HA-1neg CB samples derived from HLA-A2pos/HA-1neg or HLA-A2pos/HA-1pos mothers were analyzed for their feasibility to generate HA-1-specific cytotoxic T cells ex vivo as well as for the presence of pre-existing HA-1-specific T cells.
Materials and methods
Cord blood
After informed consent of the mother, cord blood was collected from the umbilical cord vein with the placenta still in utero. HLA-A2pos/HA-1neg CB samples derived from HLA-A2pos/HA-1neg and HLA-A2pos/HA-1pos mothers were selected after high-resolution HLA class I typing and HA-1 genomic typing as described previously.25 Cord blood mononuclear cells (CB-MNCs) were isolated by Ficoll-Isopaque density gradient centrifugation and stored in liquid nitrogen. Approval for these studies was obtained from the Institute for Transplantation Diagnostics and Cell Therapeutics institutional review board.
HLA class I/minor H antigen peptide tetrameric complexes
Expression of the T-cell receptor specific for HLA-A2/HA-1H peptide (VLHDDLLEA) complexes was analyzed by staining T cells with phycoerythrin (PE)-conjugated HLA-A2/HA-1 tetrameric complexes (HA-1A2) in combination with allophycocyanin (APC)-conjugated anti-CD8 monoclonal antibody (BD Biosciences, Amsterdam, The Netherlands). Tetramers were generated as previously described.26 Specificity analysis of the HA-1A2 tetramer was performed in parallel experiments using HA-1-specific and HA-1-nonspecific cytotoxic T-lymphocyte (CTL) clones (data not shown).
Culture, retroviral transduction, and maturation of CD34+-derived dendritic cells
CD34+ cells were isolated via positive selection using CD34 magnetic-activated cell sorting (MACS) beads (Miltenyi GmBH, Bergisch Gladbach, Germany). CD34+ cells were cultured in complete Iscove modified Dulbecco medium (IMDM) containing 10% pooled human serum (HS), 250 U/mL granulocyte macrophage colony-stimulating factor (GM-CSF) (Leucomax, Novartis, Arnhem, The Netherlands), 25 μg/mL stem cell factor (SCF) (Peprotech, London, United Kingdom), 100 U/mL tumor necrosis factor (TNF)-α (Peprotech), and 50 μg/mL FLt3 ligand (R&D Systems, Minneapolis, MN). Dendritic cells were transduced with retroviral supernatants containing HA-1H-encoding cDNA, as previously described,27 and further cultured in complete medium supplemented with 300 U/mL interleukin-4 (IL-4) (Peprotech). Maturation of HA-1H-transduced dendritic cells was induced by co-culturing immature dendritic cells for 3 days with irradiated CD40 ligand-transfected fibroblasts.
Generation of HA-1-specific cytotoxic T cells from cord blood
A slightly modified protocol, originally designed for the induction of minor H antigen-specific cytotoxic T cells from adult peripheral blood mononuclear cells (PBMCs), was applied.11 In short, CD8pos T cells were isolated via positive selection using CD8 MACS beads (Miltenyi) and cultured with irradiated autologous HA-1H-transduced dendritic cells at a CD8-to-dendritic cell ratio of 5:1 or 10:1 in IMDM supplemented with 10% HS, 0.5 U/mL IL-2 (Cetus, Emeryville, CA), and 1 U/mL IL-12 (R&D Systems). After 3 days, irradiated autologous T helper cells were added to the culture at a CD8-to-T helper ratio of 10:1. T helper cells were generated by stimulating CD34/CD8-depleted CB-MNCs with anti-CD3/CD28 beads according to supplier's protocol (Dynal Biotech, Smestad, Norway). This mode of stimulation results in at least 80% activated CD4pos T cells that produce interferon-γ (IFN-γ), TNF-α, and IL-2 (data not shown). From day 7 onward, oligoclonal T-cell lines were restimulated weekly using irradiated dendritic cells and T helper cells at a CD8-to-T helper-to-dendritic cell ratio of 10:1:1. Fresh medium containing IL-2 was added every 3 to 4 days.
Bulk T-cell lines were tested for the presence of HA-1A2 tetramerpos CD8pos T cells after 2 to 4 rounds of stimulation with T helper cells and dendritic cells. HA-1A2 tetramer-staining cells were subsequently sorted on a FACS Vantage cell sorter (Becton Dickinson, San Jose, CA) and cloned by limiting dilution. Cloned T cells were expanded in the presence of 5 × 104 irradiated allogeneic PBMCs and 5 × 103 HLA-A2pos/HA-1pos Epstein-Barr virus (EBV)-transformed B cells-lymphoblastoid cell lines (EBV-LCL) and 30 U/mL IL-2.
Direct isolation and culture of HA-1-specific cytotoxic T cells from cord blood
The protocol for detection of circulating minor H antigen-specific cytotoxic T cells in PBMCs from healthy multiparous female blood donors was applied.12 In short, CB-MNCs were depleted for various cell subsets using CD4, CD14, CD19, CD16, and glycophorinA MACS beads (Miltenyi). The depleted fraction was subsequently stained with CD8-APC, CD45RA-FITC (BD Biosciences), and PE-conjugated HA-1A2 tetrameric complexes. Further enrichment of HA-1A2 tetramerpos CD8pos cells was performed on a FACS Vantage cell sorter using the “enrich mode,” whereby cells are sorted at 20 000 events per second. The enriched fraction was reanalyzed and resorted immediately at 10 000 events per second using the more stringent “normal-R” mode. Double FACS-sorted cells were expanded in the presence of irradiated autologous HA-1neg CB-MNC cells, 1% phytohemag-glutinin, and 30 U/mL IL-2. Fresh IL-2-containing medium was added every 3 to 4 days.
Cell-mediated lympholysis assay
Standard 4-hour 51Cr-release assays were performed as previously described.13
Results
Ex vivo generation of HA-1-specific cytotoxic T-cell lines from HLA-A2pos/HA-1neg cord blood
Six HLA-A2pos CB samples, 4 HA-1neg and 2 HA-1pos, were used for the ex vivo generation of HA-1-specific cytotoxic T-cell lines. CD8pos T cells were isolated and cultured with autologous HA-1H-transduced dendritic cells and T helper cells. The generation of HA-1-specific cytotoxic T-cell lines was successful in 3 of 4 HLA-A2pos/HA-1neg CB samples, whereas no growth at all was observed in the 2 HLA-A2pos/HA-1pos CB samples. Results of 2 HLA-A2pos/HA-1neg CB samples (I and II) are shown in Figure 1. The percentages of HA-1A2 tetramer and CD8 double-positive T cells are shown after 14 days of culture (Figure 1A-B). HA-1A2 tetramer-staining CD8pos T cells were further enriched after additional rounds of stimulation (Figure 1C-D). The latter populations were FACS sorted, expanded for 14 days, and functionally analyzed. Strong HA-1-specific lytic activity is demonstrated for both CB-derived T-cell cultures (Figure 1E-F).
Ex vivo generation of HA-1-specific cytotoxic T cells from HLA-A2pos/HA-1neg cord blood. Purified CB CD8pos T cells were cultured in the presence of autologous HA-1H-expressing dendritic cells and T helper cells. Results of 2 different CB cultures are shown (I and II). Samples were stained with HA-1A2 tetramers (x-axis) and CD8 antibodies (y-axis). The percentages of HA-1A2 tetramerpos CD8pos T cells are shown after 14 (A, B), 28 (C), or 21 days of culture (D). HA-1A2 CD8pos T cells were FACS sorted (indicated by arrow), expanded, and tested for cytoxic activity (E, F). Target cells: HLA-A2pos/HA-1neg EBV-LCL (•), HLA-A2pos/HA-1neg EBV-LCL pulsed with HA-1H peptide (▪), and HLA-A2pos/HA-1pos EBV-LCL (▴). Data are presented as mean percentage of lysis ± SD.
Ex vivo generation of HA-1-specific cytotoxic T cells from HLA-A2pos/HA-1neg cord blood. Purified CB CD8pos T cells were cultured in the presence of autologous HA-1H-expressing dendritic cells and T helper cells. Results of 2 different CB cultures are shown (I and II). Samples were stained with HA-1A2 tetramers (x-axis) and CD8 antibodies (y-axis). The percentages of HA-1A2 tetramerpos CD8pos T cells are shown after 14 (A, B), 28 (C), or 21 days of culture (D). HA-1A2 CD8pos T cells were FACS sorted (indicated by arrow), expanded, and tested for cytoxic activity (E, F). Target cells: HLA-A2pos/HA-1neg EBV-LCL (•), HLA-A2pos/HA-1neg EBV-LCL pulsed with HA-1H peptide (▪), and HLA-A2pos/HA-1pos EBV-LCL (▴). Data are presented as mean percentage of lysis ± SD.
Origin of cord blood-derived HA-1-specific cytotoxic T cells
Since cord blood may contain maternal cells, we determined whether the HA-1-specific T cells were child or mother derived. DNA typing of the HA-1 alleles showed unequivocally that ex vivo-generated HA-1-specific T cells are from child origin (data not shown).
Hematopoietic-restricted cytolytic activity of HA-1-specific cord blood-derived T cells
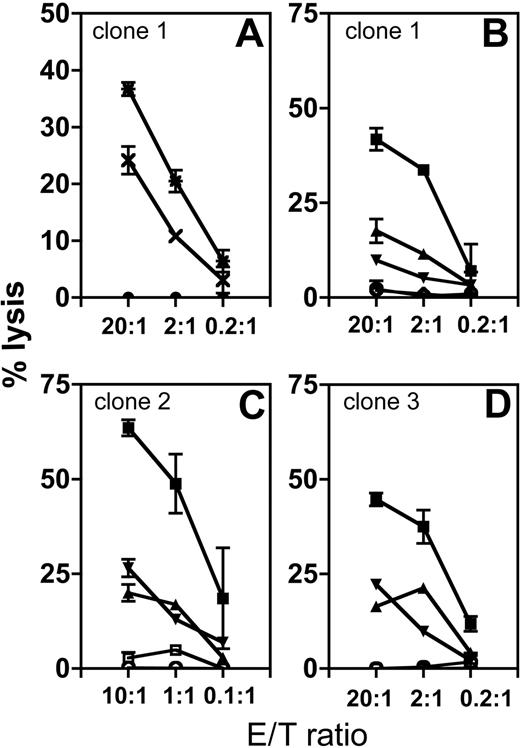
T-cell clones were generated from CB I (n = 5), CB II (n = 29), and CB III (n = 8) and analyzed for HA-1 hematopoietic-restricted specificity and leukemic cell lysis. Results of 3 representative T-cell clones (clones 1-3) are shown in Figure 2. Clone 1 lysed HLA-A2pos/HA-1pos phytohemagglutinin (PHA) blasts, but not fibroblasts, while fibroblasts pulsed with HA-1H peptide were lysed (Figure 2A). Clones 1, 2, and 3 were analyzed against a panel of HA-1pos and HA-1neg leukemic cells. Each clone recognized the 3 HLA-A2pos/HA-1pos leukemic targets, whereas HA-1neg leukemic cells and autologous HA-1neg PHA blasts, tested in parallel, were not recognized (Figure 2B-D). Thus, HA-1-specific cytotoxic T cells can be generated ex vivo from HLA-A2/HA-1neg CB samples. These T cells display specific and hematopoietic-restricted recognition.
Hematopoietic-restricted lysis of CB-derived cytotoxic T-cell clones. (A) The cytotoxic activity of one representative HA-1-specific T-cell clone (1) is shown against fibroblasts (closed circles), fibroblasts pulsed with HA-1H peptide (*), and PHA blasts (×). The fibroblasts and PHA blasts are derived from the same HLA-A2pos/HA-1pos donor. (B-D) The cytotoxic activity of 3 HA-1-specific cytotoxic T-cell clones (clones 1, 2, and 3) is shown against 3 different HLA-A2pos/HA-1pos leukemic cells (▪, ▴, and ▾ ; acute lymphocytic lymphoma cells); HLA-A2pos/HA-1neg leukemic cells (○) and autologous HLA-A2pos/HA-1neg CB PHA blasts (⋄ or □).
Hematopoietic-restricted lysis of CB-derived cytotoxic T-cell clones. (A) The cytotoxic activity of one representative HA-1-specific T-cell clone (1) is shown against fibroblasts (closed circles), fibroblasts pulsed with HA-1H peptide (*), and PHA blasts (×). The fibroblasts and PHA blasts are derived from the same HLA-A2pos/HA-1pos donor. (B-D) The cytotoxic activity of 3 HA-1-specific cytotoxic T-cell clones (clones 1, 2, and 3) is shown against 3 different HLA-A2pos/HA-1pos leukemic cells (▪, ▴, and ▾ ; acute lymphocytic lymphoma cells); HLA-A2pos/HA-1neg leukemic cells (○) and autologous HLA-A2pos/HA-1neg CB PHA blasts (⋄ or □).
Isolation of circulating HA-1-specific T cells from HLA-A2pos/HA-1neg cord blood
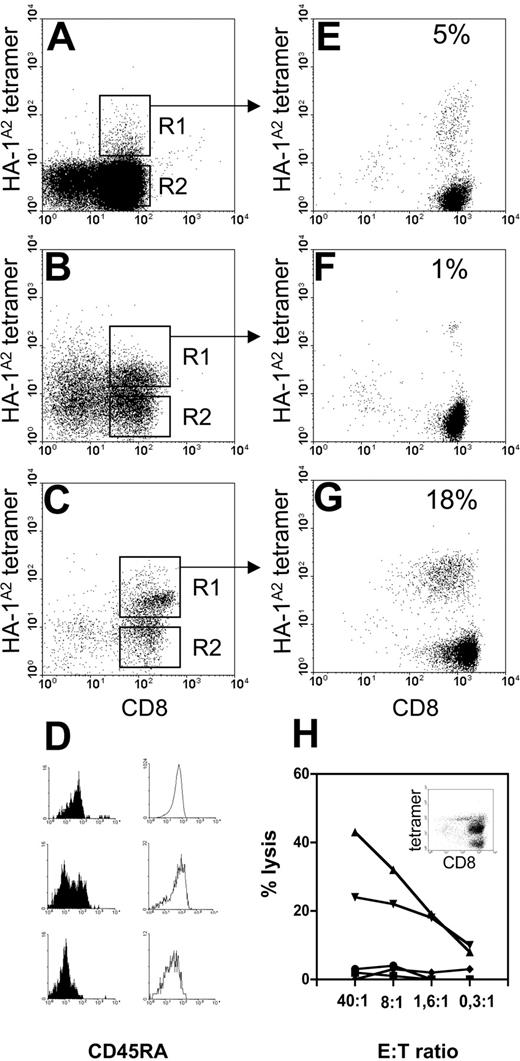
The presence of circulating HA-1-specific T cells was analyzed in 11 HLA-A2pos/HA-1neg CB samples derived from 7 HA-1pos mothers and from 4 HA-1neg mothers. All 11 CB samples were stained with HA-1A2 tetramers and CD8 antibodies, FACS sorted, and nonspecifically expanded, omitting any in vitro HA-1-specific stimulation. Subsequently, HA-1A2 tetramer analysis was performed after 21 days of expansion. Cells isolated from all 4 HA-1neg CB samples derived from HA-1neg mothers failed to grow in vitro, despite a few detectable tetramer-staining CD8pos cells. The latter observed events are most likely due to background staining. Cord blood samples contain variable percentages of CD3negCD8pos natural killer (NK) cells and erythroblasts that display nonspecific staining in our hands. The level of background tetramer staining depends on the degree of depletion of these cells by magnetic bead separation. Yet a substantial number of cells isolated from 3 of 7 HA-1neg CB samples derived from HA-1pos mothers stained with the HA-1A2 tetrameric complexes (Figure 3A-C, gate R1) and expanded to sufficient numbers for tetramer analysis. A variable percentage (1%-18%) of HA-1A2 tetramer staining CD8pos cells was observed at day 21 of nonspecific expansion (Figure 3E-G). Culture G was expanded nonspecifically for another 14 days, which resulted in a further enrichment of tetramerpos CD8pos cells (40%, insert Figure 3H). Functional analysis of the latter culture showed lysis of HLA-A2pos/HA-1pos leukemic cells and HLA-A2pos/HA-1neg EBV-LCL pulsed with HA-1H peptide, while autologous HLA-A2pos/HA-1neg PHA blasts, HLA-A2pos/HA-1neg EBV-LCL, and K562 cells were not lysed (Figure 3H).
Direct isolation of HA-1-specific cytotoxic T cells from HLA-A2pos/HA-1neg cord blood. Results from 3 (A, B, and C) HLA-A2pos/HA-1neg CB samples obtained from HLA-A2pos/HA-1pos mothers are shown. (A-C) Analysis of cells collected after the first enrichment sort for HA-1A2 tetramer and CD8pos cells. (D) CD45RA expression on HA-1A2 tetramerpos CD8pos cells (gate R1, filled histograms) and tetramerneg CD8pos cells (gate R2, open histograms) from CB samples A, B, and C, respectively. (E-G) CD8 and HA-1A2 tetramer staining of polyclonal cultures expanded after the enrichment sort followed by R1 sort of tetramerpos CD8pos cells from CB samples A, B, and C, respectively. (H) Cytotoxic activity of culture G, after a second expansion phase, against various target cells: HLA-A2pos/HA-1neg EBV-LCL (squares); HLA-A2pos/HA-1neg EBV-LCL pulsed with HA-1H peptide (triangles); HLA-A2pos/HA-1pos leukemic cells (▾); autologous HLA-A2pos/HA-1neg CB PHA blasts (♦); and K562 cells (•). Insert in H shows the corresponding HA-1A2 tetramer staining.
Direct isolation of HA-1-specific cytotoxic T cells from HLA-A2pos/HA-1neg cord blood. Results from 3 (A, B, and C) HLA-A2pos/HA-1neg CB samples obtained from HLA-A2pos/HA-1pos mothers are shown. (A-C) Analysis of cells collected after the first enrichment sort for HA-1A2 tetramer and CD8pos cells. (D) CD45RA expression on HA-1A2 tetramerpos CD8pos cells (gate R1, filled histograms) and tetramerneg CD8pos cells (gate R2, open histograms) from CB samples A, B, and C, respectively. (E-G) CD8 and HA-1A2 tetramer staining of polyclonal cultures expanded after the enrichment sort followed by R1 sort of tetramerpos CD8pos cells from CB samples A, B, and C, respectively. (H) Cytotoxic activity of culture G, after a second expansion phase, against various target cells: HLA-A2pos/HA-1neg EBV-LCL (squares); HLA-A2pos/HA-1neg EBV-LCL pulsed with HA-1H peptide (triangles); HLA-A2pos/HA-1pos leukemic cells (▾); autologous HLA-A2pos/HA-1neg CB PHA blasts (♦); and K562 cells (•). Insert in H shows the corresponding HA-1A2 tetramer staining.
CD45RA expression on CB CD8pos cells was determined directly after the first enrichment sort prior to in vitro culture (Figure 3D). The majority of tetramerneg CD8pos cells (Figure 3A-C, gate R2) expressed CD45RA (open histograms). In contrast, tetramerpos CD8pos cells (Figure 3A-C, gate R1) from 2 of the 3 CB samples clearly expressed lower levels of CD45RA (filled histograms), suggesting a primed phenotype at the time of isolation. Thus, antigen-experienced circulating T cells specific for maternal minor H antigen HA-1 can be detected in cord blood.
Discussion
Our study demonstrates the presence of circulating HA-1-specific T cells in HLA-A2pos/HA-1neg CB samples derived from children delivered by HLA-A2-matched but HA-1-mismatched mothers. We also show that HA-1-specific T cells can be generated ex vivo from HLA-A2pos/HA-1neg CB samples. CB-derived HA-1-specific T cells show the expected cytotoxic activity that includes lysis of HA-1pos leukemic cells.
The majority of unrelated CBT is performed with 1 or 2 HLA-mismatched grafts as reviewed.28 An inverse correlation between the number or type of HLA disparities and relapse risk was recently found,29 suggesting that alloreactivity to mismatched HLA antigens contributes to GvL responses. The fact that minor H antigen-specific cytotoxic T cells are generated across HLA haplo barriers and observed in fetal blood underlines the immunogenicity of the hematopoietic-specific minor H antigen HA-1. We speculate that pre-existing HA-1-specific T cells may contribute to GvL reactivity upon CBT in HLA-A2pos/HA-1pos recipients. Alternatively, HA-1-specific cytotoxic T cells can be generated ex vivo and stored for adoptive transfer in case of leukemic relapse.
The alloreactive potential of cord blood is shaped during pregnancy by fetal-maternal interactions. Both fetal and maternal HLA alloreactive T cells are, however, subjected to immune regulatory mechanisms to prevent fetal reactivity toward maternal tissues or fetal rejection.30-32 Despite the apparent immunologic tolerance, normal precursor frequencies of cytotoxic and helper T cells specific for noninherited maternal HLA antigens (NIMA) can be detected in cord blood.21,33 Similarly, noninherited maternal minor H antigens can prime fetal cytotoxic T cells, as shown in this study. Whether these T cells have any implications for the immunology of the maternal-fetal interface is a subject for further studies.
The tetramerpos CD8pos T cells directly sorted from 2 different CB samples clearly expressed lower levels of CD45RA than tetramerneg CD8pos T cells. Low CD45RA expression is indicative of recent antigen exposure, suggesting that HA-1-specific T-cell priming has occurred in utero. Similar fetal CD8 T-cell responses have been observed in case of maternal infections with Trypanosoma cruzi or cytomegalovirus,16,34 as well as allergen- or Epstein-Barr virus-specific CD4pos T-helper cells.35-37 In line with these observations, our results demonstrate that T-cell priming for minor H antigens also occurs in utero. This is a remarkable finding, since allelic variants of minor H antigens can be considered as “modified self” in contrast to foreign viral antigens. The broadness of the autosomal encoded minor H antigen repertoire in CB samples needs to be investigated.
Maternal microchimerism is frequently found in CB samples38 and in newborn tissue.39 Nucleated maternal cells have been found in the fetal circulation as early as the second trimester of pregnancy.40 Whether the presence of HA-1 cytoxic T cells is associated with the presence of maternal HA-1 microchimerism in the CB samples is as yet unknown. If so, we will analyze whether HA-1 is expressed by professional antigen-presenting cells, as we observed in another study.41 If maternal cells persist, they could provide a continuous antigen source of HA-1 that may maintain HA-1-specific cytotoxic T cells into adult life. This would explain the low but significant precursor frequencies of HA-1-specific cytotoxic T cells that we observe in some healthy blood or stem cell donors.26 Pre-existing cytotoxic T-cell precursor frequencies may facilitate the ex vivo generation of HA-1-specific T cells for adoptive immunotherapy.
Recipients of HLA-identical SCT have a poorer transplant outcome if the donor is female rather than male.42,43 The explanation of this clinical observation is that pregnancy can lead to alloimmune responses. Over decades, several types of alloimmune responses, varying from immunization against erythrocyte- and HLA-specific antibodies44 to alloimmune responses against fetal paternal minor H antigens, have been reported.45 With regard to the latter, both autosomal and Y chromosomes encoded minor H antigen responses have been observed.12,24 Evidently priming of fetal cells restricts itself to the autosomal minor H repertoire, since maternal cells lack the Y-chromosome encoded H-Y antigens. The absence of fetal anti-H-Y priming, disadvantageous for female-to-male SCT, makes cord blood an attractive stem cell source for male patients.
Prepublished online as Blood First Edition Paper, October 21, 2004; DOI 10.1182/blood-2004-07-2832.
Supported in part by grants from the German Cancer Aid and the Jan Dekker and Dr Ludgardine Bouwman Foundation.
B.M. and J.A.S.-K. contributed equally to the study.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
A. Kloosterman, M. van der Keur, and R. van der Linden are acknowledged for excellent technical assistance. We thank Drs M. Oudshoorn and M.H.M. Wauben and Professor J.J. van Rood for critical reading of the manuscript.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal