Abstract
Fetal hemoglobin (Hb F) levels increase in most patients with sickle cell disease following intermittent butyrate therapy. Although the full effects of butyrate on Hb F levels usually require multiple treatment cycles, in some patients a peak level is achieved after a few days of butyrate therapy. Our investigation of the mechanism(s) responsible for this rapid induction of Hb F by butyrate showed that reticulocyte γ-globin chain synthesis markedly increased within 24 hours of butyrate exposure, without concomitant changes in reticulocyte γ-globin mRNA levels. This suggests that butyrate might induce Hb F by increasing the efficiency of translation of γ-globin mRNA. This hypothesis was confirmed by ribosome loading studies that demonstrated enrichment of the polysomal fraction of reticulocytes with γ-globin mRNA following butyrate exposure. Thus, the induction of Hb F by butyrate may be mediated by translational effects in addition to its well-known effects on transcription of the γ-globin genes. (Blood. 2005;105:1807-1809)
Introduction
During normal ontogeny, fetal hemoglobin (Hb F) is gradually replaced by adult hemoglobin as a result of a switch from γ- to β-globin gene expression. Patients with sickle cell disease (SCD) and β-thalassemia who have genetically determined high levels of Hb F generally have a mild clinical disorder.1-3 This suggested that induction of Hb F in adult life might be an effective form of treatment for patients with SCD. Subsequent studies showed that hypomethylating agents (eg, 5-azacytidine),4,5 cytotoxic agents (eg, hydroxyurea),6-13 and inhibitors of histone deacetylase (eg, butyrate)14-17 can increase Hb F in patients with SCD.
Butyrate was previously shown to increase embryonic globin gene expression in chickens pretreated with 5-azacytidine,18 cause higher levels of Hb F at birth in infants born to diabetic mothers,19,20 delay the switch from fetal to adult hemoglobin in sheep exposed to butyrate in utero,21 and increase Hb F levels in adult baboons.22,23 In clinical trials, intravenous infusions of arginine butyrate14-16 and oral administration of sodium phenylbutrate17 increased Hb F in patients with SCD and β-thalassemia. More recently, our own studies showed that intermittent therapy with arginine butyrate resulted in a sustained marked induction of Hb F in 9 of 11 patients with SCD.14
The mechanisms by which butyrate increases γ-globin expression have not been fully elucidated. Since butyrate is a known inhibitor of histone deacetylase, it is widely assumed, although never proven, that butyrate increases Hb F levels by increasing the transcription of the γ-globin through changes in histone acetylation.23,24 In most patients who receive butyrate, Hb F levels increase gradually over several months of therapy.14 However, in some patients, Hb F levels increase within a few days of initiating butyrate therapy. The kinetics of this rapid increase are not compatible with transcriptional effects in nucleated erythroid precursors in the bone marrow. This prompted us to explore other mechanism(s) that might result in a rapid increase in Hb F levels in SCD.
Study design
Patients and treatment
According to the Declaration of Helsinki, informed consent was obtained from all patients. All procedures were approved by the Institutional Review Board of the Mount Sinai School of Medicine. Studies were performed on peripheral blood from 7 adult patients with SCD who were enrolled in a study of intermittent arginine butyrate therapy.14 Blood was collected before treatment and at timed intervals during butyrate therapy.
Reticulocyte γ-globin chain synthesis
RNAase protection assay
RNA was extracted, and γ-globin mRNA levels were measured by an RNAase protection assay (RPA) as previously described.27 The β-globin probe used for the RPA was generated by in vitro transcription of a plasmid containing a genomic DNA insert corresponding to human β-globin exon 2 and adjacent intronic segments; the exon 2-protected fragment is 205 base pairs (bp). The γ-globin probe was directly transcribed from a human γ-globin genomic DNA polymerase chain reaction (PCR) template containing a contiguous region extending from intron 1 through intron 2. This γ-globin RNA probe protected a 223-nucleotide (nt) fragment corresponding to exon 2. The intensities of the bands of interest were quantified by Phosphoimager analysis (Molecular Dynamics Storm 840; Molecular Dynamics, Sunnyvale, CA).
Polysomal distribution of γ-globin mRNA
Red blood cells were washed, lysed, centrifuged on a sucrose gradient, and fractionated as previously described.28 The gradients were collected in 15 fractions, and the γ-globin and β-globin mRNA levels in each fraction were also determined by RPA.
Results and discussion
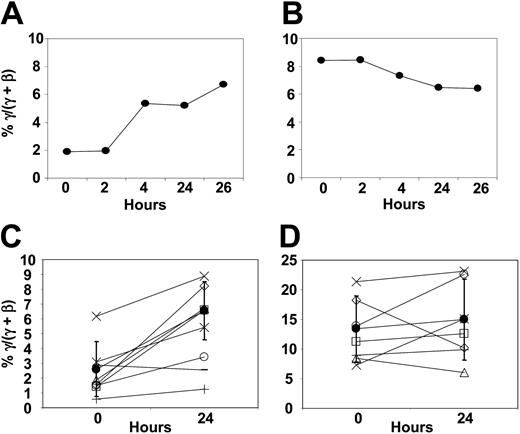
We measured γ-globin chain synthesis and mRNA levels in patients' reticulocytes before treatment and at timed intervals after initiation of butyrate infusions. Detailed data from a representative patient responsive to butyrate are presented in Figure 1A. Reticulocyte γ-globin chain synthesis increased from 1.9% to 6.7% (3.5-fold increase) at 26 hours after initiation of infusion. In contrast, there was no significant change in γ-globin mRNA levels during that time (Figure 1B). Data from this patient, 4 other patients responsive to butyrate, and 2 patients not responsive to butyrate are summarized in Figure 1C-D. In patients responsive to butyrate, mean reticulocyte γ-globin chain synthesis increased from 2.6% ± 1.9% before treatment to 6.5% ± 2.0% (P = .004) within 24 hours after initiation of infusion (Figure 1C). In contrast, there was no significant change in γ-globin mRNA levels during that time (13.4% ± 5.6% before treatment versus 15.0% ± 6.9% at 24 hours, P = .57; Figure 1D). In these patients responsive to butyrate, the increase in γ-globin protein chain synthesis was very rapid, occurring within 2 days of beginning therapy in all patients (data not shown). In contrast, there was no change in γ-globin protein chain synthesis or γ-globin mRNA in patients not responsive to butyrate (Figure 1C-D). This rapid increase in γ-globin protein chain synthesis was not associated with consistent changes in mRNA levels (Figure 1B,D). These observations suggest that the rapid increase in γ-globin chain synthesis is most likely a result of an increase in the efficiency of translation of γ-globin mRNA.
The rapid reticulocyte γ-globin response. (A-B) Kinetics in a representative patient with SCD responsive to butyrate: γ-globin protein chain synthesis (A) and γ-globin mRNA (B). (C-D) Reticulocyte γ-globin protein chain synthesis (C) and γ-globin mRNA (D), respectively, in 5 patients responsive to butyrate (○, ▵, □, ×, ⋄) and 2 patients not responsive to butyrate (+,-)(•). Mean ± 1 SD.
The rapid reticulocyte γ-globin response. (A-B) Kinetics in a representative patient with SCD responsive to butyrate: γ-globin protein chain synthesis (A) and γ-globin mRNA (B). (C-D) Reticulocyte γ-globin protein chain synthesis (C) and γ-globin mRNA (D), respectively, in 5 patients responsive to butyrate (○, ▵, □, ×, ⋄) and 2 patients not responsive to butyrate (+,-)(•). Mean ± 1 SD.
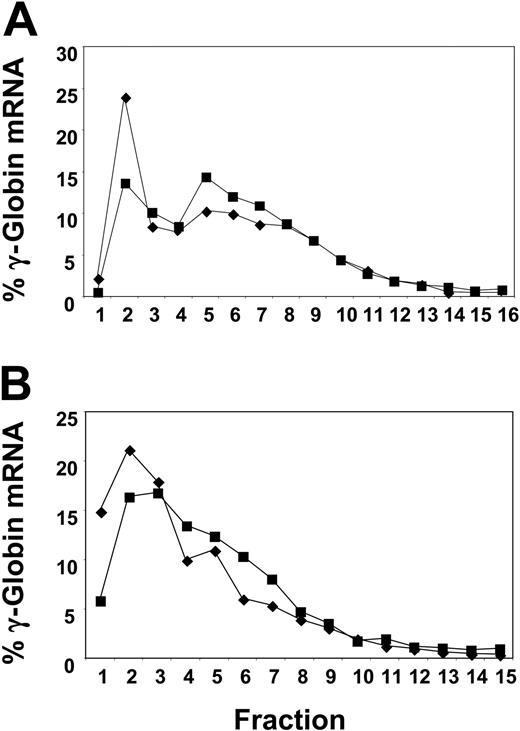
We then investigated the effect of butyrate on the efficiency of mRNA translation. In the first patient we studied, reticulocyte γ-globin protein chain synthesis increased by 4-fold (from 1.03% to 3.96%) within 24 hours of butyrate infusion. Prepolysomal reticulocyte γ-globin mRNA decreased from 25% to 14% at 24 hours after treatment, while polysomal mRNA increased from 29% to 37.6% (Figure 2A), suggesting an increased efficiency of translation of γ-globin mRNA. Interestingly, no polysomal shift was seen in this same patient during a butyrate cycle that followed a serious infection when she did not respond by increasing her Hb F level (data not shown). In another patient we studied, Hb F increased modestly from 23.1% to 25.6% during the 4-day treatment cycle. The prepolysomal γ-globin mRNA decreased from 36.1% before treatment to 22.3% at 24 hours, while the polysomal mRNA increased from 26.5% to 35.1% (Figure 2B).
Alteration in the polysomal distribution of γ-globin mRNA in reticulocytes subsequent to butyrate therapy. Before therapy (♦) and at 24 hours (▪). Fractions 1 and 2 are the prepolysomal region; fractions 5 to 8, the 1 to 3 ribosomal region. (A) Analysis of γ-globin mRNA from reticulocytes from a representative patient with SCD responsive to butyrate. (B) Analysis of γ-globin mRNA from reticulocytes from another representative patient with SCD responsive to butyrate.
Alteration in the polysomal distribution of γ-globin mRNA in reticulocytes subsequent to butyrate therapy. Before therapy (♦) and at 24 hours (▪). Fractions 1 and 2 are the prepolysomal region; fractions 5 to 8, the 1 to 3 ribosomal region. (A) Analysis of γ-globin mRNA from reticulocytes from a representative patient with SCD responsive to butyrate. (B) Analysis of γ-globin mRNA from reticulocytes from another representative patient with SCD responsive to butyrate.
These studies provide experimental evidence in support of the hypothesis that the butyrate-induced, rapid increase in Hb F levels is mediated by an increase in the efficiency of translation of γ-globin mRNA. This, of course, does not imply that butyrate induces Hb F levels solely through an effect on translation of γ-globin mRNA. The transcriptional effects of butyrate in nucleated erythroid precursors in the bone marrow would probably require a significantly longer time to increase the Hb F levels in the peripheral blood.15,29 The mechanism responsible for the increased efficiency of γ-globin mRNA translation is not yet known. Although our studies have shown that butyrate increases the efficiency of translation of γ-globin mRNA, but not that of α- or β-globin mRNA (data not shown), it is not clear whether other cellular mRNAs may be translated more efficiently in the presence of butyrate. We speculate that the effect of butyrate on the efficiency of mRNA translation may be a result of changes in the acetylation of proteins involved in mRNA translation. These questions and several others that are raised by our observations described here require further investigation.
Prepublished online as Blood First Edition Paper, October 12, 2004; DOI 10.1182/blood-2004-02-0454.
Supported by National Institutes of Health (NIH HL28381, R.S.W.; NIH HL73438, G.F.A.; NIH HL65449, S.A.L.; FD-R-001532, NIH HL-15157, and NIH HL-61208, S.P.; and NIH HL-20899, G.S.) and by a grant (MO1-RR-00071) awarded to the Mount Sinai School of Medicine General Clinical Research Center.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal