Comment on Lal et al, page 1669
In this issue, Lal and colleagues report a novel action of retinoic acid in altering cell growth and differentiation by altering RNA translation due to activation of p70 S6 kinase through a PI3-kinase– and mTOR-dependent pathway. This highlights a growing body of literature indicating an interrelation between growth factor– and nuclear receptor–mediated signaling pathways and emphasizes the growing recognition of the importance of translation in cellular growth control.
The classical view of the action of all-trans retinoic acid (ATRA) in acute promyelocytic leukemia (APL) holds that the chimeric promyelocytic leukemia/retinoic acid receptor α (PML-RARα) protein is an aberrant repressor of genes required for the white cell to transit through the promyelocyte stage of differentiation. Therapeutic doses of ATRA release corepressors from PML-RARα and its targets and allow for the recruitment of coactivators and reactivation of the target genes. This view must be modified in view of increasing data that retinoic acid may mediate a number of nontranscriptional changes in cell signaling by mechanisms apart from the direct effects of the nuclear receptor on transcription. Reciprocally, a change in the cellular milieu due to the effects of growth factors alters nuclear receptor function and responsiveness.
Retinoic acid can stimulate phosphorylation and activation of signal transducers and activators of transcription 1 (STAT1)1 within 2 hours, so rapidly that it is very unlikely that this effect is mediated by a transcriptional effect of the RAR. STAT activation is important for the biologic effects of ATRA since engineered expression of a nonphosphorylatable form of STAT1 blocks ATRA-mediated myeloid differentiation. Platanias's group2 and others previously found an interrelationship between p38 mitogen-activated protein (MAP) kinase signaling and retinoic acid. ATRA can stimulate the activity of both p38 and p42/44MAP kinase, but inhibitor studies showed that extracellular signal-related kinase (ERK) signaling was required for differentiation whereas p38 signaling blocked differentiation.2 Recently, ATRA was shown to rapidly lead to cyclic adenosine monophosphate (AMP) accumulation in APL cells, most likely by stimulation of adenyl cyclase, leading to protein kinase A (PKA) activation and enhanced expression of RAR target genes and differentiation. A PKA antagonist inhibited differentiation of these cells.3 PKC is also activated by ATRA and plays a role in differentiation. The study by Lal et al in this issue of Blood extends these observations by showing that ATRA treatment of an APL cell line rapidly led to activation of p70 S6 kinase. Using inhibitors, they mapped this activation upstream to the stimulation of mammalian target of rapamycin (mTOR) as well as activation of the phosphatidylinositol 3–kinase (PI3K) pathway. Downstream of S6 kinase, the authors found that ATRA treatment was associated with phosphorylation and deactivation of elongation factor 4E-BP1, an inhibitor of RNA translation mediated by the cap-dependent mechanism. Inhibitors of the PI3K pathway as well as rapamycin, an inhibitor of mTOR, both blocked ATRA-mediated differentiation. Together these data offer an additional mechanism for the antiproliferative and differentiation effects of ATRA.FIG1
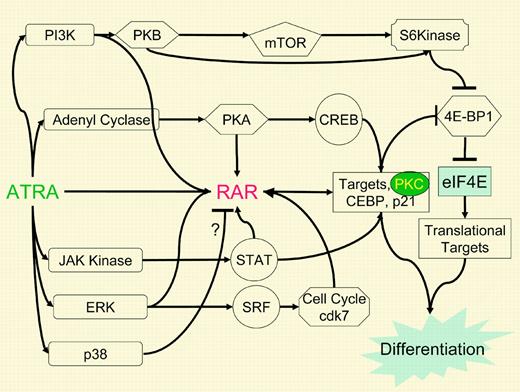
A web of ATRA actions. A flow diagram illustrating the interactions discussed in this capsule and the article by Lal et al. ATRA, by as yet unknown mechanisms, activates a number of signaling pathways usually activated by growth factors. These signaling pathways also impact RAR function. Nontranscriptional and transcriptional effects of ATRA converge at one node, the translational inhibitor 4E-BP1, through the RAR target gene PKCδ as well as p70-S6kinase, which was rapidly activated, presumably through non–RAR-mediated effects on the PI3K pathway. The net result is to augment the action of ATRA on transcription, translation, and the cellular outcome of growth and differentiation.
A web of ATRA actions. A flow diagram illustrating the interactions discussed in this capsule and the article by Lal et al. ATRA, by as yet unknown mechanisms, activates a number of signaling pathways usually activated by growth factors. These signaling pathways also impact RAR function. Nontranscriptional and transcriptional effects of ATRA converge at one node, the translational inhibitor 4E-BP1, through the RAR target gene PKCδ as well as p70-S6kinase, which was rapidly activated, presumably through non–RAR-mediated effects on the PI3K pathway. The net result is to augment the action of ATRA on transcription, translation, and the cellular outcome of growth and differentiation.
These studies illustrate the nonlinear pathway through which ATRA-mediated biology must now be viewed (see figure). However, there are persistent questions about how this all comes about. Specifically how does ATRA activate these kinases? Could ATRA action through the cytosolic retinoic acid binding proteins (CRABs) affect receptor function? Could the interaction of lipophilic retinoids with the membrane alter fluidity and membrane protein interaction? Could critical signaling molecules be retinolyated to activate their function? If ATRA effects on signaling occur rapidly, why is it dependent, at least in APL cells, upon the presence of an intact PML-RARα fusion protein? Furthermore, there are cell-type–specific effects of ATRA that remain unexplained. For example, while Lal et al found that ATRA stimulated PI3K activity in APL cells, Gianni et al4 showed in embryonic carcinoma cells that ATRA inhibited PI3 kinase activity to facilitate the activity of the RAR. These latter data indicate that empiric trials of combinations of ATRA and other agents that affect protein kinase signaling pathways must be performed in the cancer cell type of interest before assuming that the combination might be effective in therapy.
Growth factor signaling also impacts on RAR function, often forming a positive feedback loop to amplify retinoid effects on gene expression. Lal et al, in addition to finding that ATRA activated PI3K, found that inhibition of PI3K activity blocked RAR function. The N-terminal non–ligand-dependent activation domain of RARs is phosphorylated in response to multiple kinases including PKA, cdk7, p38, and ERK. This modification stimulates the transcriptional function of the RAR possibly through transcription factor II H (TFIIH) as well as engagement of the proteosomal machinery. While ATRA stimulates the STAT pathway, STAT pathways stimulate the RAR. For example IL-3 activates STAT5, which forms a complex with the RARα, stimulating gene activation and cell self-renewal.5 All told, the study of Lal et al tells us that we cannot accept the standard model for ATRA action as the whole story. Much more work is needed to fully understand the web of actions encompassed by ATRA therapy. ▪

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal