Abstract
Formation and rearrangement of disulfide bonds during the correct folding of nascent proteins is modulated by a family of enzymes known as thiol isomerases, which include protein disulfide isomerase (PDI), endoplasmic reticulum protein 5 (ERP5), and ERP57. Recent evidence supports an alternative role for this family of proteins on the surface of cells, where they are involved in receptor remodeling and recognition. In platelets, blocking PDI with inhibitory antibodies inhibits a number of platelet activation pathways, including aggregation, secretion, and fibrinogen binding. Analysis of human platelet membrane fractions identified the presence of the thiol isomerase protein ERP5. Further study showed that ERP5 is resident mainly on platelet intracellular membranes, although it is rapidly recruited to the cell surface in response to a range of platelet agonists. Blocking cell-surface ERP5 using inhibitory antibodies leads to a decrease in platelet aggregation in response to agonists, and a decrease in fibrinogen binding and P-selectin exposure. It is possible that this is based on the disruption of integrin function, as we observed that ERP5 becomes physically associated with the integrin β3 subunit during platelet stimulation. These results provide new insights into the involvement of thiol isomerases and regulation of platelet activation.
Introduction
In classical terms, reduction/oxidation systems within a cell have been represented very simply. The cytoplasmic environment is hypoxic and reducing, whereas the extracellular environment is normoxic and oxidizing. The generation of a disulfide bond from 2 cysteine residues is an oxidation reaction. To correctly generate these bonds inside the cell, there are, therefore, a group of enzymes known as the thiol isomerases. These are capable of the formation, reduction, and rearrangement of the disulfide-bonding patterns of proteins, often as part of folding of nascent proteins. The thiol isomerase enzymes are anchored to the endoplasmic reticulum via KDEL-receptor proteins.1-3 Recent studies have suggested additional functions for thiol isomerase enzymes: on the surface of cells, where they participate in receptor activation and remodeling, and substrate processing.4-6
Protein disulfide isomerase (PDI) is the best-characterized thiol isomerase to demonstrate this dual functionality. A number of cell types, including bovine aortic endothelial cells,7 rat hepatocytes,8,9 and human B cells,5,10 have been shown to secrete PDI, which associates with the cell surface. Cell-surface PDI has been implicated in the reduction of the disulfide-linked diptheria toxin heterodimer11 and events triggering the entry of HIV into lymphoid cells.6,12 On the basis of a series of investigations, initially by Detweiller and coworkers, a role for PDI in platelet physiology is now established.4,13-16 Early studies demonstrated PDI was present on the external membrane of activated and resting platelets, and proteins with thiol isomerase activity were secreted from activated platelets. Indeed, cell-surface exposure of free thiol groups, such as those from PDI, are elevated following platelet activation.17 Further studies demonstrated that inhibition of PDI with inhibitory antibodies can block a number of platelet responses, including aggregation, adhesion, fibrinogen binding, and integrin activation.16,18-20 Reagents that block cell-surface thiol groups, such as para-chloromercuriphenyl sulfonate, dithiobisnitrobenzoic acid, and bacitracin, have also been shown to inhibit these functions.19,21 This inhibition has often occurred to a greater degree than that observed for anti-PDI antibodies, indicating that additional proteins may be involved in this process.19,21 The reason for these observations has not been determined, although it has been proposed that they are based upon interaction with integrins, in particular integrins α2β1 and αIIbβ3.18,20 Studies have shown that the different affinity states for the ecto-domain of αIIbβ3 have different conformations, and evidence indicates switching between states is a redox active process with a different arrangement of disulfide bonds in the 2 conformations.22-24 It has been shown that αIIbβ3 and αVβ3 possess endogenous thiol isomerase activity,25 but it is not known if this activity is sufficient to promote the conformational change in either direction. However, there must be an additional level of regulation to prevent the receptors from being presented in a constitutively active form. It is possible that this could involve PDI, although, to date, the only physical association on the platelet surface that has been shown for PDI is with glycoprotein Ibα.17
In this study, we report the isolation of an additional thiol isomerase enzyme from human platelet membranes, which was identified as endoplasmic reticulum protein 5 (ERP5). Following platelet activation, levels of ERP5 on the platelet surface were rapidly elevated. Antibodies that block the thiol isomerase activity of ERP5 were found to inhibit platelet function. Notably, ERP5 was found to become associated with the integrin β3 subunit on platelet stimulation.
Materials and methods
Materials
Platelet agonists were collagen (Horm, type I from equine tendon; Nycomed, Munich, Germany); thrombin (Sigma, Poole, United Kingdom) and convulxin (convulxin was purified from snake venom and was a generous gift from Drs M. Leduc and C. Bon [Institut Pasteur, Paris, France]). Two venom preparations, with different potencies, were used. Horseradish peroxidase–conjugated secondary antibodies and the enhanced chemiluminescence detection system were from Amersham Biosciences (Buckinghamshire, United Kingdom); RNAse, from Roche (Lewes, United Kingdom); and bovine serum albumin, from First Link (Birmingham, United Kingdom). Anti-β3 clone AP3 was kindly provided by Prof P. Newman (Blood Research Institute of Southeastern Wisconsin, Medical College of Wisconsin, Milwaukee). Monoclonal anti-PDI (MA3-019) was from Affinity Bioreagents (Golden, CO); phycoerythrin (PE)–conjugated anti-CD62p and P-selectin, from BD Biosciences (Oxford, United Kingdom). The IV.3 hybridoma cell line (HB-217) was from ATCC (Manassas, VA). F(ab′) fragments of the IV.3 antibody were generated by means of the Immunopure kit (Pierce, Tattenhall, United Kingdom). All other reagents were purchased from Sigma. Protein concentrations were determined by Bradford assay (BioRad, Hemel Hempstead, United Kingdom).
ERP5 purification
Platelet membranes prepared from approximately 5 U blood (provided by Dr P. Smethurst, University of Cambridge, United Kingdom) were solubilized in buffer containing 1% (vol/vol) Triton X-100. Affinity chromatography was performed with 50 μg convulxin coupled to 200 μL sepharose 4B. Eluted fractions were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), Western blotted onto polyvinylidene difluoride (PVDF) membrane, and stained. Bands of approximately 50 kDa were subjected to protein sequencing by Edman degradation.
Antibody generation
Full-length ERP5 gene (cDNA clone provided by Prof M. Kikuchi, Ritsumeikan University, Japan)26,27 was cloned into the pGEX4T2 expression vector to generate an ERP5–glutathione S-transferase (ERP5-GST) fusion protein. Recombinant protein was located in inclusion bodies from Escherichia coli and isolated via urea solubilization and refolding to greater than 98% purity, as estimated by SDS-PAGE. Polyclonal antibodies were raised against the fusion protein and purified by means of protein G–sepharose. Specificity was determined by immunoblotting platelet lysates and comparing with anti-PDI, anti–calcium-binding protein 1 (anti-CaBP1) (anti-CaBP1 antibody to the rat homolog of ERP5 was a gift from Dr D. Ferrari, Max Planck Institute, Göttingen, Germany), and with antibody neutralized with recombinant ERP5. Polyclonal anti-PDI antibodies were raised in rabbits with the use of purified recombinant PDI (human PDI expression vector [pLWRP62] was provided by Dr L. W. Ruddock, Biocenter Oulu, Oulu, Finland).
Preparation of highly purified platelet plasma membranes (PMs) and intracellular membranes (IMs)
Platelet PMs and IMs were prepared as described in detail previously.28 Briefly, platelets were separated from human blood and treated with neuraminidase (type X, 0.05 U/mL) for 20 minutes at 37°C. After washing, platelets were disrupted by sonication, and the platelet homogenate was centrifuged at 42 000g for 90 minutes on a linear (1 to 3.5 M) sorbitol density gradient to obtain a mixed membrane (MM) fraction (free of granular contamination). MMs were separated into PMs and IMs by free-flow electrophoresis with an Octopus apparatus (FEE Weber, Planegg, Germany) running at 750 V, 100 mA. Two discrete peaks comprising PMs and IMs (more electronegative) were obtained. Tops of peaks were pooled, centrifuged (100 000g for 60 minutes), and resuspended in 0.4 M sorbitol, 5% glycerol, and 10 mM triethanolamine (pH 7.2).
Preparation and stimulation of washed platelets
Human platelets from drug-free volunteers were freshly prepared by differential centrifugation as described previously29 and resuspended in modified Tyrode/HEPES (Tyrode/N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid) buffer (134 mM NaCl, 0.34 mM Na2HPO4, 2.9 mM KCl, 12 mM NaHCO3, 20 mM HEPES, 5 mM glucose, and 1 mM MgCl2, pH 7.3).
Stimulation of platelets with collagen, convulxin, and thrombin was performed in an optical aggregometer (Chrono-log) at 37°C with continuous stirring. For platelet aggregation and flow cytometry studies, platelets were stimulated at 4 × 108/mL; for immunoprecipitation studies, platelets were stimulated at 1 × 109/mL. Where necessary, nonaggregating conditions were maintained by addition of EGTA (ethylene glycol tetraacetic acid) (1 mM), and secondary stimulation with released thromboxane A2 or secreted adenosine diphosphate (ADP) was prevented by inclusion of indomethacin (10 μM) and apyrase (2 U/mL), respectively.
Coimmunoprecipitation
Standard procedures for immunoprecipitation were followed.30 Platelets were lysed with ice-cold Nonidet P40 (NP-40) buffer (300 mM NaCl, 20 mM Tris [tris(hydroxymethyl)aminomethane], 10 mM EDTA [ethylenediaminetetraacetic acid], 2% NP-40, 1 mM phenylmethylsulfonyl fluoride, 2 mM Na3VO4, 10 μg/mL leupeptin, 10 μg/mL aprotonin, and 1 μg/mL pepstatin A, pH 7.3). Precleared lysates were incubated with specific antibodies (1 μg) and protein A (or G)–sepharose at 4°C with rotation for 90 minutes. Following SDS-PAGE and Western blotting onto PVDF, immunoblotting was performed by means of standard procedures.29 Primary antibodies were used at a concentration of 1 μg/mL. Horseradish peroxidase–conjugated secondary antibodies were diluted 1:10 000. Blots were stripped and reprobed to verify equivalent levels of protein loading. Enhanced chemiluminescence (ECL) images were collected on x-ray film.
Flow cytometry
Platelets were stimulated with agonist in the presence of EGTA (1 mM), indomethacin (10 μM), and apyrase (2 U/mL). Stimulation was terminated by addition of modified Tyrodes/HEPES buffer containing 1% (wt/vol) bovine serum albumin, 1 mM EGTA, and 200 μM sodium azide. Primary antibody was added at appropriate dilutions, and the buffer was incubated for 1 hour on ice. Secondary antibody (fluorescein isothiocyanate–conjugated immunoglobulin G [IgG]) was used at 1:2000 dilution and incubated for 1 hour on ice in the dark. Data were collected and analyzed by means of a Becton Dickinson (Oxford, United Kingdom) FACScan flow cytometer and CELLQuest software.
Fibrinogen binding
Fluorescein isothiocyanate (FITC)–labeled human fibrinogen binding was measured as for flow cytometry with the omission of EGTA from all buffers. Where required, samples were incubated with α-ERP5 antibodies or control preimmune antibodies prior to platelet stimulation. All samples were preincubated with saturating concentrations of a F(ab′) fragment of monoclonal antibody IV.3 to prevent signaling through the FcγRIIA receptor. Saturating concentrations were determined as the amount of IV.3 F(ab′) fragment, preincubated with platelets, required to completely inhibit platelet aggregation caused by subsequent incubation with whole IV.3 and cross-linking using anti–mouse IgG2a F(ab′)2 fragment.31
RNAse activity assay
Thiol isomerase activity was assessed by the ability to renature reduced and denatured RNAse (rdRNAse). The assay was performed as outlined by O'Neill et al25 and Pigiet and Schuster.32 Reactivated RNAse was assayed by the degradation of cyclic 2′-3′cytidine monophosphate (cCytP), measured by increase in absorbance at 284 nm in refolding buffer (50 mM NaPO4, pH 7.2; 50 mM NaCl; 1.5 mM glutathione; 500 μM glutathione disulfide; 400 μM CaCl2; and 400 μM MgCl2). Controls of rdRNAse only, cCytP substrate only (blank), protein, and cCytP substrate but no rdRNAse were run with each set of experiments. The activity was expressed relative to native RNAse, or as a percentage of inhibition of activity for antibody-blocking experiments.
Calcium mobilization assay
Mobilization of calcium from intracellular stores was measured by means of a fast-filter ratiometric technique based on fluorescence of the calcium-binding compound Fura-2.31,33 Platelets (approximately 5 × 109/mL) were loaded with Fura-2-am/HEPES (5 μM) and resuspended at 4 × 108/mL in Tyrodes/HEPES buffer.31,33 Prior to stimulation, EGTA (1 mM) was added to each sample. Data were collected on a Perkin Elmer (Beaconsfield, United Kingdom) LS50B fluorimeter and analyzed by means of FLWinLab software as described prevously.31,33
Data analysis
Data were analyzed with SPSS (Chicago, IL) software to calculate 2-tailed paired t test at a 95% confidence value.
Results
ERP5 is present in human platelets and is associated with intracellular and plasma membranes
An unknown protein was purified from human platelet membrane fractions by means of a convulxin affinity column. Convulxin, isolated from the venom of the rattlesnake, possesses a high affinity for the platelet glycoproteins (GPs) GPVI and GPIb.34,35 N-terminal sequence data were obtained for the first 15 residues of the protein (LYSSSDDVIELTPSN). A BLAST search36 revealed this to be identical to the sequence of ERP5 following cleavage of a predicted signal sequence.
ERP5 was cloned by Hayano and Kikuchi26 from a placental cDNA library while screening for proteins related to protein disulfide isomerase, PDI. The gene sequence encodes a 48-kDa protein containing 2 active thioredoxin domains (containing CGHC motifs) that share 47% amino acid sequence identity with human PDI, a C-terminal peptide-binding domain, and a KDEL sequence for retention in the endoplasmic reticulum.26 Sequence alignment studies by Ferrari and Soling (Ferrari and Soling2 ; Kramer et al37 ) suggest that ERP5 and PDI share a similar domain structure.
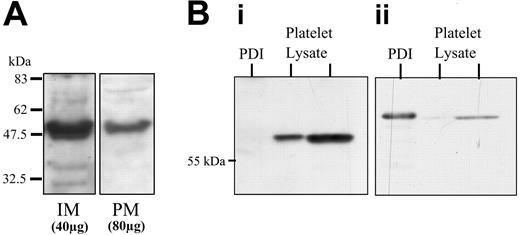
Given its expected restriction to the ER, the presence of ERP5 in membrane fractions was confirmed by immunoblot analysis (not shown). The membrane association was examined more closely with the use of IMs and PMs from resting platelets isolated by free-flow electrophoresis (Figure 1A). ERP5 was present on both intracellular and plasma membranes. The IM fraction contains membranes of intracellular origin such as endoplasmic reticulum, but excludes granule membranes and surface-connected membranes. The higher loading of PMs indicates that the predominant location of ERP5 in resting platelets is the intracellular membranes. The purity of platelet membrane fractions was assessed by complete separation of the 2 peaks following free-flow electrophoresis, the absence of sarcoplasmic/endoplasmic reticulum calcium adenosine triphosphatase 2b (SERCA 2b) in PM, and the absence of GP1b in IM (not shown). Full characterization of these fractions has been extensively reported previously.28,38 ERP5 was detected with the use of polyclonal antibodies raised to a GST-fusion protein containing full-length human ERP5. On Western blots (Figure 1B), a protein was detected of the same mobility as that recognized by antibodies to CaBP1, the rat homolog of ERP5 (not shown).37 These antibodies showed no cross-reactivity with human recombinant and platelet PDI (Figure 1B).
Localization of ERP5 and specificity of anti-ERP5 antibodies. (A) Localization of ERP5 to intracellular and plasma membranes of human platelets. Human (IM 40 μg) and PM (80 μg) membranes from resting platelets prepared by high-voltage free-flow electrophoresis were separated by SDS-PAGE and immunoblotted. ERP5 protein was detected by means of specific polyclonal antibodies. (B) Specificity of anti-ERP5 antibodies. Recombinant human PDI (4 μg) and human platelet lysates (4 μg and 20 μg) were Western blotted and probed for ERP5 (i) and PDI (ii) by means of sheep polyclonal anti-ERP5 and mouse monoclonal anti-PDI, respectively.
Localization of ERP5 and specificity of anti-ERP5 antibodies. (A) Localization of ERP5 to intracellular and plasma membranes of human platelets. Human (IM 40 μg) and PM (80 μg) membranes from resting platelets prepared by high-voltage free-flow electrophoresis were separated by SDS-PAGE and immunoblotted. ERP5 protein was detected by means of specific polyclonal antibodies. (B) Specificity of anti-ERP5 antibodies. Recombinant human PDI (4 μg) and human platelet lysates (4 μg and 20 μg) were Western blotted and probed for ERP5 (i) and PDI (ii) by means of sheep polyclonal anti-ERP5 and mouse monoclonal anti-PDI, respectively.
Flow cytometry was employed to confirm cell-surface expression of ERP5 and investigate whether this was a static or dynamic process. Washed platelets were stimulated with agonists convulxin, collagen, or thrombin, and the concentration- and time-dependent patterns of cell-surface exposure for ERP5 were studied (Figure 2). Low levels of cell-surface ERP5 were found to be substantially and rapidly increased following stimulation with each agonist in a concentration-dependent manner. To investigate the trends observed in time-dependence profiles, data were normalized to the greatest response within an individual experiment and averaged to overcome donor variability (Figure 2C). All 3 agonists demonstrate biphasic profiles, where there is an initial rapid increase in cell-surface exposure, which peaks at approximately 45 seconds, with substantial increases seen as rapidly as 15 seconds. For convulxin and thrombin, this is followed by a lag phase where exposure levels are maintained or dip slightly between 60 and 120 seconds before beginning to rise again over 150 to 300 seconds. These data suggest there are secondary effectors or mechanisms present for promoting a second wave of cell-surface exposure of ERP5 in response to these agonists. These experiments were performed in the presence of EGTA, apyrase, and indomethacin, which block the second wave of platelet aggregation responses based on fibrinogen, ADP, and thromboxane A2. Microaggregates based upon αIIbβ3 interactions have been reported to form in the presence of EGTA39 ; this may account for a second wave of signaling. A different profile was observed for the time-dependent increase in cell-surface exposure of ERP5 with the agonist collagen. Again, there was a rapid increase, which peaked at approximately 45 seconds, but these levels decreased to approximately baseline values by 300 seconds.
Increase in ERP5 cell-surface expression in a concentration- and time-dependent manner in response to platelet stimulation by convulxin, collagen, or thrombin. (A) Concentration dependence with 90-second stimulation. (i) Stimulation by convulxin (Cvx). Basal level is indicated by filled histogram; stimulation with 10 ng/mL Cvx, by an empty histogram; and stimulation with 40 ng/mL Cvx, by an empty dotted-line histogram. (ii) Stimulation with collagen (Coll). Basal level is indicated by a filled histogram; stimulation with 25 μg/mL Coll, by an empty histogram; and stimulation with 100 μg/mL Coll, by an empty dotted-line histogram. (iii) Stimulation with thrombin (Thr). Basal level is indicated by filled histogram; stimulation with 0.2 U/mL Thr, by an empty histogram; and stimulation with 1.0 U/mL Thr, by an empty dotted-line histogram. (B) Time dependence. (i) Basal level is indicated by filled histogram; 45-second stimulation with 40 ng/mL Cvx by an empty histogram; and 300-second stimulation with 40 ng/mL Cvx, by an empty dotted-line histogram. (ii) Stimulation by Coll. Basal level is indicated by a filled histogram; 45-second stimulation with 25 μg/mL Coll by an empty histogram; and 300-second stimulation with 25 μg/mL Coll, by an empty dotted-line histogram. (iii) Stimulation by Thr. Basal level is indicated by a filled histogram; 45-second stimulation with 1.0 U/mL Thr, by an empty histogram; and 300-second stimulation with 1.0 U/mL Thr, by an empty dotted-line histogram. (C) Normalized plots for the increase in cell-surface exposure observed for ERP5 over time are given for convulxin (i), collagen (ii), and thrombin (iii). Data are presented as mean ± standard error (SE) (n = 3), where 100% represents the maximal response detected.
Increase in ERP5 cell-surface expression in a concentration- and time-dependent manner in response to platelet stimulation by convulxin, collagen, or thrombin. (A) Concentration dependence with 90-second stimulation. (i) Stimulation by convulxin (Cvx). Basal level is indicated by filled histogram; stimulation with 10 ng/mL Cvx, by an empty histogram; and stimulation with 40 ng/mL Cvx, by an empty dotted-line histogram. (ii) Stimulation with collagen (Coll). Basal level is indicated by a filled histogram; stimulation with 25 μg/mL Coll, by an empty histogram; and stimulation with 100 μg/mL Coll, by an empty dotted-line histogram. (iii) Stimulation with thrombin (Thr). Basal level is indicated by filled histogram; stimulation with 0.2 U/mL Thr, by an empty histogram; and stimulation with 1.0 U/mL Thr, by an empty dotted-line histogram. (B) Time dependence. (i) Basal level is indicated by filled histogram; 45-second stimulation with 40 ng/mL Cvx by an empty histogram; and 300-second stimulation with 40 ng/mL Cvx, by an empty dotted-line histogram. (ii) Stimulation by Coll. Basal level is indicated by a filled histogram; 45-second stimulation with 25 μg/mL Coll by an empty histogram; and 300-second stimulation with 25 μg/mL Coll, by an empty dotted-line histogram. (iii) Stimulation by Thr. Basal level is indicated by a filled histogram; 45-second stimulation with 1.0 U/mL Thr, by an empty histogram; and 300-second stimulation with 1.0 U/mL Thr, by an empty dotted-line histogram. (C) Normalized plots for the increase in cell-surface exposure observed for ERP5 over time are given for convulxin (i), collagen (ii), and thrombin (iii). Data are presented as mean ± standard error (SE) (n = 3), where 100% represents the maximal response detected.
ERP5 protein has thiol isomerase activity
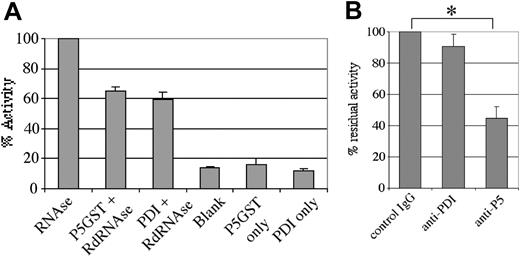
Based upon refolding of reduced denatured RNAse, previous studies have demonstrated thiol isomerase activity for bovine liver ERP5, CaBP1, and human PDI.37 To verify that human ERP5 is a functionally active thiol isomerase, we analyzed the recombinant fusion protein in this assay system (Figure 3A). The protein was found to possess thiol isomerase activity, with activity approximately 70% of that measured for molar equivalents of the PDI recombinant fusion protein. Human ERP5 immunoprecipitated from platelet samples with the use of a nonfunction-blocking antibody also demonstrated thiol isomerase activity (not shown). It was found that, under the assay conditions employed, thiol isomerase activity of both ERP5-GST and PDI-histidine was dependent on divalent cations and inhibited in the presence of EDTA (not shown). This is opposite to the observed thiol isomerase activity profile for the integrin subunit β3, which has been shown to display enhanced activity in the presence of EDTA.25 Such differential cation dependence for thiol isomerase activity may be important in the cellular regulation of the activity of these proteins and may imply distinct functions.
Thiol isomerase activity of human ERP5. Thiol isomerase activity was assessed as the ability to refold denatured, scrambled RNAse and enhance the degradation of cyclic 2′,3′-cytidine monophosphate, as followed by ultraviolet/visible (UV/vis) spectroscopy. (A) Activity was observed for a recombinant ERP5 fusion protein (21 μg/mL) and a recombinant PDI fusion protein (11 μg/mL) relative to samples containing only cyclic 2′,3′-cytidine monophosphate (blank) or only fusion protein. (B) Anti-ERP5 polyclonal antibodies (24 μg/mL) raised in sheep were able to partially inhibit the thiol isomerase activity of a recombinant ERP5 fusion protein (30 μg/mL). Preimmune IgG (24 μg/mL) and monoclonal anti-PDI antibodies (28 μg/mL) were found to possess no such inhibitory activity. Data are presented as mean ± SE from 3 different determinations. *P < .05.
Thiol isomerase activity of human ERP5. Thiol isomerase activity was assessed as the ability to refold denatured, scrambled RNAse and enhance the degradation of cyclic 2′,3′-cytidine monophosphate, as followed by ultraviolet/visible (UV/vis) spectroscopy. (A) Activity was observed for a recombinant ERP5 fusion protein (21 μg/mL) and a recombinant PDI fusion protein (11 μg/mL) relative to samples containing only cyclic 2′,3′-cytidine monophosphate (blank) or only fusion protein. (B) Anti-ERP5 polyclonal antibodies (24 μg/mL) raised in sheep were able to partially inhibit the thiol isomerase activity of a recombinant ERP5 fusion protein (30 μg/mL). Preimmune IgG (24 μg/mL) and monoclonal anti-PDI antibodies (28 μg/mL) were found to possess no such inhibitory activity. Data are presented as mean ± SE from 3 different determinations. *P < .05.
The effect on enzymatic activity of ERP5 by antibodies to ERP5 was investigated. Antibodies raised in sheep against recombinant ERP5 were found to inhibit enzyme activity, whereas preimmune IgG and monoclonal antibodies against human PDI displayed no effect on activity (Figure 3B). In addition, anti-ERP5 antibodies showed no inhibitory effect on the thiol isomerase activity of recombinant human PDI (not shown). It was not possible to completely block the thiol isomerase activity of ERP5-GST, even at very high antibody concentrations, consistent with studies performed on PDI with function-blocking antibodies.
Platelet aggregation is inhibited by inhibition of ERP5 activity
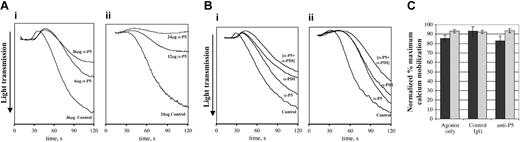
Activity-blocking anti-ERP5 antibodies were used to investigate the potential involvement of ERP5 in the regulation of platelet function. Platelets were stimulated with collagen or convulxin following incubation with anti-ERP5 antibodies or control IgG purified from preimmune serum from the animal used to raise the antibodies. Prior to addition of inhibitory antibodies, platelets were incubated with saturating concentrations of F(ab′) fragment of the monoclonal antibody IV.3 to prevent signaling through the FcγRIIa receptor.31 The traces shown in Figure 4 demonstrate that anti-ERP5 antibodies inhibit the aggregation response induced by low concentrations of convulxin and collagen. Platelet aggregation was reduced substantially by 6 μg/mL anti-ERP5 in response to 2.5 μg/mL collagen. The aggregation profile was reversible, with platelets showing signs of disaggregating after 120 seconds. Addition of higher concentrations of anti-ERP5 antibodies further decreased the level of aggregation observed. For convulxin, aggregation was reduced substantially following incubation with 12 μg/mL antibody, and it was possible to completely inhibit aggregation at higher antibody concentrations. For both collagen and convulxin, preincubation with antibodies did not inhibit shape change. At higher concentrations of collagen and convulxin, it was possible to overcome inhibitory effects of anti-ERP5 antibodies. The additive effect of anti-ERP5 and anti-PDI antibodies on platelet aggregation was investigated (Figure 4B). Submaximal antibody concentrations (with regard to inhibitory activity) were used in these experiments because at high concentrations aggregation is completely inhibited. Total antibody concentrations were controlled for using preimmune IgG in samples containing only a single inhibitory antibody. The results indicate that there is a modest additive effect on the inhibition of platelet aggregation when anti-ERP5 and anti-PDI antibodies are used in combination. This does not appear to be synergistic, as there is no enhancement of the effect when the 2 antibodies are combined.
Inhibition of platelet aggregation by an inhibitory antibody for ERP5. Platelets (4 × 108/mL) were incubated with anti-ERP5 IgG or control IgG at the given concentrations for 2.5 minutes prior to addition of agonist. Prior to the addition of antibodies, platelets were preincubated with a saturating concentration of an F(ab′) fragment of the IV.3 protein to prevent signaling through the FcγRIIa receptor. Control IgG was purified from the preimmune serum of the animal in which antibodies were raised. (A) Concentration effect of inhibitory antibodies. (i) Concentration effect of 2.5 μg/mL collagen incubated with preimmune IgG (36 μg/mL); anti-ERP5 antibody (6 μg/mL); or anti-ERP5 antibody (36 μg/mL). (ii) Concentration effect of 30 ng/mL convulxin incubated with preimmune IgG (24 μg/mL); anti-ERP5 antibody (12 μg/mL); or anti-ERP5 antibody (24 μg/mL). (B) Additive effect of inhibitory antibodies for ERP5 and PDI. (i) Additive effect of 4.0 μg/mL collagen incubated with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). (ii) Additive effect of 150 ng/mL convulxin incubated with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). Traces shown are representative of those observed for at least 3 different donors. (C) Mobilization of calcium from intracellular stores. Ca2+ release was measured by Fura-2 fluorescence in platelets stimulated with convulxin (300 ng/mL, ▪) or collagen (4 μg/mL, ▦). Prior to stimulation, platelets were incubated with anti-ERP5 antibodies or control antibodies (24 μg/mL) from preimmune sera. Data are presented as mean ± SE for 3 separate experiments.
Inhibition of platelet aggregation by an inhibitory antibody for ERP5. Platelets (4 × 108/mL) were incubated with anti-ERP5 IgG or control IgG at the given concentrations for 2.5 minutes prior to addition of agonist. Prior to the addition of antibodies, platelets were preincubated with a saturating concentration of an F(ab′) fragment of the IV.3 protein to prevent signaling through the FcγRIIa receptor. Control IgG was purified from the preimmune serum of the animal in which antibodies were raised. (A) Concentration effect of inhibitory antibodies. (i) Concentration effect of 2.5 μg/mL collagen incubated with preimmune IgG (36 μg/mL); anti-ERP5 antibody (6 μg/mL); or anti-ERP5 antibody (36 μg/mL). (ii) Concentration effect of 30 ng/mL convulxin incubated with preimmune IgG (24 μg/mL); anti-ERP5 antibody (12 μg/mL); or anti-ERP5 antibody (24 μg/mL). (B) Additive effect of inhibitory antibodies for ERP5 and PDI. (i) Additive effect of 4.0 μg/mL collagen incubated with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). (ii) Additive effect of 150 ng/mL convulxin incubated with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). Traces shown are representative of those observed for at least 3 different donors. (C) Mobilization of calcium from intracellular stores. Ca2+ release was measured by Fura-2 fluorescence in platelets stimulated with convulxin (300 ng/mL, ▪) or collagen (4 μg/mL, ▦). Prior to stimulation, platelets were incubated with anti-ERP5 antibodies or control antibodies (24 μg/mL) from preimmune sera. Data are presented as mean ± SE for 3 separate experiments.
Agonist-stimulated mobilization of calcium from intracellular stores was measured to determine whether anti-ERP5 antibodies affected platelet activation signaling itself and thereby reduced platelet activation. Mobilization of calcium from intracellular stores was not affected by incubation of platelets with anti-ERP5 antibodies (Figure 4C). Platelet activation signaling per se is therefore unaffected by anti-ERP5 treatment, but partial inhibition of ERP5 affects downstream functional responses that lead to aggregation.
ERP5 is implicated in the regulation of fibrinogen binding
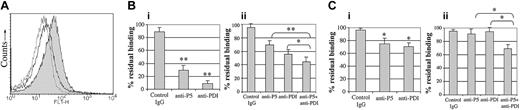
In view of the inhibition of aggregation observed following blockade of ERP5 and previous studies that have reported anti-PDI–mediated inhibition of fibrinogen binding,4,19 we investigated the ability of platelets to bind fibrinogen in the presence of inhibitory anti-ERP5 antibodies. Flow cytometry was used to measure the binding of FITC-labeled fibrinogen to collagen- and convulxin-stimulated platelets (Figure 5). A greater shift in fluorescence is observed by flow cytometry at higher agonist concentrations. Therefore, to increase the dynamic range for these experiments, higher agonist concentrations relative to the aggregation studies were used. Stimulation of platelets resulted in an increase in the level of binding of FITC-fibrinogen, consistent with an increase in affinity of integrin αIIbβ3.40,41 Incubation of platelets with anti-ERP5 antibodies or monoclonal anti-PDI antibodies resulted in a significant decrease in platelet binding to fibrinogen for both agonists. In response to 100 ng/mL convulxin, platelet binding of fibrinogen reduced by 70% and 91% for anti-ERP5 (P < .005) and anti-PDI (P < .005) antibodies, respectively. When 10 μg/mL collagen was used, the reduction in fibrinogen binding was more modest: 25% and 29% for anti-ERP5 (P < .05) and anti-PDI (P < .05), respectively. This is consistent with the more modest inhibitory effect of anti-ERP5 on collagen-stimulated platelet aggregation. Preincubation of platelets with IgG from preimmune sera had no effect on levels of FITC-fibrinogen binding. The effect of a combination of anti-ERP5 and anti-PDI antibodies on fibrinogen binding was studied (Figure 5Bii,Cii). For both convulxin- and collagen-stimulated platelets, an additive effect was observed for the reduction in platelet binding of fibrinogen at submaximal concentrations of inhibitory antibodies. For either single antibody with respect to a combination of antibodies (anti-ERP5 plus anti-PDI), the difference in fibrinogen binding is significant (P < .05), but does not appear to be synergistic. At higher concentrations of inhibitory antibodies, this effect is reduced, and a less-than-additive effect is observed (data not shown).
Inhibition of fibrinogen binding in platelets following blocking of cell-surface ERP5. Binding of FITC-labeled fibrinogen was measured with the use of flow cytometry on platelets stimulated with the agonists convulxin or collagen. Prior to stimulation, platelets were incubated with anti-ERP5 antibodies, anti-PDI antibodies, or control antibodies from preimmune sera. (A) Histogram for fluorescence of FITC-fibrinogen labeled platelets in response to the agonist convulxin (100 ng/mL). Control IgG (12 μg/mL) is indicated by a filled histogram; anti-ERP5 antibody (12 μg/mL), by an empty histogram; and anti-PDI antibody (33 μg/mL), by a dotted-line empty histogram. (Bi) Residual binding of FITC-fibrinogen following incubation of platelets with preimmune IgG (12 μg/mL), anti-ERP5 antibody (12 μg/mL), or anti-PDI antibody (33 μg/mL). Agonists were 100 ng/mL convulxin. (Bii) Additive effect of inhibitory antibodies on residual binding of FITC-fibrinogen following incubation of platelets with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). Agonists were 300 ng/mL convulxin. (Ci) Residual binding of FITC-fibrinogen following incubation of platelets with antibodies as described for panel Bi. Agonists were 10 μg/mL collagen. (Cii) Additive effect of inhibitory antibodies on residual binding of FITC-fibrinogen following incubation of platelets with antibodies as described for panel Bii. Agonists were 4 μg/mL collagen. Data are presented as mean ± SE for 4 separate experiments (*P < .05; **P < .005).
Inhibition of fibrinogen binding in platelets following blocking of cell-surface ERP5. Binding of FITC-labeled fibrinogen was measured with the use of flow cytometry on platelets stimulated with the agonists convulxin or collagen. Prior to stimulation, platelets were incubated with anti-ERP5 antibodies, anti-PDI antibodies, or control antibodies from preimmune sera. (A) Histogram for fluorescence of FITC-fibrinogen labeled platelets in response to the agonist convulxin (100 ng/mL). Control IgG (12 μg/mL) is indicated by a filled histogram; anti-ERP5 antibody (12 μg/mL), by an empty histogram; and anti-PDI antibody (33 μg/mL), by a dotted-line empty histogram. (Bi) Residual binding of FITC-fibrinogen following incubation of platelets with preimmune IgG (12 μg/mL), anti-ERP5 antibody (12 μg/mL), or anti-PDI antibody (33 μg/mL). Agonists were 100 ng/mL convulxin. (Bii) Additive effect of inhibitory antibodies on residual binding of FITC-fibrinogen following incubation of platelets with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). Agonists were 300 ng/mL convulxin. (Ci) Residual binding of FITC-fibrinogen following incubation of platelets with antibodies as described for panel Bi. Agonists were 10 μg/mL collagen. (Cii) Additive effect of inhibitory antibodies on residual binding of FITC-fibrinogen following incubation of platelets with antibodies as described for panel Bii. Agonists were 4 μg/mL collagen. Data are presented as mean ± SE for 4 separate experiments (*P < .05; **P < .005).
α-granule secretion is inhibited by anti-ERP5
P-selectin exposure, a marker of α-granule secretion,42,43 was inhibited in response to the agonists collagen and convulxin by blocking ERP5 or PDI with specific function-blocking antibodies (Figure 6). In response to 100 ng/mL convulxin, there was a decrease in surface exposure for P-selectin of 73% and 94% in the presence of anti-ERP5 (P < .01) and anti-PDI (P < .001) antibodies, while in response to 10 μg/mL collagen a decrease of 39% (P < .02) and 46% (P < .005) was measured, respectively. The effect of a combination of anti-ERP5 and anti-PDI antibodies on P-selectin exposure was also studied (Figure 6Bii,Cii.). For both convulxin- and collagen-stimulated platelets, an additive effect was observed for the reduction in the cell-surface expression of P-selectin at submaximal concentrations of inhibitory antibodies. In a manner similar to fibrinogen binding, a combination of antibodies led to results significantly different (P < .05) from those obtained by the use of a single antibody. This held for all examples except for anti-ERP5 following stimulation with convulxin. Again at higher concentrations of inhibitory antibodies, a less-than-additive effect was observed (not shown).
Inhibition of P-selectin expression in platelets following blocking of cell-surface ERP5. Binding of PE-conjugated anti-CD62p was measured by means of flow cytometry on platelets stimulated with the agonists convulxin or collagen. Prior to stimulation, platelets were incubated with anti-ERP5 antibodies, anti-PDI antibodies, or control antibodies from preimmune sera. (A) Histogram for fluorescence of PE anti-CD62p–labeled platelets in response to the agonist convulxin (100 ng/mL). Control IgG (12 μg/mL) is indicated by a filled histogram; anti-ERP5 antibody (12 μg/mL), by an empty histogram; and anti-PDI antibody (33 μg/mL), by a dotted-line empty histogram. (B,C) Data are presented as mean ± SE for 4 separate experiments. *P < .05. **P < .005. (Bi,Ci) Residual binding of PE anti-CD62p following incubation of platelets with preimmune IgG (12 μg/mL), anti-ERP5 antibody (12 μg/mL), anti-PDI antibody (33 μg/mL). Agonists were 100 ng/mL convulxin (Bi); and 10 μg/mL collagen (Ci). (Bii,Cii) Additive effect for inhibitory antibodies on residual binding of PE anti-CD62p following incubation of platelets with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). Agonists were 300 ng/mL convulxin (Bii) and 4 μg/mL collagen (Cii).
Inhibition of P-selectin expression in platelets following blocking of cell-surface ERP5. Binding of PE-conjugated anti-CD62p was measured by means of flow cytometry on platelets stimulated with the agonists convulxin or collagen. Prior to stimulation, platelets were incubated with anti-ERP5 antibodies, anti-PDI antibodies, or control antibodies from preimmune sera. (A) Histogram for fluorescence of PE anti-CD62p–labeled platelets in response to the agonist convulxin (100 ng/mL). Control IgG (12 μg/mL) is indicated by a filled histogram; anti-ERP5 antibody (12 μg/mL), by an empty histogram; and anti-PDI antibody (33 μg/mL), by a dotted-line empty histogram. (B,C) Data are presented as mean ± SE for 4 separate experiments. *P < .05. **P < .005. (Bi,Ci) Residual binding of PE anti-CD62p following incubation of platelets with preimmune IgG (12 μg/mL), anti-ERP5 antibody (12 μg/mL), anti-PDI antibody (33 μg/mL). Agonists were 100 ng/mL convulxin (Bi); and 10 μg/mL collagen (Ci). (Bii,Cii) Additive effect for inhibitory antibodies on residual binding of PE anti-CD62p following incubation of platelets with preimmune IgG (36 μg/mL); anti-ERP5 (12 μg/mL) plus preimmune IgG (19 μg/mL); anti-PDI (6 μg/mL) plus preimmune IgG (19 μg/mL); or anti-ERP5 (12 μg/mL) plus anti-PDI (6 μg/mL). Agonists were 300 ng/mL convulxin (Bii) and 4 μg/mL collagen (Cii).
The data obtained in the thiol isomerase assay (Figure 3; also O'Neill et al25 ) demonstrate that inhibitory antibodies are incapable of completely blocking enzyme activity. Therefore, even in the presence of high concentrations of inhibitory antibodies, there will still be low levels of thiol isomerase activity on resting and activated platelets. Thus, it is hard to determine the relative contributions of ERP5 and PDI proteins to fibrinogen binding and P-selectin exposure assays used here. However, these data strongly implicate cell-surface ERP5 and PDI in the regulation of platelet thrombus formation.
ERP5 associates with integrin β3 in stimulated platelets
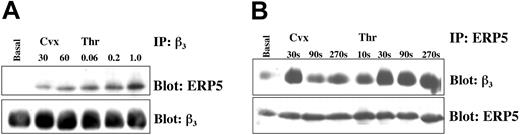
To investigate whether there was a direct association between ERP5 and the fibrinogen receptor, integrin αIIbβ3, coimmunoprecipitation studies were performed. The integrin β3 subunit was found to associate with ERP5 in platelets activated by the agonists convulxin and thrombin (Figure 7). This association was observed with the use of complementary experimental techniques (ie, for immunoprecipitation using either anti-ERP5 or anti-β3 antibodies). The degree of association was agonist-concentration and time dependent, increasing with increasing concentrations of agonist, and peaking at approximately 30 seconds after stimulation. The data shown in Figure 2C for the cell-surface exposure of ERP5, demonstrate there is a peak at approximately 45 seconds for the exposure of ERP5 in response to both of the agonists convulxin and thrombin.
Stimulation-dependent association of ERP5 with integrin β3. Platelets (1 × 109/mL) were stimulated in the presence of EGTA, apyrase, and indomethacin at varying concentrations of convulxin (ng/mL) or thrombin (U/mL) for 90 seconds (panel A), or at fixed concentrations of agonist (100 ng Cvx, 1.0 U Thr) for increasing durations (panel B). Following sample lysis, proteins were precipitated and separated with specific antibodies and protein A–sepharose. Immunoblotting was used to show interacting proteins. Blots were stripped and reprobed to verify equivalent levels of target antigen in each sample lane.
Stimulation-dependent association of ERP5 with integrin β3. Platelets (1 × 109/mL) were stimulated in the presence of EGTA, apyrase, and indomethacin at varying concentrations of convulxin (ng/mL) or thrombin (U/mL) for 90 seconds (panel A), or at fixed concentrations of agonist (100 ng Cvx, 1.0 U Thr) for increasing durations (panel B). Following sample lysis, proteins were precipitated and separated with specific antibodies and protein A–sepharose. Immunoblotting was used to show interacting proteins. Blots were stripped and reprobed to verify equivalent levels of target antigen in each sample lane.
Similar experiments were performed to examine the potential interaction of PDI with β3, but no such interaction was observed. Interactions between ERP5 and PDI were also not observed.
Discussion
Recent studies have developed the concept of redox-controlled receptor remodeling as part of the activation process in platelets. It has been proposed that these reactions are based on thiol isomerase activity: the ability to generate, reduce, or rearrange disulfide bonds in proteins.23-25 Resting platelets display low levels of thiol isomerase activity on the cell surface, and these levels are enhanced dramatically when platelets are stimulated by agonists.17 The functional importance of this activity is demonstrated by the fact that blocking thiol isomerases inhibits a number of key events in the platelet activation process, including adhesion, aggregation, fibrinogen binding, and P-selectin exposure.4,19,20 The only thiol isomerase enzyme previously characterized in platelets is PDI. We report the presence of an additional thiol isomerase enzyme, ERP5, on the surface of platelets. The contribution to the cell-surface thiol isomerase activity by other enzymes, such as ERP5, could be the basis for the observation that chemical modification reagents consistently inhibit platelet activation markers to a greater extent than specific antibodies that inhibit PDI.
In theory, a small number of thiol isomerases could activate a large number of receptors, as they do not have to form long-term stable complexes. The balance in this scenario will be time, because fewer proteins will take longer to activate all receptors. Indeed, limiting surface expression could be seen as another form of setting the gain, or threshold, for platelet activation by modulating the response time for complete activation. These characteristics of extended periods of shape change and slower onset of aggregation are observed when platelets are incubated with low levels of inhibitory antibodies.
Shuttling of receptors between internal organelles and the cell surface is a common phenomenon, and recent studies have shown that cell-surface expression of GluR5 kainate receptors is regulated by an endoplasmic reticulum retention signal.44 Both ERP5 and PDI are recruited to the cell surface, as shown in Figure 2 and Burgess et al,17 respectively, although the intracellular source, whether α-granules or IMs, is presently unclear. The kinetics of recruitment of ERP5 are similar to α-granule secretion. In response to convulxin and thrombin, there is a biphasic profile with an initial peak at approximately 60 seconds followed by prolonged increase in exposure. For collagen, following an initial peak in exposure, cell-surface levels of ERP5 return to basal after 5 minutes. It may be that stimulation with collagen is unable to mobilize a second wave of cell-surface exposure for ERP5, but given that collagen and Cvx are both able to stimulate platelet activation via GPVI, this would be surprising.
Until recently, it would have been easy to attribute the differences observed in the time-dependent exposure profiles to the fact that these agonists stimulate platelets through different signaling pathways: thrombin through G-protein–coupled receptor pathways via protease-activated receptor 1 (PAR1) and PAR4,45,46 collagen through GPVI and the integrin α2β1,47,48 and convulxin through the receptor GPVI.34 However, recent reports have suggested that this distinction is less clear; convulxin has been shown to bind GP1b,35 and it has been proposed that the central receptor responsible for collagen signaling is GPVI,49,50 with integrin α2β1 being responsible primarily for adhesion to collagen. Thus, one may expect thrombin, but not collagen, to be distinct. Previous reports have indicated a link between thiol isomerase activity of PDI and integrin activation.18 It is possible, therefore, that the different profiles seen are based on a separate pathway following activation via integrins as opposed to other stimuli. In platelets, Wang et al51 have observed internalization of soluble collagen via the integrin α2β1 over a period of 30 minutes. It is possible that ERP5 may internalize with α2β1, although under the conditions presented in this study, the kinetics of disappearance of ERP5 from the platelet surface are faster. Another possible mechanism for the loss from the platelet surface is that ERP5 is released from collagen-stimulated platelets. Indeed, platelets stimulated with thrombin have been shown to release 10% of total PDI.14
The observation that small, thiol-reactive reagents modulate platelet function suggests the enzymatic activity of the proteins underlie their function on the cell surface.18 Little is known of the mechanism by which this occurs. Essex et al18 have proposed PDI acts downstream of the primary activation process, but prior to activation of the integrin receptor αIIbβ3. They observed that anti-PDI inhibitory antibodies were able to block conversion of integrin αIIbβ3 to the activated state recognized by pituitary adenylate cyclase–activating polypeptide 1 (PAC-1) antibody, but not block activation via a peptide (LSARLAF) that has been shown to bind αIIbβ3 and directly stimulate aggregation and secretion. We believe this is a good starting point, which can be developed further to incorporate the presence of additional thiol isomerase proteins on the platelet cell surface, such as ERP5. A question introduced by the discovery of ERP5 is whether it and PDI act through a common mechanism. When a combination of inhibitory antibodies to ERP5 and PDI are used at submaximal concentrations (Figures 4, 5, 6), an additive effect on platelet function was detected. If a common pathway is involved, the effects of competitive inhibition may promote a less-than-additive effect. At higher concentrations of inhibitory antibody, a less-than-additive effect is observed for the fibrinogen-binding and P-selectin–exposure assays. It is possible this indicates that both ERP5 and PDI operate through a common mechanism, although they may be acting at different points. Future work to establish the respective substrate preferences of ERP5 and PDI will be required to shed more light on this issue.
The ability of ERP5 to regulate the binding of fibrinogen, cell-surface exposure of P-selectin, and coassociation of β3 integrin (Figures 5, 6, 7), highlight the potential interrelationships between different thiol isomerase enzymes and receptor activation on the platelet surface. Following preincubation of platelets with function-blocking antibodies to either ERP5 or PDI, the binding of fibrinogen and cell-surface exposure of P-selectin was found to be significantly inhibited in platelets. Differences were observed in agonist and inhibitory antibody responses, with greater inhibition observed for convulxin than for collagen, and for blocking PDI than for ERP5. The different levels of inhibition observed may be based on different potencies of the agonists used, different activation pathways, and the respective amounts of ERP5 and PDI on the platelet surface at the time of assay. Lahav et al19 have reported an approximate 55% decrease in fibrinogen binding and an approximate 30% decrease in P-selectin exposure for collagen-stimulated platelets preincubated with the monoclonal anti-PDI antibody RL-90. These results are consistent with the data we have obtained showing a 29% decrease in fibrinogen binding and a 47% decrease in P-selectin exposure in response to blocking with the monoclonal anti-PDI antibody Ma3-019. There is no statistical significance (P > .25) between the reductions in fibrinogen binding and P-selectin exposure in this study. This suggests that the differences observed in the relative levels of inhibition for the 2 sets of data are based on experimental protocol. The greater inhibition observed in this study for PDI relative to ERP5 may be due to different affinities and function-blocking properties of the antibodies used. Interestingly, the extent of inhibition of fibrinogen binding and P-selectin exposure is closer for anti-PDI and anti-ERP5 blocking in response to collagen (P > .2) than in response to convulxin (P < .05), suggesting that PDI is more important than ERP5 upon convulxin stimulation. This may be due to different signaling pathways stimulated by these agonists. Notably, convulxin is capable of stimulating GPVI and GP1b, and Burgess et al17 have demonstrated a physical association between PDI and GP1b.
Remodeling of the integrin αIIbβ3 from an inactive to a ligand-binding state involves a conformational change in which the disulfide bonding pattern of the receptor is changed.40,41 The β3 subunit of the integrin possesses inherent thiol isomerase activity,25 although it is unknown whether this activity is sufficient to promote the conformational change in either direction. We have demonstrated a coassociation of ERP5 with the β3 subunit of integrin αIIbβ3 in activated platelets. We suggest that, when associated with β3, ERP5 is able to assist in the conformational change of the integrin from an inactive to an active state. The mechanism through which this is regulated is uncertain, with increased cell-surface exposure and β3 association likely to be involved. The interaction with, and regulation by, other molecules, such as the interaction of PDI, or calreticulin,52 may also play a role. However, it is possible to inhibit integrin ligation and still trigger expression of P-selectin (or vice versa). Therefore, ERP5 may play a role upstream of both in the activation process.
Prepublished online as Blood First Edition Paper, October 5, 2004; DOI 10.1182/blood-2004-02-0608.
Supported by grants from the British Heart Foundation (K.S.A., J.M.G.), the Biotechnology and Biological Sciences Research Council (BBSRC) (J.M.G.), and the Medical Research Council (MRC) (J.M.G.).
An Inside Blood analysis of this article appears in the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We wish to thank Dr Gwenda Graham (University of Reading) and Dr Sheila Hassock (King's College) for technical assistance.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal