Abstract
Thioredoxin truncated at its carboxy terminal (Trx80) acts as a cytokine that stimulates monocytes and eosinophils. In the present study, Trx80 was shown to induce differentiation of human CD14+ monocytes into a cell type not described previously, which we designate as Trx80-activated monocytes (TAMs). TAMs resemble immature dendritic cells (iDCs) generated in the presence of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin 4 (IL-4) in that both these cell populations exhibit increased proportions of CD1a+ and mannose receptor (MR)+ cells. However, in contrast to iDCs, TAMs express high proportion of CD14 and lower proportion of CD83 and HLA-DR. Functional assays revealed that, in comparison to iDCs, TAMs 1) exhibit a higher pinocytic capacity; 2) release significantly higher amounts of the proinflammatory cytokines tumor necrosis factor-α (TNFα), IL-1β, and IL-6 and of the anti-inflammatory cytokine IL-10; and 3) induce a significantly lower proliferative response in allogeneic peripheral blood mononuclear cells (PBMCs). Indeed, Trx80 appears to be the first endogenous substance shown to have the capacity on its own to induce IL-10 production by monocytes. Analysis of the mitogen-activated protein (MAP) kinase signaling pathway revealed that Trx80 induces phosphorylation of p38, extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK). We propose that Trx80 is an early signal in response to danger, and that TAMs may play a major role in triggering innate immune responses.
Introduction
The 12-kDa human cytosolic thioredoxin (Trx) protein contains a redox-active dithiol moiety in its conserved active-site sequence -Cys32-Gly33-Pro34-Cys35-.1,2 In resting cells, thioredoxin resides in the cytosol, but activation by a wide variety of stimuli leads to translocation to the nucleus or secretion of this compound.1,3,4 The general thiol-disulfide-oxidoreductase activity of Trx is involved in most of the functions of this protein,5 including regulation of the activity of transcription factors such as nuclear factor κβ (NF-κβ), activator protein-1 (AP-1), and the glucocorticoid receptor via thiol redox control.3,5-7 Moreover, reduced Trx inhibits apoptosis by binding to apoptosis signaling kinase 1 (ASK-1).8 In the extracellular milieu Trx acts as a chemokine and co-cytokine, stimulating cytokine secretion and cell proliferation.9-11
A truncated form of thioredoxin (Trx80) has been detected in the plasma of patients suffering from severe schistosomiasis.12,13 Produced primarily by monocytes, nanomolar concentrations of Trx80 enhances eosinophil cytotoxic function.14-17 Furthermore, it has been demonstrated that Trx80 is present in the plasma of healthy blood donors and that recombinant Trx80 evokes proliferation of both normal human peripheral blood mononuclear cells (PBMCs) and purified human monocytes.18,19
Moreover, exposure of monocytes to Trx80 elicits elevated expression of several surface antigens, ie, CD14, CD40, CD54, and CD86, as well as inducing production of interleukin 12 (IL-12) by CD40+ monocytes in the presence of T cells.18 In addition, Trx80 stimulates production of interferon γ (IFN-γ) by PBMCs and acts as a chemokine for monocytes, T cells, and polymorphonuclear cells (PMNs).18,20,21 Site-directed mutagenesis of the Cys32 and Cys35 residues in Trx80 (corresponding to the Cys residues present in the active-site of full-length Trx) to serine residues does not alter these biologic activities of Trx80, strongly suggesting that these effects do not involve a disulfide-reductase mechanism.22 Macrophages have been reported to cleave full-length Trx to yield the truncated Trx80 form,23 which is present mainly at the surface of monocytes, in contrast to the cytosolic localization of the full-length protein.17
In this investigation we present novel evidence that Trx80 elicits differentiation and activation of normal human monocytes into a population of cells, which has not previously been described, that exhibit increased pinocytic activity and pronounced release of inflammatory cytokines. We refer to these cells as Trx80-activated monocytes (TAMs).
Materials and methods
Reagents
AIM-V and RPMI 1640 cell culture medium, l-glutamine, penicillin and streptomycin (Gibco BRL, Life Technologies, Paisley, Scotland); fetal calf serum (FCS; Hyclone, Logan, UT); Ficoll-Paque (Pharmacia Biotec, Uppsala, Sweden); polymyxin B (PMB) sulfate, 2-mercaptoethanol, ultralow attachment cell culture plates, lipopolysaccharide (LPS) from Escherichia coli 055:B5, and dextran conjugated with fluorescein isothiocyanate (FITC) (average molecular weight 40 000 kDa; Sigma Chemical, St Louis, MO); [3H]-thymidine (Amersham Pharmacia Biotech, Uppsala, Sweden); granulocyte-macrophage colony-stimulating factor (GM-CSF; Schering Plough, Kenilworth, NJ, and Biosource International, Camarillo, CA); recombinant interleukin-4 (rIL-4; Nordic BioSite, Stockholm, Sweden); and CD14+ magnetic activated cell sorting microbeads (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) were purchased from the sources indicated.
Monoclonal antibodies
The following mouse monoclonal antibodies (mAbs) conjugated with FITC, phycoerythrin (PE), and allophycocyanin (APC) were used for flow cytometric analysis: anti-CD1a (PE; T6-RD1; Beckman Coulter, Fullerton, California) and anti-CD14 (FITC, APC; MϕP9), anti–HLA-DR (FITC; L243), and anti-CD83 (FITC; HB15e); the mannose receptor (MR/CD206; PE; 19.2) and isotype-matched antibodies were used as negative controls (Becton Dickinson, San Diego, CA).
Preparation of Trx80
Recombinant Trx80 was prepared as described in detail earlier.19 Briefly, this protein was overexpressed in E coli, where it was found in inclusion bodies. Subsequently, Trx80 was solubilized from these bodies using urea, and the resulting solution was dialyzed and subjected to ion-exchange and reverse-phase chromatography, followed by gel filtration through a Sephadex G-75 column. The purity of the final preparation was confirmed by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis, and circular dichroic spectra were taken to ensure that proper refolding had occurred after the urea denaturation.19 Finally, to eliminate contamination by endotoxin, these Trx80 preparations were first passed through a polymyxin B column and then in a positively charged sterile filter. The limulus assay performed by the authorized laboratory in Karolinska Hospital revealed that the endotoxin content of the final protein sample was negligible, resulting in less than 100 pg/mL in cell cultures exposed to 100 nM Trx80. Stock solutions were maintained at –20° C until use.
Purification of CD14+ cells
Following approval by the Ethics Committee of the Karolinska Institutet and Karolinska University Hospital, human PBMCs were prepared from the blood of healthy donors (Blood Bank, Karolinska University Hospital) by the standard procedure of Ficoll-Paque centrifugation. The cells recovered were counted by trypan blue exclusion, revealing a routine viability of more than 99%. Thereafter, CD14+ cells were isolated using the CD14+ MACS procedure, according to the manufacturer's instructions. Purified CD14+ cells were resuspended in complete RPMI 1640 medium containing 10% heat-inactivated FCS, 2 mM l-glutamine, 100 U penicillin and 100 U streptomycin/mL, and 50 μM 2-mercaptoetanol. The purity of these monocytes was always more than 97%, as revealed by flow cytometry.
Culture conditions
Following resuspension in complete medium at a concentration of 106 cells/mL, CD14+ cells were cultured in the absence or presence of 100 nM Trx80. As a positive control for the production of immature dendritic cells (iDCs), other CD14+ monocytes (4 × 105 cells/mL) were cultured in complete medium supplemented with GM-CSF (550 U/mL) and IL-4 (800 U/mL). All cells were cultured in a final volume of 3 to 5 mL on ultralow attachment cell culture plates, at 37°C in humidified incubators containing air with 5% CO2. The development and structure of the cells were examined with an Axiovert25 phase contrast light microscope equipped with 20 ×/3.0 and 40 ×/0.5 objective lenses (Zeiss, Jena, Germany). Images were obtained using a Nikon DXM1200 color CCD camera and ACT-1 software run on a PC (Nikon, Tokyo, Japan). Images were resized, cropped, and assembled using Adobe Photoshop version 6.0 (Adobe Systems, San Jose, CA).
Detection of cytokine in the culture medium
Medium was collected from monocyte cultures described in “Culture conditions” after 3 and 5 days and maintained at –20°C until being analyzed. TNFα, IL-1β, IL-6, IL-8, IL-10, and IL-12p70 were all quantitated using the Cytometric Bead Array (CBD) human inflammation kit (Becton Dickinson) according to the manufacturer's protocol. The lower limit of detection using this procedure was 20 pg/mL.
Flow cytometric analysis
Cell cultures were terminated after 3 and 5 days by the addition of cold phosphate-buffered saline (PBS) followed by incubation for 10 minutes on ice. Subsequently, the cells were resuspended using a Pasteur pipette and harvested. Approximately 5 × 105 cells were then incubated with either FITC-, PE-, or APC-conjugated mAbs diluted in PBS for 45 minutes on ice. After this incubation, the cells were washed once with 1 mL PBS and finally suspended in 500 μL of the same buffer. For triple marker staining, cells were incubated with a combination of FITC-conjugated CD83, PE-conjugated CD1a, and APC-conjugated CD14.
Thereafter, 5 to 10 thousand cells were acquired on a FACSCalibur flow cytometer (Becton Dickinson) and analyzed using CellQuest software (Becton Dickinson). An appropriate gate was set on the basis of the characteristic forward-scatter pattern of these cells, and only cells within this gate were analyzed. Cells exhibiting a higher mean fluorescence index (MFI) value than control cells incubated with an irrelevant isotype-matched antibody were considered positive.
The flow cytometer was calibrated according to the manufacturer's instructions prior to each acquisition. Incubation with FITC-, PE-, or APC-conjugated immunoglobulin G (IgG) isotype-matched irrelevant antibodies from Becton Dickinson were used as negative controls. Dead cells were excluded on the basis of their staining with propidium iodide (PI) and/or scatter properties. PI staining indicated that more than 99% of the cells in the population examined were viable at the time of analysis.
Endocytosis of FITC-labeled particles
Monocytes were cultured in RPMI 1640 medium as described “Culture conditions,” either alone or in the presence of 100 nM Trx80 or GM-CSF + IL-4 for 3 and 5 days. After harvesting, the cells were resuspended in cold PBS at a concentration of 2 to 6 × 105 cells/mL cold PBS. FITC-conjugated dextran (FITC-Dx; 40 μg) was then added to 0.5 mL of this suspension, and the cells were incubated at 4°C or 37°C for 1 hour. Following 2 washes with 1 mL cold PBS, 104 cells were acquired on a FACSCalibur flow cytometer and analyzed using CellQuest software.
Western blotting
CD14+ cells were resuspended in RPMI 1640 medium at a concentration of 106/mL as described in “Purification of CD14+ cells” and thereafter treated with 100 nM Trx80 for different periods of time from 0 to 60 minutes. After subsequent harvesting the cell pellets were resuspended thoroughly in lysis buffer (1% Triton X-100, 50 mM HEPES [N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid] pH 7.5, 150 mM NaCl, 10% glycerol, 2 mM EDTA [ethylenediaminetetraacetic acid], 1 mM sodium orthovanadate, 1 mM PMSF [phenylmethylsulfonyl fluoride], and 40 μL/mL of the protease inhibitor cocktail solution [Roche, Penzberg, Germany]) and incubated on ice for 30 minutes. These cell lysates (25 μg total protein per lane) were submitted to SDS–polyacrylamide gel electrophoresis (PAGE) (on 12 gels), transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA) and then probed with antibodies. Six different rabbit polyclonal antibody preparations that either selectively recognize the phosphorylated, activated forms of p38 (Thr180/Tyr182), extracellular signal-regulated kinase (ERK; Thr202/Tyr204), or c-Jun N-terminal kinase (JNK; Thr183/Tyr185) or, alternatively, all forms of p38, ERK (p44/42), and JNK (p46/54) were used according to the manufacturer's instructions (Cell Signaling Technology, Beverly, MA). Binding of these primary antibodies was visualized with goat anti–rabbit immunoglobulins coupled to horseradish peroxidase (DakoCytomation, Glostrup, Denmark) and enhanced chemiluminescence (ECL; Perkin Elmer, Boston, MA).
Stimulation of allogeneic lymphocytes (mixed lymphocyte reaction; MLR)
CD14+ monocytes were cultured in complete media as described in “Culture conditions,” either alone or in the presence of 100 nM Trx80 or GM-CSF + IL-4 for 3 days, harvested, counted in the presence of trypan blue, and then irradiated (with 30 Gy γ-radiation). These irradiated monocytes (stimulators) were subsequently mixed with fresh allogeneic PBMCs, (responders) from healthy blood donors in responder-to-stimulator ratios varying from 10:1 to 10 000:1. These cell mixtures were cocultured in triplicate on 96-well plates for 5 days, with addition of 1 μCi [0.037MBq] [3H]-thymidine per well 8 hours prior to termination of this incubation. Thereafter, the plates were maintained at 20°C until determination of [3H]-thymidine incorporation by scintillation counting. The results are presented in the form of a stimulatory index (SI), which was obtained by dividing the radioactivity (cpm) incorporated by responder cells cultured in the presence of stimulator cells exposed to GM-CSF + IL-4, 100 nM Trx80, or medium alone by the level of radioactivity (cpm) incorporated by responders cells cultured alone.
Statistical analysis
Data obtained from the phenotypic and MLR analyses were analyzed using the nonparametric Wilcoxon matched-pairs test, with a P value less than .05 being considered statistically significant.
Results
Phenotypic characterization of Trx80-activated monocytes (TAMs)
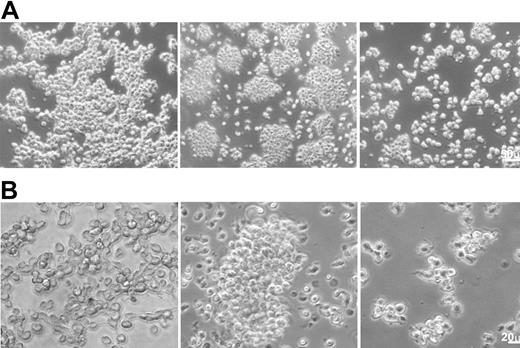
The structure of the cells during the 5 days of culture was monitored by daily observation under a phase contrast microscope (Figure 1). Most CD14+ cells cultured in medium alone adhered to the plastic plates after 2 hours in culture and remained attached for the entire 5 days in culture. In contrast, TAMs produced by culturing CD14+ cells in the presence of 100 nM Trx80 formed cell aggregates with a low degree of attachment to the plastic plates. Small cell aggregates of this nature were observed already after 1 to 2 hours in culture, with subsequent formation during the following hours of larger cell aggregates, which then remained throughout the remainder of the incubation.
The structure of TAMs as monitored by phase contrast microscopy. CD14+ cells were cultured for 3 days in medium alone (left column) or in the presence of 100 nM Trx80 (to generate TAMs; middle column) or GM-CSF + IL-4 (right column). Panels A and B show different magnifications (see the size bars).
The structure of TAMs as monitored by phase contrast microscopy. CD14+ cells were cultured for 3 days in medium alone (left column) or in the presence of 100 nM Trx80 (to generate TAMs; middle column) or GM-CSF + IL-4 (right column). Panels A and B show different magnifications (see the size bars).
During the first 2 hours of incubation in the presence of GM-CSF + IL-4, approximately half of the cells attached to the plastic plates. The number of adherent cells was markedly reduced after 1 day in culture, and by day 5 all of the cells were suspended in the medium with approximately two thirds of the cells forming small and medium-sized aggregates, and the remainder was floating as single cells.
TAMs exhibited significant up-regulation of the expression of the phagocyte-associated receptor CD14 compared with unstimulated cells. Thus, approximately 90% of the TAMs from 8 different blood donors expressed CD14 after 3 and 5 days in culture (Table 1), whereas the corresponding value was approximately 50% for CD14+ cells not activated by Trx80, and only 2% to 5% for cells exposed to GM-CSF + IL-4. Furthermore, TAMs demonstrated a 3-fold increase in their MFI for CD14 after 3 days in culture, compared with cells cultured in medium alone.
Phenotypic characterization of TAMs
. | Control cells in medium alone (range) . | . | TAMs (range) . | . | Cells exposed to GM-CSF + IL-4 (range) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Surface marker* . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | |||
| CD1a | |||||||||
| % + | 9 (2-19) | 13 (5-19) | 33† (9-75) | 40† (3-53) | 32† (11-56) | 50† (20-83) | |||
| MFI | 21 (16-30) | 19 (16-29) | 36 (14-71) | 26 (12-50) | 142† (20-241) | 316† (100-755) | |||
| CD14 | |||||||||
| % + | 53 (23-78) | 51 (26-80) | 92†,‡ (57-98) | 89†,‡ (79-97) | 5† (4-22) | 2† (1-6) | |||
| MFI | 103 (23-152) | 89 (35-166) | 343†,‡ (40-584) | 180† (36-446) | 271 (13-388) | 224 (8-450) | |||
| CD83 | |||||||||
| % + | 19‡ (12-23) | 25‡ (18-69) | 10‡ (5-49) | 20‡ (3-51) | 59 (27-67) | 51 (40-66) | |||
| MFI | 16 (11-23) | 16 (11-72) | 17 (11-33) | 13 (10-38) | 19 (14-26) | 19 (17-27) | |||
| MR/CD206 | |||||||||
| % + | 68 (21-85) | 66 (17-80) | 86 (49-99) | 92† (40-99) | 95† (90-99) | 96† (92-97) | |||
| MFI | 54 (29-156) | 50 (22-96) | 115 (29-209) | 164† (29-305) | 110 (47-265) | 15† (53-293) | |||
| HLA-DR | |||||||||
| % + | 54‡ (31-94) | 33‡ (13-69) | 67‡ (52-96) | 69‡ (15-97) | 99 (98-100) | 99 (96-100) | |||
| MFI | 143‡ (33-252) | 77‡ (25-445) | 128‡ (84-238) | 128‡ (33-281) | 423 (215-979) | 433 (291-725) | |||
. | Control cells in medium alone (range) . | . | TAMs (range) . | . | Cells exposed to GM-CSF + IL-4 (range) . | . | |||
|---|---|---|---|---|---|---|---|---|---|
| Surface marker* . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | |||
| CD1a | |||||||||
| % + | 9 (2-19) | 13 (5-19) | 33† (9-75) | 40† (3-53) | 32† (11-56) | 50† (20-83) | |||
| MFI | 21 (16-30) | 19 (16-29) | 36 (14-71) | 26 (12-50) | 142† (20-241) | 316† (100-755) | |||
| CD14 | |||||||||
| % + | 53 (23-78) | 51 (26-80) | 92†,‡ (57-98) | 89†,‡ (79-97) | 5† (4-22) | 2† (1-6) | |||
| MFI | 103 (23-152) | 89 (35-166) | 343†,‡ (40-584) | 180† (36-446) | 271 (13-388) | 224 (8-450) | |||
| CD83 | |||||||||
| % + | 19‡ (12-23) | 25‡ (18-69) | 10‡ (5-49) | 20‡ (3-51) | 59 (27-67) | 51 (40-66) | |||
| MFI | 16 (11-23) | 16 (11-72) | 17 (11-33) | 13 (10-38) | 19 (14-26) | 19 (17-27) | |||
| MR/CD206 | |||||||||
| % + | 68 (21-85) | 66 (17-80) | 86 (49-99) | 92† (40-99) | 95† (90-99) | 96† (92-97) | |||
| MFI | 54 (29-156) | 50 (22-96) | 115 (29-209) | 164† (29-305) | 110 (47-265) | 15† (53-293) | |||
| HLA-DR | |||||||||
| % + | 54‡ (31-94) | 33‡ (13-69) | 67‡ (52-96) | 69‡ (15-97) | 99 (98-100) | 99 (96-100) | |||
| MFI | 143‡ (33-252) | 77‡ (25-445) | 128‡ (84-238) | 128‡ (33-281) | 423 (215-979) | 433 (291-725) | |||
Purified CD14+ monocytes were exposed to either 100 nM Trx80 (to produce TAMs), GM-CSF + IL-4, or medium alone and cultured for 3 and 5 days in ultralow attachment plates.
The proportion of positive cells (% +) and the mean fluorescence index (MFI) were determined by flow cytometry. The data presented are medians and ranges of the values obtained with CD14+ monocytes from 8 different blood donors during the periods of incubation indicated.
P < .05 compared with cells cultured in medium alone as analyzed by using the nonparametric Wilcoxon matched-pairs test.
P < .05 compared with cells exposed to GM-CSF + IL-4 as analyzed by using the nonparametric Wilcoxon matched-pairs test.
Furthermore, the proportion TAMs that were MR+ was significantly higher than in the case of untreated monocytes after 5 days of culture (Table 1). In cultures exposed to GM-CSF + IL-4, more than 90% of the cells were MR+. After 5 days in culture the MFI value for TAMs or monocytes exposed to GM-CSF + IL-4 was 3-fold higher than that observed for cells cultured in medium alone (Table 1).
In addition, the expression of CD1a on the surface of cultured TAMs was significantly elevated after both 3 and 5 days in comparison to unstimulated cells (Table 1). The proportion of TAMs and cells exposed to GM-CSF + IL-4 that were CD1a+ were similar after 3 days in culture (median, around 33%), whereas 9% of the untreated monocytes expressed this same receptor. However, at this same time point, the surface density of CD1a on GM-CSF + IL-4–treated cells (as reflected in the MFI) was 4-fold higher than that observed for TAMs. After additional 2 days in culture, TAMs exhibited no major further change in their expression of CD1a, whereas even more of the cells exposed to GM-CSF + IL-4 expressed CD1a at an even further enhanced surface density (Table 1).
Moreover, after 3 and 5 days of culture TAMs and monocytes cultured in medium alone exhibited similar low proportions of CD83+ cells (Table 1). In contrast, significantly higher percentage of the cells exposed to GM-CSF + IL-4 were CD83+, even though the MFI values were similar in all 3 cases (Table 1).
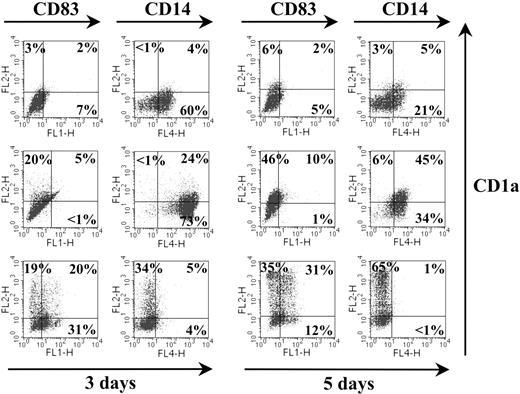
Triple marker staining was performed to clarify at the single-cell level the proportion of Trx80–treated cells expressing the markers CD1a, CD14, and CD83. After 3 days in culture, Trx80 elicited a subpopulation of cells positive for both CD1a and CD14 (median, 17%; range, 11%-24%; n = 5). This population did not coexpress CD83 (Figure 2). After 5 days in culture, there was a tendency to an increased proportion of CD1a+CD14+ cells (median, 24%; range, 7%-45%; n = 5). After 3 and 5 days, the level of expression of CD83 was slightly higher compared with the background levels expressed in the nonstimulated cell population. Cells exposed to GM-CSF + IL-4 showed the typical pattern of CD1a and CD83 expression, lacking the CD14 marker (Figure 2).
Phenotypic characterization of TAMs. Purified CD14+ monocytes were exposed to medium alone (top row), 100 nM Trx80 (middle row), or GM-CSF + IL-4 (bottom row) and cultured for 3 and 5 days in ultralow attachment plates. For triple marker staining, cells were incubated with a combination of FITC-conjugated CD83, PE-conjugated CD1a, and APC-conjugated CD14 and analyzed by flow cytometry. The proportion of positive cells is indicated. One representative experiment of 5 is shown.
Phenotypic characterization of TAMs. Purified CD14+ monocytes were exposed to medium alone (top row), 100 nM Trx80 (middle row), or GM-CSF + IL-4 (bottom row) and cultured for 3 and 5 days in ultralow attachment plates. For triple marker staining, cells were incubated with a combination of FITC-conjugated CD83, PE-conjugated CD1a, and APC-conjugated CD14 and analyzed by flow cytometry. The proportion of positive cells is indicated. One representative experiment of 5 is shown.
Finally, TAMs and monocytes activated by GM-CSF + IL-4 showed striking differences in their expression of HLA-DR (Table 1). After 3 and 5 days in culture, 99% of the later were HLA-DR+, whereas only 68% of the TAMs expressed this surface antigen. In addition, the MFI of HLA-DR in cells exposed to GM-CSF + IL-4 was more than 3-fold higher than that observed for TAMs (Table 1).
Uptake of FITC-labeled dextran particles by TAMs
The elevated expressions of CD14 and MR observed on the surface of TAMs indicated that these cells might be more efficient than untreated monocytes or monocytes stimulated with GM-CSF + IL-4 in carrying out pinocytosis. To examine this possibility, we evaluated the capacity of these different cells to internalize FITC-Dx (Table 2). After 3 days of culture followed by exposure to FITC-Dx for 1 hour at either 4°C (a background control for nonspecific binding) or 37°C (active uptake), no more than 40% of the cells cultured in medium alone actively internalized FITC-Dx, whereas the corresponding value for TAMs was 75%. Of the cells exposed to GM-CSF + IL-4, 16% were positive for FITC-Dx. After 5 days in culture, TAMs still demonstrated the highest endocytotic capacity, with 68% actively taking up FITC-Dx.
Endocytosis of FITC-labeled dextran particles by TAMs
. | Period of culture . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | 3 d . | . | 5 d . | . | |||
| Fluorescent staining for monocytes . | % + . | MFI . | % + . | MFI . | |||
| Control cells in medium alone | |||||||
| CD14 | 50 | 62 | 34 | 58 | |||
| MR | 55 | 59 | 53 | 53 | |||
| FITC-Dx at 4°C | 3 | 47 | 3 | 50 | |||
| FITC-Dx at 37°C* | 42 | 45 | 17 | 57 | |||
| TAMs | |||||||
| CD14 | 92 | 168 | 77 | 60 | |||
| MR | 96 | 266 | 97 | 317 | |||
| FITC-Dx at 4°C | 3 | 27 | 3 | 38 | |||
| FITC-Dx at 37°C* | 75 | 34 | 68 | 43 | |||
| Stimulated with GM-CSF + IL-4 | |||||||
| CD14 | 7 | 39 | 1 | 44 | |||
| MR | 91 | 122 | 84 | 126 | |||
| FITC-Dx at 4°C | 3 | 31 | 3 | 46 | |||
| FITC-Dx at 37°C* | 16 | 44 | 20 | 61 | |||
. | Period of culture . | . | . | . | |||
|---|---|---|---|---|---|---|---|
| . | 3 d . | . | 5 d . | . | |||
| Fluorescent staining for monocytes . | % + . | MFI . | % + . | MFI . | |||
| Control cells in medium alone | |||||||
| CD14 | 50 | 62 | 34 | 58 | |||
| MR | 55 | 59 | 53 | 53 | |||
| FITC-Dx at 4°C | 3 | 47 | 3 | 50 | |||
| FITC-Dx at 37°C* | 42 | 45 | 17 | 57 | |||
| TAMs | |||||||
| CD14 | 92 | 168 | 77 | 60 | |||
| MR | 96 | 266 | 97 | 317 | |||
| FITC-Dx at 4°C | 3 | 27 | 3 | 38 | |||
| FITC-Dx at 37°C* | 75 | 34 | 68 | 43 | |||
| Stimulated with GM-CSF + IL-4 | |||||||
| CD14 | 7 | 39 | 1 | 44 | |||
| MR | 91 | 122 | 84 | 126 | |||
| FITC-Dx at 4°C | 3 | 31 | 3 | 46 | |||
| FITC-Dx at 37°C* | 16 | 44 | 20 | 61 | |||
Monocytes were cultured in the absence (control) or presence of GM-CSF + IL-4 or 100 nM Trx80 (TAMs) for 3 or 5 days. After harvesting, washing, and resuspension in 0.5 mL ice-cold PBS to obtain a concentration of 2-6 × 105 cells/mL, 40 μg FITC-conjugated dextran (FITC-Dx) was added, and the cells were incubated for an additional hour at 4°C (as control for nonspecific binding) or 37°C. Finally, the cells were washed and their fluorescence was analyzed by flow cytometry. The values presented are from 1 representative experiment of 3 performed with cells from different blood donors.
Release of inflammatory cytokines by TAMs
TAMs generated by exposure of CD14+ cells to 100 nM Trx80 for 3 or 5 days release the proinflammatory cytokines TNFα, IL-1β, IL-6, IL-8, and the anti-inflammatory cytokine IL-10 (Table 3). Higher levels of TNFα and IL-10 were observed in the culture medium of TAMs cultured for 3 days than following 5 days of exposure to Trx80. As we have reported previously, no IL-12 was released by these isolated CD14+ cells stimulated with Trx80.18 The levels of TNFα, IL-1β, IL-6, and IL-10 in the media from the untreated cells and monocytes exposed to GM-CSF + IL-4 were close to or under the lower limit of detection of the assay used (20 pg/mL). High levels of the chemokine IL-8 were detected in all of the culture supernatant (Table 3).
Release of cytokines by monocytes cultured for 3 or 5 days in the presence of Trx80, GM-CSF + IL-4, or medium alone
Culture conditions . | TNFα . | . | IL-1β . | . | IL-6 . | . | IL-8 . | . | IL-10 . | . | IL-12 . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | ||||||
| Medium alone | < 20 | < 20 | < 20 | < 20 | < 20 | < 20 | > 5000 | > 5000 | < 20 | < 20 | < 20 | < 20 | ||||||
| GM-CSF + IL-4 | < 20 | < 20 | 32 | 27 | < 20 | < 20 | > 5000 | > 5000 | < 20 | < 20 | < 20 | < 20 | ||||||
| 100 nM Trx80 | 166 | 92 | 1621 | 1621 | > 5000 | > 5000 | > 5000 | > 5000 | 220 | 139 | < 20 | < 20 | ||||||
Culture conditions . | TNFα . | . | IL-1β . | . | IL-6 . | . | IL-8 . | . | IL-10 . | . | IL-12 . | . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | 3 d . | 5 d . | ||||||
| Medium alone | < 20 | < 20 | < 20 | < 20 | < 20 | < 20 | > 5000 | > 5000 | < 20 | < 20 | < 20 | < 20 | ||||||
| GM-CSF + IL-4 | < 20 | < 20 | 32 | 27 | < 20 | < 20 | > 5000 | > 5000 | < 20 | < 20 | < 20 | < 20 | ||||||
| 100 nM Trx80 | 166 | 92 | 1621 | 1621 | > 5000 | > 5000 | > 5000 | > 5000 | 220 | 139 | < 20 | < 20 | ||||||
Culture media from TAMs generated in the presence of 100 nM Trx80 and from CD14+ monocytes incubated in medium alone (at 106 cells/mL) or in the presence of GM-CSF + IL-4 (4 × 105 cells/mL) were collected after 3 and 5 days. Cytokine concentrations were determined by using the Cytometric Bead Array (CBD) assay and are expressed as picogram per milliliter. The lower limit of detection of this assay was 20 pg/mL. Data from 1 representative experiment of 3 performed with cells from different blood donors are shown.
Trx80 induces activation of p38, ERK, and JNK in TAMs
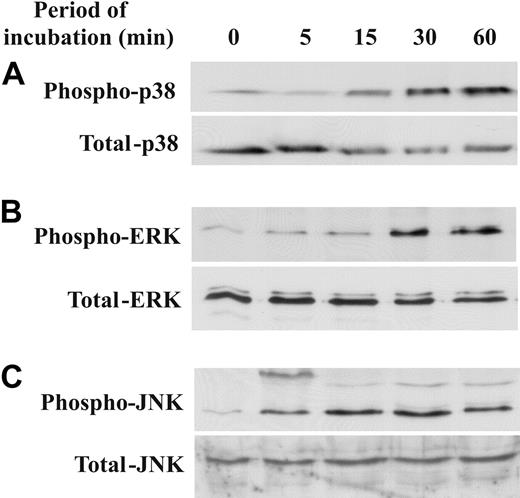
Activation of mitogen-activated protein (MAP) kinases is responsible for up-regulation of the expression of both proinflammatory and anti-inflammatory cytokines. Therefore, we analyzed whether Trx80 causes activation of the MAP kinases p38, ERK, and JNK by phosphorylation (Figure 3A-C). Indeed, the level of phosphorylation of all 3 of these MAP kinases was higher in TAMs than in monocytes cultured in medium alone. After only 5 minutes of exposure to Trx80, the level of phosphorylated ERK in TAMs was elevated, and this level continued to increase throughout the 60-minute incubation period studied. The level of phosphorylated p38 was enhanced after 15 minutes of stimulation with Trx80 and also continued to increase during the time period studied. In addition Trx80 induced phosphorylation of JNK, which reached a maximum after 15 to 30 minutes (Figure 3A-C; Table 4).
Trx80 induces phosphorylation of p38, ERK, and JNK in TAMs. TAMs were produced by exposure of CD14+ monocytes to 100 nM Trx80 for the time periods indicated. The levels of phosphorylation of p38 (A, top), ERK (B, top), and JNK kinase (C, top) were determined by Western blotting of whole-cell lysates using antibodies specific for the phosphorylated, activated forms. The corresponding Western blots for the total levels of these kinases are depicted in the bottom panels. One representative experiment is shown.
Trx80 induces phosphorylation of p38, ERK, and JNK in TAMs. TAMs were produced by exposure of CD14+ monocytes to 100 nM Trx80 for the time periods indicated. The levels of phosphorylation of p38 (A, top), ERK (B, top), and JNK kinase (C, top) were determined by Western blotting of whole-cell lysates using antibodies specific for the phosphorylated, activated forms. The corresponding Western blots for the total levels of these kinases are depicted in the bottom panels. One representative experiment is shown.
Relative OD ratios
Period of incubation, min . | Relative OD, mean ± SD . | . | . | ||
|---|---|---|---|---|---|
| . | Phospho-p38 . | Phospho-ERK . | Phospho-JNK . | ||
| 5 | 1.5 ± 0.7 | 2.2 ± 0.4 | 8.8 ± 3.7 | ||
| 15 | 3.4 ± 1.2 | 2.1 ± 0.7 | 16.8 ± 11.9 | ||
| 30 | 4.8 ± 0.7 | 6.7 ± 2.5 | 16.3 ± 6.4 | ||
| 60 | 6.0 ± 2.6 | 6.8 ± 5.3 | 15.6 ± 5.1 | ||
Period of incubation, min . | Relative OD, mean ± SD . | . | . | ||
|---|---|---|---|---|---|
| . | Phospho-p38 . | Phospho-ERK . | Phospho-JNK . | ||
| 5 | 1.5 ± 0.7 | 2.2 ± 0.4 | 8.8 ± 3.7 | ||
| 15 | 3.4 ± 1.2 | 2.1 ± 0.7 | 16.8 ± 11.9 | ||
| 30 | 4.8 ± 0.7 | 6.7 ± 2.5 | 16.3 ± 6.4 | ||
| 60 | 6.0 ± 2.6 | 6.8 ± 5.3 | 15.6 ± 5.1 | ||
The relative optical density (OD) values represent the ratios between the OD of the phosphorylated band in Trx80-treated cells and the OD of the corresponding band in untreated cells. The relative OD mean and SD of 3 experiments are shown.
Allostimulatory capacity of TAMs
Finally, the allostimulatory capacity of TAMs was compared with that of monocytes cultured in medium alone or exposed to GM-CSF + IL-4. Monocytes stimulated with GM-CSF + IL-4 exhibited a median SI of 14 with respect to their allostimulatory effect on PBMCs (range, 5-617, n = 14). In contrast, the allostimulatory capacity of TAMs was equally low as that of monocytes cultured in medium alone (SI = 2.6; [range, 1-68] and 2.1 [range, 1-37], respectively; n = 14) (Figure 4).
Stimulation of allogeneic PBMCs by TAMs and monocytes exposed to GM-CSF or medium alone. CD14+ monocytes isolated from 4 different donors were cultured for 3 days in medium alone or in the presence of either 100 nM Trx80 (TAMs) or GM-CSF + IL-4. These cells were subsequently harvested, irradiated, and tested as stimulators of freshly separated allogeneic PBMCs (responders). Each such stimulator cell preparation was tested with 3 or 4 different responder preparations, to give a total number of 14. For this purpose, the PBMCs (106/mL) were cultured in triplicate on 96-well plates in the presence of 1/10 to 1/10 000 as many stimulator cells. Following 5 days of such culture, 1 μCi (0.037 MBq) [3H]thymidine was added to each well 8 hours prior to termination of the incubation. The SI was calculated as the ratio of the radioactivity incorporated (cpm) by the responder cells in the presence of stimulator cells to the corresponding value for the responder cells cultured alone. The data presented are the median values obtained with the 14 different cell preparations of the responders which incorporated between 180 and 665 cpm tritiated thymidine when cultured alone. • indicates responders cells in the presence of stimulator cells exposed to GM-CSF + IL-4; ▵, responder cells cultured with TAMs; ♦, responder cells cultured together with stimulator cells exposed only to medium. The stimulatory index for stimulator cells cultured in the presence of GM-CSF + IL-4 was significantly higher than that for TAMs or monocytes cultured in medium alone (P < .05), as determined by the Wilcoxon matched-pairs test.
Stimulation of allogeneic PBMCs by TAMs and monocytes exposed to GM-CSF or medium alone. CD14+ monocytes isolated from 4 different donors were cultured for 3 days in medium alone or in the presence of either 100 nM Trx80 (TAMs) or GM-CSF + IL-4. These cells were subsequently harvested, irradiated, and tested as stimulators of freshly separated allogeneic PBMCs (responders). Each such stimulator cell preparation was tested with 3 or 4 different responder preparations, to give a total number of 14. For this purpose, the PBMCs (106/mL) were cultured in triplicate on 96-well plates in the presence of 1/10 to 1/10 000 as many stimulator cells. Following 5 days of such culture, 1 μCi (0.037 MBq) [3H]thymidine was added to each well 8 hours prior to termination of the incubation. The SI was calculated as the ratio of the radioactivity incorporated (cpm) by the responder cells in the presence of stimulator cells to the corresponding value for the responder cells cultured alone. The data presented are the median values obtained with the 14 different cell preparations of the responders which incorporated between 180 and 665 cpm tritiated thymidine when cultured alone. • indicates responders cells in the presence of stimulator cells exposed to GM-CSF + IL-4; ▵, responder cells cultured with TAMs; ♦, responder cells cultured together with stimulator cells exposed only to medium. The stimulatory index for stimulator cells cultured in the presence of GM-CSF + IL-4 was significantly higher than that for TAMs or monocytes cultured in medium alone (P < .05), as determined by the Wilcoxon matched-pairs test.
Discussion
The highly conserved small redox protein thioredoxin is present in archebacteria, eubacteria, and eukaryotes and plays a basic role in connection with DNA synthesis by acting as an electron donor for the synthesis of deoxyribonucleotides by ribonucleotide reductase.5,24 From an evolutionary perspective, thioredoxin developed prior to the immune system.
Cleavage of thioredoxin in association with its secretion may represent an early type of alarmone function, ie, a priming signal for the immune system. Trx80 might thus activate monocytes to secrete a specific set of cytokines, as well as stimulate these same cells to differentiate into specific cell types. In the present investigation, we have demonstrated how Trx80 can stimulate human CD14+ to differentiate into a novel cell population that we have designated Trx80-activated monocytes (TAMs).
The increased expression of CD14 on the surface of monocytes exposed to Trx80 demonstrated in the present study is a confirmation of our earlier findings.18 CD14 plays important roles as a receptor binding endotoxin and in connection with phagocytosis of apoptotic cells without inflammation.25,26 In addition, exposure of CD14+ monocytes to 100 nM Trx80, a level we have observed in the plasma of healthy blood donors,19 induces expression of CD1a (a marker for immature dendritic cells) and the MR. Indeed, TAMs exposed in this manner for 3 days contain a proportion of CD1a+ cells similar to that observed with monocytes exposed to GM-CSF and IL-4, which is the “golden standard” procedure for generating monocyte-derived dendritic cells (MDDCs),27,28 also referred to as immature dendritic cells (iDCs). However, in contrast to iDCs, which are negative for CD14, the CD1a+ TAM cells are also positive for CD14.
The MR has been reported to be the most important, if not the only, receptor involved in endocytosis of FITC-Dx.29 Our findings reveal that TAMs are highly efficient in taking up FITC-Dx, even more active in this respect than CD14+ monocytes exposed to GM-CSF and IL-4. The significance of the MR in connection with innate immunity is exemplified by its capacity to bind a wide variety of microorganisms.30-35
The present study confirms our previous observation that Trx80 alone cannot induce IL-12 production in purified CD14+ monocytes. However, in contrast to other endogenous factors that activate monocytes, Trx80 alone can induce the production and release of the proinflammatory cytokines TNFα, IL-1β, IL-6, and IL-8 and of the anti-inflammatory cytokine IL-10. Other reports have demonstrated that upon CD40 binding, monocytes primed with macrophage colony-stimulating factor (M-CSF) produce IL-1β and IL-10, whereas monocytes primed with IFN-γ produced primarily higher levels of TNFα and lower levels of IL-1β and IL-10.36 TNFα promotes the activation of monocytes and in the presence of IL-4 the differentiation of cells into dendritic cells.37,38
To our knowledge Trx80 is the first endogenous substance that has the capacity to induce by itself production of IL-10 by CD14+ monocytes. The major function of IL-10 appears to be to limit and ultimately terminate inflammatory responses. This cytokine exerts autoregulatory effects on activated monocytes and iDCs by inhibiting their antigen-presentation capacity, cytokine production, expression of costimulatory molecules, and chemokine secretion.38-41 The production of IL-10 during the first 3 days of exposure to Trx80 observed here may thus represent a self-controlling mechanism of activation that limits the extent of the inflammatory reaction, thereby preventing undesirable chronic inflammation. IL-10 also plays an essential role in the development of regulatory T (Tr) cells, which are key actors in controlling immune responses and tolerance in vivo.40,42 Induction of the development of Tr cells by Trx80 may be an additional mechanism for controlling immune responses.
Our findings that TAMs elicit only a small proliferative response in allogeneic PBMCs can be explained by the observations that these cells express low levels of HLA-DR and release the anti-inflammatory cytokine IL-10. In comparison with iDCs, this is a striking difference. TAMs and iDCs share the capacity to endocyte antigens. Once loaded with their cargo of antigens, iDCs migrate to the neighboring lymph nodes and become mature dendritic cells where they present peptides derived from antigens to T lymphocytes. Our preliminary studies adding TNF-α, anti-CD40, and LPS have shown that TAMs do not differentiate into dendritic cells. If further confirmed, these observations would imply that the primary role of TAMs would be as effectors of the innate immunity by phagocytosing infectious agents, rather than inducing an adaptive immune response.
Previous studies have shown that activation of p38 induces the maturation of MDDCs, whereas ERK inhibits this maturation.43,44 In contrast, in connection with the development of TAMs, p38, ERK, and JNK are all activated by phosphorylation. This activation is acquired early, during the first hour of exposure to Trx80. A deeper analysis will help to clarify which kinases are involved in the activation of specific cytokines. The phenotypic characteristics of these cells described here (after 3 and 5 days in culture) and in our previous study18 may be attributed to this activation.42,45
Both full-length Trx and Trx80 exhibit chemokine activity.9,20 The level of the full-length Trx protein has been shown to be elevated both in the synovial fluid of patients with arthritis and in atherosclerotic plaques.46,47 Moreover, production of both full-length Trx and Trx80 is increased upon stimulation of monocytes with, ie, endotoxins or hydrogen peroxide, and macrophages can cleave full-length Trx to produce truncated Trx in vitro.3,12-15,23,48
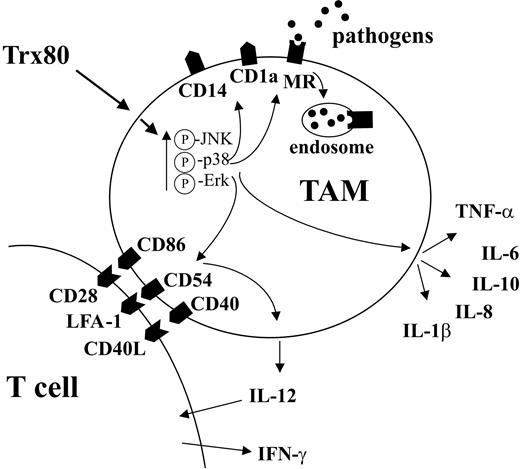
Thus, at the site of infection/inflammation, cells can be induced to produce full-length Trx and/or Trx80, whose chemoattractant properties could result in recruitment of monocytes and T cells.9,20 These monocytes can cleave full-length Trx to Trx80, as well as produce their own Trx80.23 This situation would then lead to activation of the monocytes by Trx80, up-regulating expression of MR, CD14, CD40, CD86, and CD54 and the release of cytokines and chemokines. Cross-talk between TAMs and T cells will stimulate the TAMs to produce IL-12, which, in turn, will subsequently trigger the production of IFN-γ by the T lymphocytes (Figure 5).18 Since CD14 and the MR play important roles in connection with innate immunity, as well as for the engulfment of apoptotic cells, these effects could be beneficial in helping the organism clear the infection.25,26,31-35
Proposed mechanism by which Trx80 stimulates differentiation of CD14+ monocytes into TAMs and the functional consequences of this differentiation. A schematic model of how Trx80 induces differentiation of CD14+ monocytes into TAMs. Please note that for IL-12 production, interaction with T cells is required. For further details, see “Discussion.”
Proposed mechanism by which Trx80 stimulates differentiation of CD14+ monocytes into TAMs and the functional consequences of this differentiation. A schematic model of how Trx80 induces differentiation of CD14+ monocytes into TAMs. Please note that for IL-12 production, interaction with T cells is required. For further details, see “Discussion.”
The findings described here shed new light on the functions of Trx80 in connection with both the inflammatory process and defenses against infectious microbes. On the basis of these findings, we propose that TAMs may play a major role in innate immunity and that Trx80 is one of the earliest stress signals elicited by the invasion of a foreign intruder.
Prepublished online as Blood First Edition Paper, October 19, 2004; DOI 10.1182/blood-2004-04-1577.
Supported by the Swedish Cancer Society (961), the Swedish Medical Research Council (project numbers 03X-3529, 03XS-13005, 74EF-15193, and 74X-7924), the Karolinska Institutet, the Swedish Asthma and Allergy Association's Research Foundation, the Swedish Council for Work Life Research, the Swedish Foundation for Health Care Sciences and Allergy Research, the Swedish Society for Medicine, and AMF-Sjukförsäkrings Jubilee Foundation for Research in National Diseases.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank Theresa Neimert-Andersson for her help in performance of the cytokine analysis and Gunilla Jacobsson Ekman for her help in producing the phase-contrast photographs.

![Figure 4. Stimulation of allogeneic PBMCs by TAMs and monocytes exposed to GM-CSF or medium alone. CD14+ monocytes isolated from 4 different donors were cultured for 3 days in medium alone or in the presence of either 100 nM Trx80 (TAMs) or GM-CSF + IL-4. These cells were subsequently harvested, irradiated, and tested as stimulators of freshly separated allogeneic PBMCs (responders). Each such stimulator cell preparation was tested with 3 or 4 different responder preparations, to give a total number of 14. For this purpose, the PBMCs (106/mL) were cultured in triplicate on 96-well plates in the presence of 1/10 to 1/10 000 as many stimulator cells. Following 5 days of such culture, 1 μCi (0.037 MBq) [3H]thymidine was added to each well 8 hours prior to termination of the incubation. The SI was calculated as the ratio of the radioactivity incorporated (cpm) by the responder cells in the presence of stimulator cells to the corresponding value for the responder cells cultured alone. The data presented are the median values obtained with the 14 different cell preparations of the responders which incorporated between 180 and 665 cpm tritiated thymidine when cultured alone. • indicates responders cells in the presence of stimulator cells exposed to GM-CSF + IL-4; ▵, responder cells cultured with TAMs; ♦, responder cells cultured together with stimulator cells exposed only to medium. The stimulatory index for stimulator cells cultured in the presence of GM-CSF + IL-4 was significantly higher than that for TAMs or monocytes cultured in medium alone (P < .05), as determined by the Wilcoxon matched-pairs test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-04-1577/6/m_zh80040574000004.jpeg?Expires=1769082176&Signature=wEJ-56gsdYfM2KsrWeUSZC9UPyIKVCkZ3p8MHq4fj~izY3FdjM3OPfIJTSRNQiuDVPuyb-yOdS-oAx-opd0vyQU~-RkInIe0RkRfDJ4VcI3dFdb9WubmQJpPSRqPfsVdsZMcRUy40Df-FDUPCmcBt6NFFKYkm6ZeZ58cDCuJdiU3lJG4QMaqhnWia7iWORFdcAE~~tFUz~ya7R6mnrJ4W4rjyxF3ogoThETeFLejO8TvRz5OXST80l6LrZ0mHrHBoV6zR31dVRJySioGiB7yZ3AtI-u3Z6nY~L0YlH9d91MkSg92OzbUNQPDsy6tySDIFhKmCUDJBnTT0ksxAypU5w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal