Abstract
Glycoprotein VI (GPVI) is an essential platelet collagen receptor; therefore, the inhibition of GPVI-collagen interactions may be an attractive antithrombotic strategy. We have previously shown that targeting of GPVI with antibodies leads to the depletion of the receptor and to long-term antithrombotic protection in mice. An alternative agent to interfere with GPVI-collagen interactions might be soluble GPVI acting as a competitive inhibitor, thereby averting undesired effects on platelets. To test this, we expressed soluble dimeric human GPVI, comprising the extracellular domain of the receptor fused to the human immunoglobulin Fc domain (GPVI-Fc), and compared its antithrombotic potential with that of anti-GPVI antibodies in mice. In contrast to a recent report, we found by intravital fluorescence microscopy and ultrasonic flow measurements that GPVI-Fc had no effect on platelet adhesion and thrombus formation at the injured arterial wall, whereas anti-GPVI antibodies profoundly inhibited these processes. Similar results were obtained with a fusion protein comprising the extracellular domain of mouse GPVI and human IgG-Fc. This indicates that direct targeting of GPVI provides significantly stronger protection against arterial thrombosis than soluble GPVI dimer.
Introduction
Rupture of the atherosclerotic plaque leads to the exposure of subendothelial fibrillar collagens to the flowing blood, which triggers the adhesion, aggregation, and procoagulant activity of platelets.1-4 Platelet-collagen interactions are complex and involve a number of different receptors and signaling pathways. Besides glycoprotein Ib-V-IX (GPIb-V-IX), which indirectly interacts with collagen through von Willebrand factor,5 the direct collagen receptor GPVI has been identified as playing a central role in this process because it mediates the activation of integrins, thereby enabling firm platelet adhesion and thrombus growth.6 GPVI is a 62-kDa type 1 transmembrane receptor that belongs to the immunoglobulin superfamily7,8 and is noncovalently associated with the signal-transducing FcRγ chain.9,10 A few patients with GPVI deficiency have been described who have mild bleeding diatheses, but their platelets show severely impaired responses to collagen.11-13 Similarly, platelets from GPVI-14 or FcRγ/GPVI-deficient15,16 mice are unresponsive to collagen, but no major bleeding has been observed in those animals, suggesting that GPVI might be an interesting target for safe antithrombotic therapy. Experimental evidence in support of this hypothesis came from studies using anti-GPVI monoclonal antibodies (mAbs) in mice. Injection of such mAbs results in down-regulation of the receptor and abolished collagen responses in circulating platelets.17,18 Such GPVI-depleted mice are for at least 2 weeks protected against collagen-dependent thromboembolism and arterial thrombus formation but only show slightly increased bleeding times.17,19 Similarly, FcRγ chain–deficient mice, which lack GPVI,16 are protected against arterial thrombosis and subsequent neointima formation20 but display no bleeding phenotype.21
There is growing evidence that anti-GPVI treatment may also induce the down-regulation of the receptor in humans because patients with GPVI deficiency developed highly specific antibodies against the apparently absent receptor, suggesting that they acquired GPVI deficiency based on antibody-induced clearing of the receptor from their platelets.11,22
In addition to the direct inhibition or depletion of GPVI, it could principally be a promising strategy to use a soluble form of the receptor for competitive inhibition of platelet-collagen interactions at sites of injury. We have previously shown that monomeric GPVI has virtually no affinity for fibrillar collagen, whereas the dimeric form of GPVI, comprising the extracellular domain of the receptor fused to human immunoglobulin Fc domain (GPVI-Fc), binds to collagen and collagen-related peptides (CRPs) with high affinity.23 Such a GPVI-Fc fusion protein was also recently generated by Massberg et al24 and reportedly reduced platelet adhesion and aggregation at the injured carotid artery of mice, indicating that soluble GPVI dimer might confer similar antithrombotic protection as direct anti-GPVI treatment.
In the current study, we compared the antithrombotic efficacy of anti-GPVI antibodies with soluble GPVI dimer (GPVI-Fc) in 2 models of arterial thrombosis in mice. Although anti-GPVI antibodies markedly inhibited platelet adhesion and thrombus formation in injured arteries, no significant protection was achieved with GPVI-Fc.
Materials and methods
Animals
Wild-type mice and mice deficient in the FcRγ chain were of C57Bl/6 genetic background and used at the age of 10 to 16 weeks. To induce GPVI depletion, wild-type mice were injected with 2 mg/kg JAQ1 (antimouse GPVI16 ) intraperitoneally and were used for analysis on day 5. The local Animal Care and Use Committee approved all experiments on animals.
Human and murine GPVI-Fc fusion proteins
The extracellular domain of human GPVI was obtained by polymerase chain reaction (PCR) from cDNA using the GPVI cDNA (kindly provided by G. Dickneite, ZLB Behring, Marburg, Germany) as the template and the oligonucleotide ATTAGCTAGCATGTCTCCATCCATC (with the NheI site underlined) and GGATCCTTATTGGTGTAGTACTGG (with the BamHI site underlined) as the forward and reverse primers, respectively. The PCR product was purified using a QIAquick gel extraction kit (Qiagen, Hilden, Germany) digested with NheI and BamHI, purified again, and ligated to the Signal plg plus (R&D Systems Europe, Abingdon, United Kingdom) vector containing the human immunoglobulin Fc domain. The ligation mixture was transformed to Escherichia coli DH5α. The obtained construct was verified by restriction enzyme digestion and DNA sequencing.
For cloning of extracellular domain of mouse GPVI, RNA from mouse bone marrow was isolated using the RNeasy Mini Kit (Qiagen) according to the manufacturer's protocol, and reverse transcription was performed (Titan One Tube RT-PCR-Kit; Roche, Ingelheim, Germany). One hundred nanograms RNA were used as template and the oligonucleotides GGATCCACATTCCCCTTG (with the BamHI site underlined) and ATACGCTAGCATGTCTCC (with the NheI site underlined) were designed for PCR as the forward and reverse primers, respectively. The PCR product was purified using a QIAquick gel extraction kit (Qiagen) digested with NheI and BamHI, purified again, and ligated to the Signal plg plus vector containing the human immunoglobulin Fc domain. The ligation mixture was transformed to E coli DH5α. The obtained construct was verified by restriction enzyme digestion and DNA sequencing.
Human embryonic kidney (HEK) 293 cells were transfected with these constructs using the calcium-phosphate method. Stable cell lines expressing recombinant human GVPI-Fc (hGPVI-Fc) or mouse GVPI-Fc (mGPVI-Fc) were selected in medium containing 700 μg/mL geniticin. For the purification of h/mGPVI-Fc protein, the culture medium was filtered and the obtained supernatant was applied to a protein-G–Sepharose column (Amersham Biosciences, Freiburg, Germany). After it was extensively washed with phosphate-buffered saline (PBS), h/mGPVI-Fc was eluted with 0.1 M glycine buffer, pH 2.7. The eluted protein was applied to a JAQ1-coupled Sepharose column. After the column was washed with PBS, the protein was eluted by 0.1 M glycine buffer, pH 2.7, and then extensively dialyzed against PBS.
Binding studies
Microplates (BD Discovery Labware, Bedford, MA) were coated with Horm-type collagen (50 μg/mL; Nycomed, Munich, Germany), collagen-related peptide (CRP; 2 μg/mL; kindly provided by S.P. Watson, University of Birmingham, Birmingham, United Kingdom), or bovine serum albumin (BSA), and binding of GPVI-Fc was detected with horseradish peroxidase (HRP)–conjugated antibodies against human immunoglobulin G-Fc (IgG-Fc) (Jackson ImmunoResearch, West Grove, PA) and TMB. In another assay, plates were coated with 5 μg/mL mouse GPVI-Fc and specific binding of HRP-conjugated JAQ1, JAQ2, and JAQ3 (anti-GPVI) compared with irrelevant control mAbs or buffer was measured. To determine the in vivo concentration of GPVI-Fc, 50 μL blood was drawn at the indicated time points after intravenous application of the construct, and diluted plasma was transferred onto plates coated with polyclonal antihuman IgG-Fc (5 μg/mL). Binding of GPVI-Fc was then detected with HRP-conjugated antihuman IgG-Fc and TMB.
Enzyme-linked immunosorbent assay and immunoblotting
Flexible microtiter plates (BD Discovery Labware) were coated with human or mouse GPVI-Fc at a concentration of 2 μg/mL overnight. After blocking with 5% BSA in PBS, the plates were incubated with the indicated HRP-labeled antibodies (1 μg/mL) for 1 hour and were developed with TMB. Antibodies used were JAQ1, JAQ2, JAQ3 (anti-GPVI18 ), Xia.G5 (antimouse GPIb; emfret Analytics, Würzburg, Germany), and p0p4 (antimouse GPIb).
For immunoblotting, 1 μg GPVI-Fc protein was run on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and was transferred to a polyvinylidene difluoride membrane. The membrane was incubated with the indicated HRP-labeled antibodies (1 μg/mL) for 1 hour, and proteins were visualized by enhanced chemiluminescence (ECL).
Platelet aggregation
To determine platelet aggregation, platelet-rich plasma (PRP; 200 μL with 0.5 × 106 platelets/μL) was stimulated with fibrillar collagen (Nycomed, München, Germany) or CRP in the presence of GPVI-Fc (20 μg/mL) or human IgG (20 μg/mL; Sigma, Deisenhofen, Germany). Light transmission was recorded on a Fibrintimer 4-channel aggregometer (APACT Laborgeräte und Analysensysteme, Hamburg, Germany) over 10 minutes and was expressed as arbitrary units with 100% transmission adjusted with plasma.
Adhesion under flow conditions
Mouse blood (1 vol) was collected into 0.5 vol HEPES (N-2-hydroxyethylenepiperazine-N′-2-ethanesulfonic acid) buffer containing 20 U/mL heparin. Human blood was collected from the antecubital veins of volunteer donors into syringes containing 0.5 vol 20 U/mL heparin. Coverslips (24 × 60 mm) were coated with fibrillar collagen (0.2 mg/mL) and were blocked for 1 hour with 1% BSA. Perfusion studies were then performed as described.6
Preparation of platelets for intravital microscopy
Experiments were performed with a Zeiss Axiovert 200 inverted microscope equipped with a 63×/0.75 objective lens (Zeiss, Göttingen, Germany). Phase-contrast images were recorded with a Panasonic AG-7355 recorder (Matsushida Electric, Osaka, Japan) and were evaluated with MetaVue computer-assisted image analysis software (Visitron, München, Germany). The platelet adhesion results are expressed as the mean percentage of total area covered per microscopic field.
Mouse blood (1 vol) was collected into 0.5 vol HEPES buffer containing 20 U/mL heparin. The blood was centrifuged at 250g for 10 minutes, and platelet-rich plasma was gently transferred to a fresh tube. Platelets were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (DCF) and were adjusted to a final concentration of 200 × 106 platelets/250μL.19
Intravital microscopy
Intravital microscopy of the injured carotid artery was performed essentially as described.25 Briefly, mice were anesthetized by intraperitoneal injections of ketamine/xylazine (100 mg/kg ketamine; Parke-Davis, Karlsruhe, Germany) (5 mg/kg xylazine; Bayer AG, Leverkusen, Germany). Polyethylene catheters (Portex, Hythe, England) were implanted into each right jugular vein, and fluorescent platelets (200 × 106/250 μL) were infused intravenously. Where indicated, mice received mouse or human GPVI-Fc or vehicle before platelets were administered. Carotid injury for endothelial denudation was induced by vigorous ligation. Before and after vascular injury, the fluorescent platelets were visualized in situ by in vivo videomicroscopy of the right common carotid artery using a Zeiss Axiotech microscope (20 × water immersion objective, W 20 ×/0.5; Zeiss, Göttingen, Germany) with a 100 W HBO mercury lamp for epiillumination. Platelet adhesion was recorded for 5 minutes after the induction of injury, and the videotaped images were evaluated using a computer-assisted image analysis program (Visitron, Munich, Germany). The number of adherent platelets was assessed by counting the cells that did not move or detach from the vascular wall for at least 10 seconds. In each mouse, 3 nonoverlapping fields were analyzed for 30 seconds (2.5-3.0 minutes after injury) in a slow motion mode. Clusters of 2 or more platelets were defined as microaggregates.
Occlusion time
The abdominal cavity of anesthetized mice was longitudinally opened, and the abdominal aorta was prepared. An ultrasonic flow probe was placed around the aorta, and thrombosis was induced by a firm compression with a forceps. Blood flow was monitored until complete occlusion occurred. The experiment was stopped manually after 30 minutes.
Statistical evaluation
Statistical analysis was performed using the unpaired Student t test.
Results
Soluble human GPVI dimer has a significant but limited inhibitory effect on collagen-induced platelet aggregation
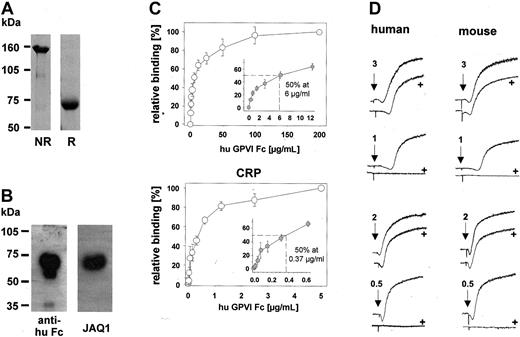
To determine the antithrombotic potential of soluble GPVI in comparison with direct anti-GPVI treatment, we fused the extracellular domain of human GPVI to the human immunoglobulin Fc domain and expressed the resultant GPVI-Fc fusion protein (hGPVI-Fc) in a secreted soluble form, as described.23,24 GPVI-Fc had an apparent molecular mass of approximately 70 kDa under reducing and approximately 150 kDa under nonreducing conditions, as visualized by Coomassie blue stain (Figure 1A).23,24 The construct was specifically detected by HRP-conjugated antibodies against the human IgG Fc part and the anti-GPVI antibody, JAQ1, in Western blot analysis (Figure 1B) and enzyme-linked immunosorbent assay (ELISA) (not shown). hGPVI-Fc bound to immobilized fibrillar collagen and the GPVI-specific ligand, CRP, in a dose-dependent and saturable manner (Figure 1C). Half-maximal binding to collagen occurred at a final hGPVI-Fc concentration of approximately 6 μg/mL, whereas on CRP this was observed already at approximately 0.37 μg/mL (Figure 1C). Next we tested the inhibitory effect of hGPVI-Fc on collagen- and CRP-induced platelet aggregation. The fusion protein, at a concentration of 20 μg/mL, blocked aggregation of human platelets in response to low doses of collagen (1 μg/mL) or CRP (0.5 μg/mL). However, when agonist concentrations were increased, the inhibitory effect of hGPVI-Fc was rapidly overcome. Similar results were obtained when the experiment was performed with mouse platelets (Figure 1D). In parallel experiments, we obtained similar results with a second hGPVI-Fc fusion protein that was generated independently and was initially described by Miura et al23 (hGPVI-Fc*) (not shown). Together, this confirmed that soluble GPVI dimer binds to fibrillar collagen and CRP23,24 and demonstrated that it has a significant, but limited, inhibitory effect on platelet aggregation induced by either agonist.
Characterization of hGPVI-Fc. (A) Three micrograms purified hGPVI-Fc was subjected to SDS-PAGE under nonreducing (NR) and reducing (R) conditions, and the gel was stained with Coomassie brilliant blue. (B) One microgram purified hGPVI-Fc was subjected to SDS-PAGE under reducing conditions and was immunoblotted with HRP-conjugated antihuman Fc antibodies or JAQ1. (C) Dose-dependent binding of hGPVI-Fc to immobilized collagen (50 μg/mL) and CRP (2 μg/mL) was measured by ELISA (mean ± SD of 3 individual experiments). (D) hGPVI-Fc partly inhibits collagen- and CRP-induced aggregation of human and mouse platelets. Under stirring conditions, human or mouse PRP stimulated with the indicated concentrations of collagen or CRP in the presence (+) or absence of 20 μg/mL GPVI-Fc. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 experiments.
Characterization of hGPVI-Fc. (A) Three micrograms purified hGPVI-Fc was subjected to SDS-PAGE under nonreducing (NR) and reducing (R) conditions, and the gel was stained with Coomassie brilliant blue. (B) One microgram purified hGPVI-Fc was subjected to SDS-PAGE under reducing conditions and was immunoblotted with HRP-conjugated antihuman Fc antibodies or JAQ1. (C) Dose-dependent binding of hGPVI-Fc to immobilized collagen (50 μg/mL) and CRP (2 μg/mL) was measured by ELISA (mean ± SD of 3 individual experiments). (D) hGPVI-Fc partly inhibits collagen- and CRP-induced aggregation of human and mouse platelets. Under stirring conditions, human or mouse PRP stimulated with the indicated concentrations of collagen or CRP in the presence (+) or absence of 20 μg/mL GPVI-Fc. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 experiments.
Effect of hGPVI-Fc on platelet adhesion on collagen under flow conditions in vitro
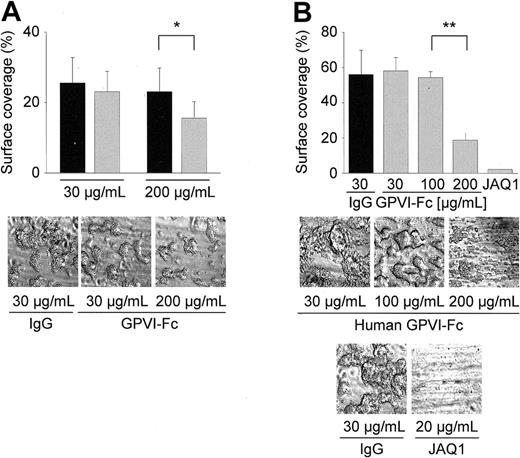
Next, we assessed the effect of hGPVI-Fc on platelet adhesion on collagen under flow conditions. For this, human and mouse blood was perfused over a collagen-coated surface at a wall shear rate of 1000 seconds–1 in the presence of different concentrations of hGPVI-Fc or control IgG.6 At a concentration of 30 μg/mL, no effect of hGPVI-Fc on adhesion and thrombus formation of human platelets was detectable (Figure 2A). Only when the protein was added at a concentration of 200 μg/mL was a significant but limited reduction (36.3%; P < .05) of thrombus formation observed. Similar results were obtained with hGPVI-Fc* (not shown). When the experiments were performed with mouse blood, comparable effects of hGPVI-Fc were found. Although 30 and 100 μg/mL of the protein were without detectable effect, a significant reduction (62.9%; P < .01) of thrombus formation was observed at 200 μg/mL (Figure 2B). In contrast, virtually complete inhibition of platelet adhesion and thrombus formation was achieved by direct GPVI inhibition with JAQ1 (20 μg/mL), confirming previous results.6
Effect of hGPVI-Fc on platelet adhesion. Heparinized whole blood from healthy volunteers (A) or wild-type mice (B) was incubated with the indicated concentrations of hGPVI-Fc ( ) or control IgG (▪) for 2 minutes and perfused at wall shear rates of 1000 seconds–1 (4 minutes) over a collagen-coated surface. Mouse blood was also tested in the presence of JAQ1 (20 μg/mL). Surface area covered by thrombi is summarized in the upper panels (mean ± SD of 5 experiments per group). Control bar (B) represents blood from wild-type mice incubated with 30 μg/mL of irrelevant IgG, but similar results were also obtained with higher concentrations (not shown). Bottom panels show representative phase-contrast images taken at the end of the experiment. (*P < .05; **P < .01)
) or control IgG (▪) for 2 minutes and perfused at wall shear rates of 1000 seconds–1 (4 minutes) over a collagen-coated surface. Mouse blood was also tested in the presence of JAQ1 (20 μg/mL). Surface area covered by thrombi is summarized in the upper panels (mean ± SD of 5 experiments per group). Control bar (B) represents blood from wild-type mice incubated with 30 μg/mL of irrelevant IgG, but similar results were also obtained with higher concentrations (not shown). Bottom panels show representative phase-contrast images taken at the end of the experiment. (*P < .05; **P < .01)
Effect of hGPVI-Fc on platelet adhesion. Heparinized whole blood from healthy volunteers (A) or wild-type mice (B) was incubated with the indicated concentrations of hGPVI-Fc ( ) or control IgG (▪) for 2 minutes and perfused at wall shear rates of 1000 seconds–1 (4 minutes) over a collagen-coated surface. Mouse blood was also tested in the presence of JAQ1 (20 μg/mL). Surface area covered by thrombi is summarized in the upper panels (mean ± SD of 5 experiments per group). Control bar (B) represents blood from wild-type mice incubated with 30 μg/mL of irrelevant IgG, but similar results were also obtained with higher concentrations (not shown). Bottom panels show representative phase-contrast images taken at the end of the experiment. (*P < .05; **P < .01)
) or control IgG (▪) for 2 minutes and perfused at wall shear rates of 1000 seconds–1 (4 minutes) over a collagen-coated surface. Mouse blood was also tested in the presence of JAQ1 (20 μg/mL). Surface area covered by thrombi is summarized in the upper panels (mean ± SD of 5 experiments per group). Control bar (B) represents blood from wild-type mice incubated with 30 μg/mL of irrelevant IgG, but similar results were also obtained with higher concentrations (not shown). Bottom panels show representative phase-contrast images taken at the end of the experiment. (*P < .05; **P < .01)
Direct comparison of hGPVI-Fc and anti-GPVI antibodies in arterial thrombosis in vivo
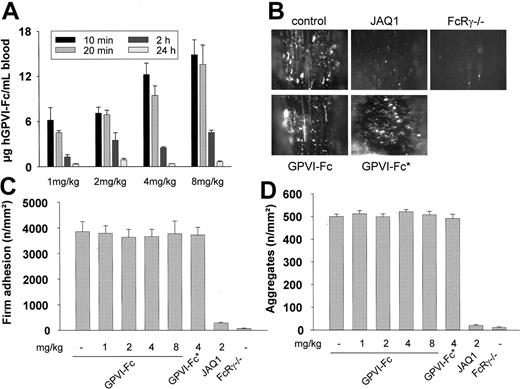
The results just described demonstrated that 200 μg/mL or more hGPVI-Fc is required to achieve partial inhibition of thrombus formation on collagen in vitro. This is in agreement with the study by Massberg et al24 in which human GPVI-Fc was used at a concentration of 800 μg/mL to achieve significant, yet incomplete (84%), inhibition of thrombus formation of human platelets on collagen. In the same study, human GPVI-Fc was found to strongly attenuate platelet adhesion and aggregation at sites of injury in vivo when given at a dose of 2 mg/kg. Assuming a body weight of 30 g and a blood volume of approximately 2 mL per mouse, this dose corresponds to a theoretical maximum concentration of approximately 30 μg/mL blood, indicating that hGPVI-Fc could be a more potent inhibitor of platelet adhesion in vivo than in vitro. In a first set of experiments, we tested the bioavailability of GPVI-Fc on intravenous injection. For this, mice received different amounts of hGPVI-Fc intravenously (1, 2, 4, and 8 mg/kg), and the plasma levels of the protein were determined at different time points. In mice treated with 2 mg/kg, plasma levels were 7.1 ± 0.8 and 6.9 ± 0.6 μg/mL at 10 and 30 minutes and progressively decreased to 0.9 ± 0.2 μg/mL after 24 hours (Figure 3A), and the protein bound to collagen and CRP ex vivo (not shown). Similar kinetics of GPVI-Fc clearing were also observed in the groups treated with 1, 4, or 8 mg/kg protein.
Effect of hGPVI-Fc or anti-GPVI antibodies on platelet adhesion in vivo. (A) Mice received the indicated amounts of hGPVI-Fc intravenously, and blood was drawn at the indicated time points. The plasma concentration of hGPVI-Fc was determined by ELISA. Results are given as mean ± SD from triplicate readings of 3 individual experiments. (B-D) Intravital microscopy. Mice received the indicated amounts of hGPVI-Fc, hGPVI-Fc*, or vehicle intravenously, and platelet-vessel wall interactions after vascular injury were examined by in vivo fluorescence microscopy of the common carotid artery in situ. In parallel, JAQ1-treated (2 mg/kg) and FcRγ–/– mice were tested. (B) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury in mice treated with vehicle, hGPVI-Fc (4 mg/kg), hGPVI-Fc* (4 mg/kg), or JAQ1 (2 mg/kg). Platelet adhesion (C) and aggregate formation (D) shown as mean ± SD of 7 experiments per group.
Effect of hGPVI-Fc or anti-GPVI antibodies on platelet adhesion in vivo. (A) Mice received the indicated amounts of hGPVI-Fc intravenously, and blood was drawn at the indicated time points. The plasma concentration of hGPVI-Fc was determined by ELISA. Results are given as mean ± SD from triplicate readings of 3 individual experiments. (B-D) Intravital microscopy. Mice received the indicated amounts of hGPVI-Fc, hGPVI-Fc*, or vehicle intravenously, and platelet-vessel wall interactions after vascular injury were examined by in vivo fluorescence microscopy of the common carotid artery in situ. In parallel, JAQ1-treated (2 mg/kg) and FcRγ–/– mice were tested. (B) The photomicrographs show representative in vivo fluorescence microscopy images 3 minutes after injury in mice treated with vehicle, hGPVI-Fc (4 mg/kg), hGPVI-Fc* (4 mg/kg), or JAQ1 (2 mg/kg). Platelet adhesion (C) and aggregate formation (D) shown as mean ± SD of 7 experiments per group.
To directly compare the antithrombotic potential of hGPVI-Fc and anti-GPVI antibodies, mice received 1, 2, 4, or 8 mg/kg GPVI-Fc intravenously, and platelet recruitment was directly assessed in a carotid injury model using intravital fluorescence microscopy, as described.24,25 In parallel, mice were tested in which GPVI had been depleted by injection of JAQ1 (2 mg/kg).17 Platelets were purified from donor mice, fluorescently labeled, and injected into recipient mice of the same genotype. Vascular injury was induced by vigorous ligation of the carotid artery, which consistently causes disruption of the endothelial layer and, frequently, breaching of the internal elastic lamina, followed by rapid platelet adhesion and aggregate formation at the site of injury.25 Unexpectedly, in vivo fluorescence microscopy revealed that the extent of platelet adhesion was indistinguishable between control mice and mice treated with hGPVI-Fc, regardless of the injected dose (Figure 3C). Furthermore, in control and hGPVI-Fc–treated mice, firmly adherent platelets recruited additional platelets from the circulation, leading to the formation of microaggregates; the number of microaggregates was similar in all groups of mice (Figure 3D). This finding was confirmed when mice were treated with hGPVI-Fc*23 (4 mg/kg; Figure 3C), and platelet adhesion and aggregate formation on injury were comparable to those in the control (Figure 3D). In contrast, platelet adhesion and aggregate formation were virtually abolished in JAQ1-treated mice, confirming previous results.19,25 To further substantiate the role of GPVI in this thrombosis model, we tested FcRγ chain–deficient mice, which lack GPVI.16 As in JAQ1-treated mice, platelet adhesion and aggregation at sites of injury was dramatically reduced in FcRγ chain–deficient mice (Figure 3C-D). Together, these findings demonstrated that under conditions of injury in the carotid artery, where soluble GPVI dimer (up to 8 mg/kg) is without significant effect, anti-GPVI treatment provides virtually complete inhibition of thrombus formation.
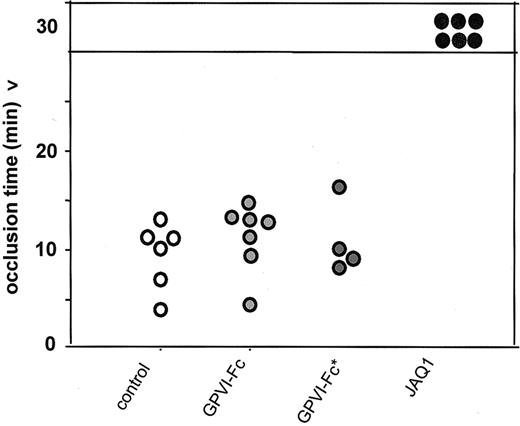
To further compare the antithrombotic potential of direct and competitive GPVI inhibition, we used a second injury model in which thrombosis was induced in the aorta and time to occlusion was determined. Mice received 4 mg/kg GPVI-Fc or GPVI-Fc* intravenously, and thrombosis was induced in the aorta by 1 firm compression with a forceps. Blood flow was monitored with an ultrasonic flow probe until complete occlusion occurred. In parallel, JAQ1-treated (2 mg/kg) mice were tested. In mice treated with hGPVI-Fc, hGPVI-Fc*, or vehicle, arterial occlusion occurred after 11.5 ± 3.7 minutes, 10.9 ± 3.6 minutes, and 10.1 ± 2.3 minutes, respectively, whereas no occlusion was observed in JAQ1-treated mice for up to 30 minutes (Figure 4).
Arterial occlusion model. Mice received vehicle (n = 6), GPVI-Fc (4 mg/kg, n = 7), GPVI-Fc* (4 mg/kg, n = 4), or JAQ1 (2 mg/kg, n = 6), and thrombosis was induced in the aorta by one firm compression with a forceps. Blood flow was monitored with an ultrasonic flow probe until complete occlusion. The experiment was stopped after 30 minutes. Each symbol represents one individual.
Arterial occlusion model. Mice received vehicle (n = 6), GPVI-Fc (4 mg/kg, n = 7), GPVI-Fc* (4 mg/kg, n = 4), or JAQ1 (2 mg/kg, n = 6), and thrombosis was induced in the aorta by one firm compression with a forceps. Blood flow was monitored with an ultrasonic flow probe until complete occlusion. The experiment was stopped after 30 minutes. Each symbol represents one individual.
Limited inhibitory effect of murine GPVI-Fc on platelet adhesion and aggregation on collagen in vitro
To examine whether species-specific differences in the GPVI-collagen interaction may contribute to the weak antithrombotic potential of hGPVI-Fc in mice, we fused the extracellular domain of mouse GPVI to the human immunoglobulin Fc domain and expressed the resultant GPVI-Fc fusion protein (mGPVI-Fc) in a secreted soluble form. As did hGPVI-Fc, mGPVI-Fc had an apparent molecular mass of approximately 70 kDa under reducing and approximately 150 kDa under nonreducing conditions, as visualized by Coomassie blue stain (Figure 5A). The construct was specifically detected by HRP-conjugated JAQ1, JAQ2, and JAQ3 and by antibodies against the human IgG Fc part in ELISA (Figure 5B) and Western blot analysis (not shown). Half-maximal binding of mGPVI-Fc on collagen occurred at approximately 11.2 μg/mL, whereas on CRP this was observed already at approximately 0.44 μg/mL (Figure 5C). The construct, at a concentration of 20 μg/mL, blocked aggregation of mouse platelets in response to low doses of collagen (1 μg/mL) or CRP (0.5 μg/mL) (Figure 5D). However, when agonist concentrations were increased, the inhibitory effect of mGPVI-Fc was rapidly overcome.
Characterization of mGPVI-Fc. (A) Three micrograms purified mGPVI-Fc was subjected to SDS-PAGE under nonreducing (NR) and reducing (R) conditions, and the gel was stained with Coomassie brilliant blue. (B) Mictrotiter plates were coated with mGPVI-Fc and then incubated with the indicated HRP-labeled antibodies followed by TMB development (JAQ1, 2, and 3 [anti-GPVI], p0p4 [anti-GPIbα]). (C) Dose-dependent binding of GPVI-Fc to immobilized collagen (50 μg/mL) and CRP (2 μg/mL) was measured by ELISA (mean ± SD of 3 experiments). (D) mGPVI-Fc partly inhibits collagen- and CRP-induced mouse platelet aggregation. Under stirring conditions, mouse PRP was preincubated with 20 μg/mL GPVI-Fc and then stimulated with the indicated concentrations of collagen or CRP. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 experiments.
Characterization of mGPVI-Fc. (A) Three micrograms purified mGPVI-Fc was subjected to SDS-PAGE under nonreducing (NR) and reducing (R) conditions, and the gel was stained with Coomassie brilliant blue. (B) Mictrotiter plates were coated with mGPVI-Fc and then incubated with the indicated HRP-labeled antibodies followed by TMB development (JAQ1, 2, and 3 [anti-GPVI], p0p4 [anti-GPIbα]). (C) Dose-dependent binding of GPVI-Fc to immobilized collagen (50 μg/mL) and CRP (2 μg/mL) was measured by ELISA (mean ± SD of 3 experiments). (D) mGPVI-Fc partly inhibits collagen- and CRP-induced mouse platelet aggregation. Under stirring conditions, mouse PRP was preincubated with 20 μg/mL GPVI-Fc and then stimulated with the indicated concentrations of collagen or CRP. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 experiments.
Next, we tested the effect of mGPVI-Fc on mouse platelet adhesion to collagen in whole blood perfusion experiments (1000 seconds–1). Although the fusion protein, at concentrations of 30 and 100 μg/mL, had no detectable effect on adhesion and thrombus formation, a significant but limited reduction of thrombus formation was observed with 200 μg/mL (40.5%; P < .05) (Figure 6). This demonstrated that mGPVI-Fc binds to type 1 fibrillar collagen at an efficiency similar to that of hGPVI-Fc and consequently exhibits a comparable inhibitory effect on platelet adhesion and aggregation on the matrix molecule.
Effect of mGPVI on platelet adhesion. Heparinized whole blood from wild-type mice was incubated with the indicated concentration of mouse GPVI-Fc (gray bars) or the same concentration of control IgG (black bar) for 2 minutes and perfused at wall shear rates of 1000 seconds–1 (4 minutes) over a collagen-coated surface. Surface area covered by thrombi is summarized in the upper panels (mean ± SD of 5 experiments per group). Control bar represents blood from wild-type mice incubated with 30 μg/mL of irrelevant IgG, but similar results were also obtained with higher concentrations (not shown). Lower panels show representative phase-contrast images taken at the end of the experiment. (*P < .05)
Effect of mGPVI on platelet adhesion. Heparinized whole blood from wild-type mice was incubated with the indicated concentration of mouse GPVI-Fc (gray bars) or the same concentration of control IgG (black bar) for 2 minutes and perfused at wall shear rates of 1000 seconds–1 (4 minutes) over a collagen-coated surface. Surface area covered by thrombi is summarized in the upper panels (mean ± SD of 5 experiments per group). Control bar represents blood from wild-type mice incubated with 30 μg/mL of irrelevant IgG, but similar results were also obtained with higher concentrations (not shown). Lower panels show representative phase-contrast images taken at the end of the experiment. (*P < .05)
Effect of mGPVI-Fc on platelet adhesion and aggregate formation in the injured carotid artery
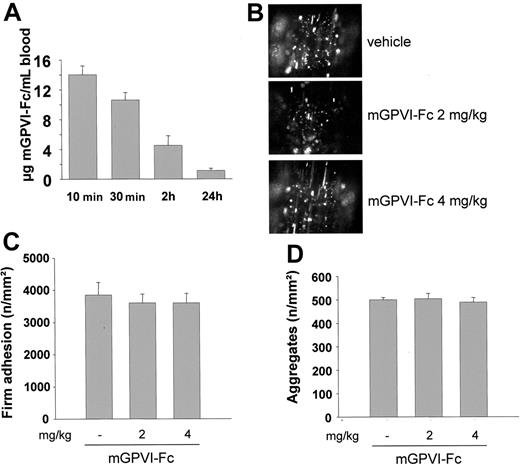
The in vivo effects of mGPVI-Fc were examined. First, mice received 2 mg/kg mGPVI-Fc intravenously, and plasma concentrations were determined at different time points by ELISA. After 10 minutes, the protein was detectable at a concentration of 14.0 ± 1.2 μg/mL, and the levels decreased to 10.6 ± 1.0 and 1.1 ± 0.3 μg/mL after 30 minutes and 24 hours, respectively (Figure 7A). To test the antithrombotic effect of mGPVI-Fc, mice received 2 mg/kg or 4 mg/kg of the fusion protein, followed by fluorescence-tagged platelets, and injury was induced by ligation of the carotid artery as described. Platelet adhesion at the vessel wall was visualized and quantitated by in vivo fluorescence microscopy. No significant reduction in the number of adherent platelets was detectable in mGPVI-Fc treated mice compared with control mice (Figure 7B,C).
Effect of mGPVI-Fc on platelet adhesion in vivo. (A) Mice received 2 mg/kg mGPVI-Fc intravenously, and blood was drawn at the indicated time points. The plasma concentration of mGPVI-Fc was determined by ELISA. Results are given as mean ± SD from triplicate readings of 3 experiments. (B-C) Mice received the indicated amounts of GPVI-Fc or vehicle intravenously, and platelet–vessel wall interactions after vascular injury were examined by in vivo fluorescence microscopy of the common carotid artery. Platelet adhesion (B) and aggregate formation (C) of 7 experiments per group are summarized. Photomicrographs show representative images 3 minutes after injury in mice treated with vehicle or mGPVI-Fc (2 or 4 mg/kg).
Effect of mGPVI-Fc on platelet adhesion in vivo. (A) Mice received 2 mg/kg mGPVI-Fc intravenously, and blood was drawn at the indicated time points. The plasma concentration of mGPVI-Fc was determined by ELISA. Results are given as mean ± SD from triplicate readings of 3 experiments. (B-C) Mice received the indicated amounts of GPVI-Fc or vehicle intravenously, and platelet–vessel wall interactions after vascular injury were examined by in vivo fluorescence microscopy of the common carotid artery. Platelet adhesion (B) and aggregate formation (C) of 7 experiments per group are summarized. Photomicrographs show representative images 3 minutes after injury in mice treated with vehicle or mGPVI-Fc (2 or 4 mg/kg).
Discussion
Coronary artery thrombosis is frequently initiated by abrupt disruption of the atherosclerotic plaque and activation and aggregation of platelets on subendothelial collagens in the disrupted plaque. GPVI has been identified as the central platelet collagen receptor that mediates the activation of integrins and the release of platelet granules. These processes are crucial for platelet adhesion and thrombus formation, making the GPVI-collagen interaction a promising target for new therapeutics to prevent ischemic cardiovascular events in patients with advanced atherosclerosis.
In the present study, we compared the antithrombotic potential of direct anti-GPVI treatment and competitive inhibition of the GPVI-collagen interaction by soluble GPVI dimer (GPVI-Fc). In 2 different models of arterial thrombosis, anti-GPVI treatment with a monoclonal antibody (JAQ1) resulted in virtually complete inhibition of adhesion and thrombus formation. These results confirm and extend previous findings by us19,25 and others,20 suggesting a crucial role of the GPVI/FcRγ chain complex in thrombus formation in large arteries. Despite the importance of the GPVI-collagen interaction for thrombus formation in these 2 models, the competitive inhibitor GPVI-Fc, at doses up to 8 mg/kg body weight, was without significant effect. This indicates that, although at high concentrations soluble GPVI dimer can reduce platelet adhesion on collagen in vitro (Figure 2B),24 it may have a lower antithrombotic potential in vivo than direct anti-GPVI agents.
Our in vivo results stand in contrast to a recent study in which the investigators used a human GPVI-Fc fusion protein to significantly inhibit platelet adhesion at sites of arterial injury in mice.24 One possible explanation for this discrepancy could be that the proteins have different binding characteristics. This appears, however, unlikely because the investigators reported half-maximal binding of their GPVI-Fc to collagen at a concentration of approximately 6 μg/mL, which is virtually identical to the results we obtained with our 2 fusion proteins (Figure 1 and data not shown, respectively). Furthermore, no major differences in bioavailability appear to exist between the proteins. Massberg et al26 reported plasma concentrations of approximately 6, approximately 5, and 1 μg/mL at 10 minutes, 30 minutes, and 24 hours, respectively, after injection of 60 μg GPVI-Fc per mouse (corresponding to approximately 2 mg/kg body weight). These results are similar to our observations (Figure 3) demonstrating that the pharmacokinetics of the different hGPVI-Fc proteins in mice are comparable. In contrast, clearing of mGPVI-Fc was slightly delayed compared with that of hGPVI-Fc, suggesting that the nature of the GPVI portion of the fusion protein partly determines its in vivo half-life (Figure 7). This indicates that hGPVI-Fc could display significantly different pharmacokinetics in humans than in mice. However, because even mGPVI-Fc displayed only a minor inhibitory effect in our thrombosis model (Figure 7), it appears that even optimal bioavailability of GPVI-Fc may not result in antithrombotic protection comparable to that achieved by anti-GPVI treatment.
Anti-GPVI agents can fully block or deplete the receptor before injury occurs, resulting in complete inhibition of GPVI-collagen interactions in vivo. In vitro studies have shown that direct inhibition or absence of GPVI impairs firm platelet adhesion and abolishes thrombus formation on collagen under flow conditions.6,14,22,27 In principle, similar effects can be achieved by competitive inhibition of GPVI-collagen interactions with GPVI-Fc. In vitro, GPVI-Fc can indeed significantly inhibit platelet adhesion to collagen, but only when used at concentrations of 200 μg/mL or more. This observation is in line with the study by Massberg et al,24 who used GPVI-Fc at a concentration of 800 μg/mL to inhibit thrombus formation on collagen in a whole blood perfusion system (ie, more than 100 times the concentration present in vivo). The requirement for such high concentrations of GPVI-Fc to effectively inhibit platelet adhesion to collagen may not be surprising. A number of observations suggest that partial impairment of the GPVI interaction with its binding motif (glycine-proline-hydroxyprolin [GPO]) within collagen has a minor effect or no effect on activation and adhesion. This is best exemplified by studies in mice in which adhesion to collagen is maintained in platelets that express 50% or 20% of the control level of GPVI.28 Conversely, the low frequency of GPO motifs in monomeric collagen is still sufficient to induce thrombus formation under flow conditions in a GPVI-dependent manner.5,6 When injected intravenously, soluble GPVI does not have access to collagen before injury. Thus, in the case of injury, soluble GPVI directly competes with platelet GPVI for binding sites on the newly exposed collagen. This competitive inhibition under flow conditions may be further complicated by the fact that binding of platelet GPVI to collagen at high shear is strongly facilitated by and dependent on GPIb–von Willebrand factor (VWF) interactions.5 This process leads to a relative concentration of platelets and, therefore, platelet GPVI in the vicinity of the exposed extracellular collagen, further increasing the concentration of GPVI-Fc required for competitive inhibition. Besides its low antithrombotic potential, GPVI-Fc could even display platelet-activating properties in humans as the fusion protein presents its Fc portion to tethering/rolling platelets at sites of injury. Human platelets express the activating Fcγ receptor RIIa (FcγRIIa, CD32), which mediates powerful cellular activation when stimulated appropriately. We did not see such effects in our in vivo experiments, but this may be explained by the fact that mouse platelets do not express FcγRIIa.
The significant but limited ability of GPVI-Fc to inhibit GPVI-collagen interactions was further revealed in aggregation studies. In agreement with previous results,8,23 human and mouse GPVI-Fc at 20 μg/mL inhibited aggregation induced by low doses of collagen or CRP. However, this inhibition was rapidly overcome when agonist concentrations were increased, though under conditions of standard aggregometry soluble GPVI dimer should have better access to collagen than platelet GPVI. Our results are similar to those of Massberg et al,26 who also found a limited inhibitory effect of their GPVI-Fc on collagen-induced aggregation. In contrast, platelets from JAQ1-treated mice are completely unresponsive to collagen, and similar observations have been made in patients with autoantibody-induced clearing of GPVI.22 Together, these in vitro observations suggest that competitive inhibition of the GPVI-collagen interaction may be difficult to achieve and, even at very high concentrations of the inhibitor, is likely less effective than direct anti-GPVI treatment. This may explain why we found such clear differences in the antithrombotic efficacy of GPVI-Fc and anti-GPVI treatment (JAQ1) in vivo. In both models tested, the GPVI/FcRγ chain complex plays a crucial role for pathologic thrombus formation as JAQ1-treated and, in some experiments, also FcRγ chain–deficient mice, which lack GPVI,16 were largely protected. Under these conditions, different GPVI-Fc protein preparations at doses up to 8 mg/kg were without significant protective effect. This suggests that the type of injury induced in these models might have been too strong or otherwise inadequate to detect the antithrombotic activity of GPVI-Fc. The injury model used by Massberg et al24 to demonstrate an inhibitory effect of GPVI-Fc on platelet adhesion may thus produce a different type of lesion than seen in our models. This assumption is strongly supported by the observation that Massberg et al24 only find thin layers of activated platelets at the lesion site, whereas in our carotid injury model the formed thrombi are consistently large and do, in many cases, occlude the vessel.25 A similar type of lesion is induced in the aorta model in which, within approximately 10 minutes, the thrombi consistently occlude the vessel (Figure 4).
In addition to the depletion of GPVI, antibodies against the receptor may exert different effects on platelets that could contribute to their high antithrombotic potential. It has been shown that patients with autoantibody-induced GPVI deficiency have strongly reduced FcRγ chain levels in their platelets,22 and there is evidence that the FcRγ chain associates with proteins other than GPVI in the cells, most notably GPIb-V-IX.29 Such an association is also supported by reports showing that GPIb-V-IX induces tyrosine phosphorylation of the FcRγ chain in platelets along with a number of other proteins, including Syk and PLCγ2.29-31 On the other hand, Goto et al27 found reduced adhesion of GPVI/FcRγ chain–deficient human platelets to immobilized VWF under high shear, an effect that cannot be achieved with GPVI-Fc, even at very high concentrations.24 Together, this indicates that anti-GPVI treatment may to some extent indirectly affect the function of GPIb-V-IX and, possibly, other receptors or signaling pathways, which could partly explain the strong antithrombotic protection seen in at times.
Studies in mice suggest that targeting of GPVI with antibodies (or other molecules) may generally result in irreversible down-regulation of the receptor,17,18 and there is increasing evidence that similar mechanisms of GPVI depletion also exist in humans.13,22 Such a GPVI-deficient phenotype can be maintained for several weeks in mice and for years in humans and does not result in a major bleeding phenotype. This suggests that anti-GPVI treatment might be associated with a relatively low risk for clinical hemorrhage while providing maximum antithrombotic protection. In contrast, whereas GPVI-Fc may be similarly safe as an anti-GPVI agent,24 our results indicate that its antithrombotic potential is lower. The requirement for high concentrations of the protein to inhibit platelet adhesion on the one hand and its rapid clearing in vivo on the other strongly suggests that GPVI-Fc would only be usable in acute clinical situations, such as to decrease the ischemic complications of percutaneous coronary interventions (PCIs) or unstable angina. In such clinical settings, however, it may be desirable to achieve maximum platelet inhibition, as shown by the successful use of GPIIb/IIIa inhibitors, which are currently considered the most powerful inhibitors of platelet function.32 Therefore, complete inhibition of GPVI-collagen interactions by direct targeting of GPVI may also be advantageous under these conditions.
Taken together, we have shown that treatment of mice with anti-GPVI antibodies results in virtually complete protection in 2 mouse models of arterial thrombosis, whereas soluble GPVI dimer (GPVI-Fc) was without significant effect under the same conditions. This shows that direct targeting of GPVI provides significantly stronger antithrombotic protection than competitive inhibitors of GPVI function, such as GPVI-Fc. These findings may have important implications for the development of antithrombotics that target GPVI-collagen interactions.
Prepublished online as Blood First Edition Paper, October 26, 2004; DOI 10.1182/blood-2004-06-2391.
Supported by grant Ni556/4-1 from the Deutsche Forschungsgemeinschaft (B.N.).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.
We thank S. Hartmann for excellent technical assistance.

![Figure 5. Characterization of mGPVI-Fc. (A) Three micrograms purified mGPVI-Fc was subjected to SDS-PAGE under nonreducing (NR) and reducing (R) conditions, and the gel was stained with Coomassie brilliant blue. (B) Mictrotiter plates were coated with mGPVI-Fc and then incubated with the indicated HRP-labeled antibodies followed by TMB development (JAQ1, 2, and 3 [anti-GPVI], p0p4 [anti-GPIbα]). (C) Dose-dependent binding of GPVI-Fc to immobilized collagen (50 μg/mL) and CRP (2 μg/mL) was measured by ELISA (mean ± SD of 3 experiments). (D) mGPVI-Fc partly inhibits collagen- and CRP-induced mouse platelet aggregation. Under stirring conditions, mouse PRP was preincubated with 20 μg/mL GPVI-Fc and then stimulated with the indicated concentrations of collagen or CRP. Light transmission was recorded on a Fibrintimer 4-channel aggregometer. Results shown are representative of 6 experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/105/4/10.1182_blood-2004-06-2391/6/m_zh80040573890005.jpeg?Expires=1767867931&Signature=YRzo-O2ZZgU9kUn2RgoCyuu-2cxHpD2RbvzOhIT5AmA~qNc8fPGiLPwQXMrGv7afovGJ9Qs0fVX59FyOrqBCLUCpbJnE6M~xcaIO9DoJOVk~6Dsn7E9Q8HezFsZk9-UYCBLFJl1D7FyeetszJa5w~xCmIYYuhRXmA7Wq00oGqasQcbgS4KDoOYZPfXWAZOH326-U91HYLYxmxgCJW8THsY06pRcbS8tjYChKjSaHw-CeoduMy3kBSnYS87d9K6t0tG1ubLvyZdrgVgo0iLekWtEab975zra7Rj8wCoqhxhkXBrRbT3bJBVJ8lyEN7mPCvnoXhVAz6mY0r4ZegBKhtQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal